Abstract
Flavin-containing Trx reductase (TrxR) of Thermoplasma acidophilum (Ta), a thermoacidophilic facultative anaerobic archaeon, lacks the structural features for the binding of 2′-phosphate of nicotinamide adenine dinucleotide phosphate (NADPH), and this feature has justified the observed lack of activity with NADPH; NADH has also been reported to be ineffective. Our recent phylogenetic analysis identified Ta-TrxR as closely related to the NADH-dependent enzymes of Thermotoga maritima and Desulfovibrio vulgaris, both being anaerobic bacteria. This observation instigated a reexamination of the activity of the enzyme, which showed that Ta-TrxR is NADH dependent; the apparent Km for NADH was 3.1 μM, a physiologically relevant value. This finding is consistent with the observation that NADH:TrxR has thus far been found primarily in anaerobic bacteria and archaea.
Introduction
The thioredoxin (Trx) system, which is composed of Trx, Trx reductase (TrxR), and a cognate reductant, is a key component of thiol-based metabolic regulation in almost all living systems. It is often crucial for the survival of these organisms through fluctuations in the availability of energy nutrients and exposure to oxidants in their habitats.1−5 The Trx system implements this regulation through the oxidation and reduction of specific redox-active cysteine pairs of target proteins by the action of Trx, a small redox protein, thereby influencing their catalytic or physical properties.1−5 In the process of reducing a target protein, the Cys residues of the redox-active Cys-X-X-Cys motif (X, variable amino acid residue) of Trx are oxidized. The resulting disulfide of Trx is then reduced by TrxR,1−5 which is of two types: ferredoxin:Trx reductase5 that carries a [Fe4-S4] center and receives electrons from reduced ferredoxin; and flavoprotein Trx reductase that is often dependent on nicotinamide adenine dinucleotide phosphate (NADPH) and consequently known as NADPH:TrxR or NTR.6 Interestingly, certain flavin-containing TrxRs from anaerobic organisms do not use NADPH,7−10 and following are examples of this group (electron donor for TrxR, source organism(s)) (Table 1): reduced ferredoxin,7Clostridium pasteurianum, a fermentative and obligate anaerobic bacterium; NADH,8,9Thermotoga maritima and Desulfovibrio vulgaris, a hyperthermophilic bacterium and a mesophilic sulfate-reducing bacterium, respectively; NADH and NADPH,10,11Pyrococcus horikoshii and Deinococcus radiophilus, a hyperthermophilic archaeon and an aerobic mesophilic bacterium, respectively; reduced coenzyme F420 or F420H2,12Methanocaldococcus jannaschii, a phylogenetically deeply rooted hyperthermophilic methane-producing archaeon. The flavin-containing TrxR of Thermoplasma acidophilum, a thermoacidophilic and facultative anaerobic euryarchaeon, has also been identified as one such enzyme, as a recombinant form of this enzyme (Ta-TrxR) has been shown to be unable to utilize either NADPH or NADH as reductant.16 This heterotroph utilizes peptides and carbohydrates via aerobic or anaerobic respiration. In the absence of oxygen, it uses elemental sulfur as a terminal electron acceptor and generates hydrogen sulfide.13,14T. acidophilum genome carries only one Trx and one TrxR and thus the electron donor of Ta-TrxR would be a key player in the response of the organism to nutrient limitation and oxidative stress. Curiously, our recent bioinformatics analysis suggested the possibility that Ta-TrxR is NADH dependent (Figure 1). The results of our study with purified recombinant Ta-TrxR as presented in this report indeed supported this hypothesis.
Table 1. Biochemical Properties of NADH-Thioredoxin Reductases (TrxR) in Archaea and Bacteriaa.
| characteristics
of flavin-dependent TrxR |
|||||||
|---|---|---|---|---|---|---|---|
|
Km, μM |
kcat/Km of the electron donor (M–1 s–1) | alternate substrate | |||||
| host (archaeon, A; bacterium, B; optimal growth temperature, °C) | ORF number for flavin-dependent TrxR (cognate Trx) studied | electron donor | Trx | NADPH, NADH | references | ||
| Thermoplasma acidophilum (A, 59) | Ta_0984 (Ta_0866) | NADH | ND | -, 3.12 | -, 1.3 × 106 | DTNB (Km, 2.93 mM) | (16) & this study |
| Desulfovibrio vulgaris (B, 37) | Dvu_0377 (Dvu_0378) | NADH | 1.12 | ND, 5.6 | 1.3 × 106 | ND | (10) |
| Pyrococcus horikoshii (A, 98) | Ph_1426 (Ph_0178) | NADPH and NADH | 0.6 | 2.7, 26 | ND | - | (9) |
| Thermotoga maritima (B, 80) | Tm_0869 (Tm_0868) | NADH and NADPH | ND | 780, 73 | 8.4 × 104/8.7 × 106 | DTNB, benzyl viologen, O2 | (8, 19) |
| Deinococcus radiophilus (B, 30–37) | ND | NADH and NADPH | ND | 12.5, 30.2 | ND | DTNB (Km = 463 μM) | (11) |
ND, not determined; -, activity not detectable; Thermococcus onnurineus NA1 carries a NAD(P)H-dependent TrxR which is yet to be analyzed for kinetic constants.32
Figure 1.
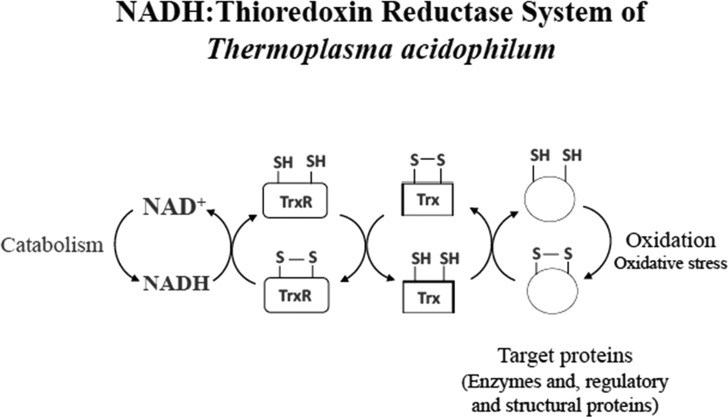
NADH-Thioredoxin system of Thermoplasma acidophilum. Trx system of T. acidophilum composed of a thioredoxin reductase (TrxR) and a thioredoxin (Trx) encoded by ta_0984 and ta_0866orfs, respectively, and NADH, the reductant. Trx reduces disulfide bonds of target proteins, and oxidized Trx is reduced by TrxR with NADH.
Results
Generation of Homogeneous Recombinant Forms of Thermoplasma acidophilum Trx and TrxR (Ta-Trx and Ta-TrxR) Free of E. coli NTR
Ta-Trx and Ta-TrxR of Thermoplasma acidophilum were expressed in E. coli SHuffle T7 Express (NEB, Ipswich, MA) in soluble forms and purified via Ni2+–nitrilotriacetic acid (Ni–NTA) affinity chromatography to homogeneity (Figure 2). This E. coli strain lacks trxB that encodes NTR,6 and this design eliminated the chance of contamination of E. coli NTR in purified proteins. The purified Ta-TrxR preparations exhibited a typical A280/460nm value of 5, which is comparable to that of an E. coli NTR preparation with full incorporation of FAD.15 The Ta-Trx preparation was proficient in reducing insulin with dithiothreitol (DTT) as reductant.16
Figure 2.
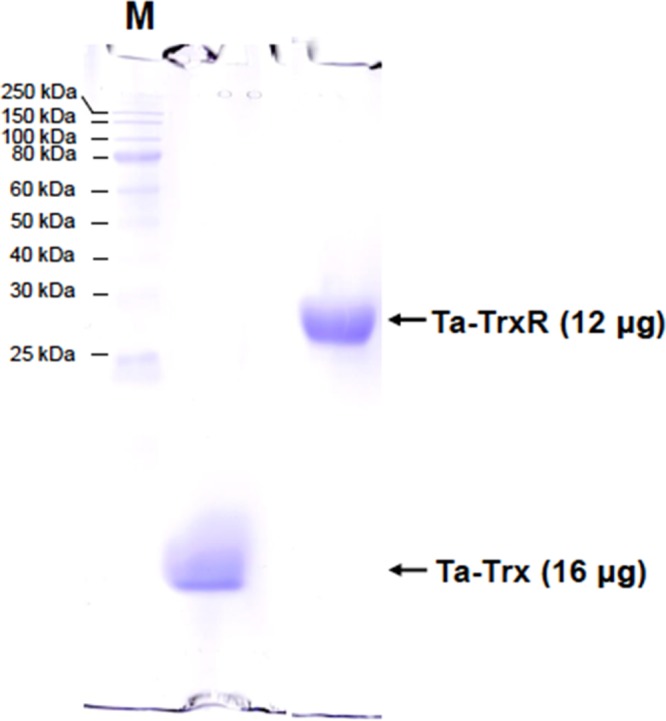
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles of recombinant Ta-Trx and TrxR. A 18% polyacrylamide gel was used. M, Broad Range Protein Ladder (NEB, Ipswich, MA). Ta-Trx and TrxR in a solution containing 100 mM potassium phosphate buffer (pH 7.0) were analyzed.
Catalytic Properties of Ta-TrxR
The first attempt to assay Trx reductase activity of Ta-TrxR involved coupling it with insulin reduction, as with DTT as reductant for Ta-Trx-reduced insulin. However, likely due to a low activity at 25 °C, Ta-TrxR did not exhibit observable activity with NADH or NADPH as reductant; a high-temperature assay was not possible due to denaturation of insulin. The next effort involved spectrophotometric observation of NADH or NADPH oxidation, and the reduction of Ta-Trx was coupled to the reduction of oxidized glutathione, as described previously.12 This coupling helped to maintain the level of oxidized Trx constant.17 In this assay, NADH, but not NADPH, supported activity; the reaction was monitored at 340 nm.12 With a fixed NADH concentration of 40 μM and at Ta-Trx concentrations of 10 and 20 μM, Ta-TrxR exhibited specific activities of 2.2 ± 0.3 and 3.5 ± 0.2 μmol min–1 mg–1, respectively; the activity values represented an average over two measurements with a Ta-TrxR concentration of 50 nM.
For the reasons elaborated in Discussion, the further kinetic analysis of the NADH-dependent activity of Ta-TrxR was performed with 5,5-dithio-bis-2-nitrobenzoic acid (DTNB) as the disulfide substrate in place of Ta-Trx. In this assay, the temperature and pH optima for the activity were found to be 70 °C and 7.5, respectively. With the concentration of NADH varying in the range of 1.6–40 μM and at a fixed concentration of 4 mM DTNB, the apparent values of Km for NADH and Vmax were found to be 3.12 ± 0.45 μM and 7.28 ± 0.32 μmol min–1 mg–1, respectively (Figure 3A). Similarly, assays at a fixed NADH concentration of 40 μM and in the DTNB concentration range of 0.05–4.5 mM yielded an apparent Km value of 2.93 ± 0.16 mM for DTNB (Figure 3B), and the corresponding Vmax value was 10.08 ± 0.29 μmol min–1 mg–1. Selenite, lipoic acid, and oxidized glutathione were not reduced by Ta-TrxR with NADH.
Figure 3.
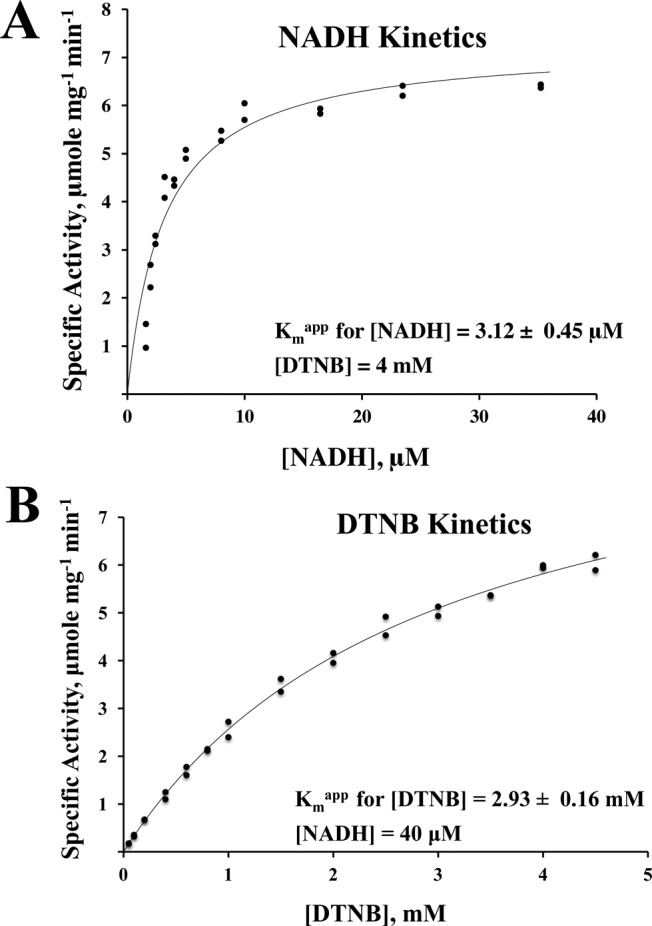
Kinetic analysis of Ta-TrxR. The substrates were 5,5-dithio-bis-2-nitrobenzoic acid (DTNB) and NADH. The assays were performed anaerobically under N2 atmosphere (1.3 × 105 Pa) at 60 °C in the assay mixture containing 100 mM potassium phosphate buffer (pH 7.0), 2 mM ethylenediaminetetraacetic acid (EDTA), and NADH and DTNB at concentrations as indicated. The assays were performed in duplicate, and in each case, the actual values from both assays have been plotted. (A) NADH kinetics; (B) DTNB kinetics. Each solid curve represents the best fit of the respective initial velocity data to the Henri–Michaelis–Menten hyperbola, v = Vmax[S]/(Km + [S]), where S is the substrate for which the concentration was varied. ±, standard deviation.
Comparative Analysis of Primary Structures of Ta-TrxR and Selected TrxRs
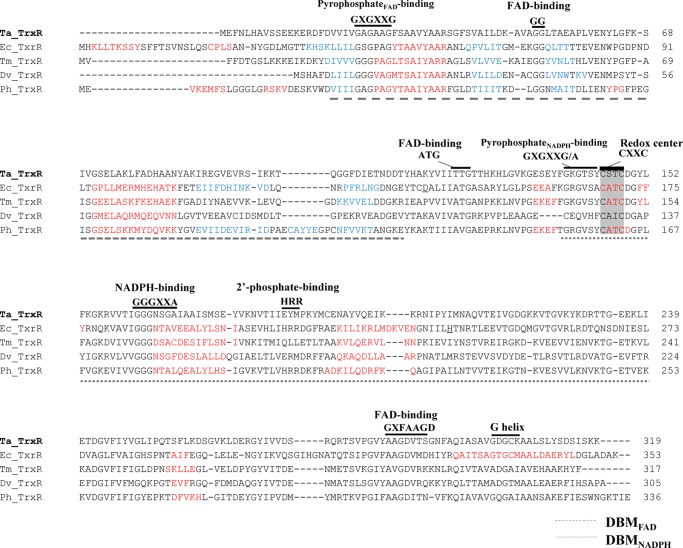
A primary sequence alignment of Ta-TrxR, Ec-NTR, and selected TrxRs of anaerobes is shown in Figure 4. All of these enzymes have been characterized biochemically at the purified enzyme stage,6,8−10,16 and a comparison of Ta-TrxR with the rest at the amino acid sequence level yielded the following values (source organism, electron donor, %identity, and %similarity to Ta-TrxR): P. horikoshii, NADH and NADPH, 40.0 and 53.9%; T. maritima, NADH, 39.1, 55.7%; D. vulgaris, NADH, 31.3 and 48.8%; and E. coli, NADPH, 25.7, 43.7%. Three key structural features of an NTR are the dinucleotide-binding motifs for FAD and NADPH (DBMFAD and DBMNADPH, respectively) and a redox-active Cys-X-X-Cys motif, where X is a variable amino acid residue (Figure 4). The comparison presented in Figure 4 and the X-ray crystallographic structure of the enzyme16 show that most of the DBMFAD-related sequence features of NTR are conserved in Ta-TrxR. However, this was not the case for DBMNADPH,16,18 and the potential implications of the observed differences are presented in Discussion.
Figure 4.
Amino acid sequence alignment of Ta-TrxR and selected TrxRs. The sequence alignment was constructed employing PROMALS3D31 with the use of three-dimensional structures of E. coli NTR (pdb id 1CL0) and Ta-TrxR (pdb id 3CTY) as guides.16,18 Abbreviations (organism name, NCBI accession number): Ta, Thermoplasma acidophilum, WP_010901395; Tm, Thermotoga maritima, AAD35951.1; Dv, Desulfovibrio vulgarisstr. Hildenborough, AAS94860.1; Ph, Pyrococcus horikoshii OT3, BAA30532; Ec, Escherichia coli, EGJ07907.1. Conserved amino acids are shown in bold. Consensus predicted secondary structures: red h, α helix; blue e, β strand. Gray shading: the redox-active CXXC motif. Black bar: conserved motifs as found in low-molecular-weight NTR and flavoproteins and labeled as such. DBM: dinucleotide-binding motif.
Discussion
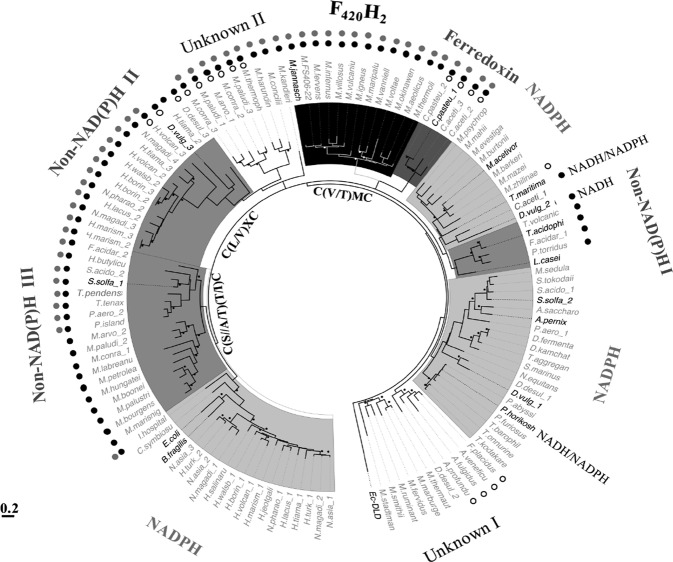
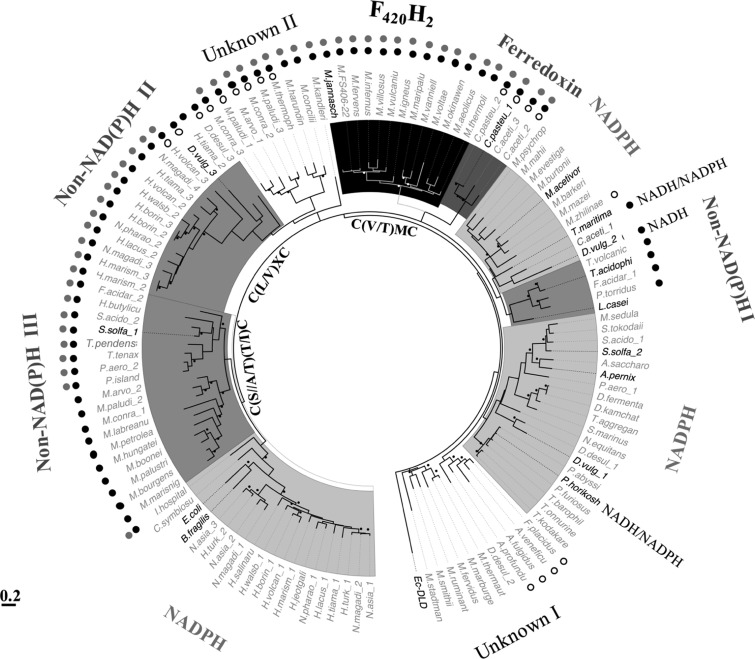
This study was instigated by the results of our recent phylogenetic analysis of low-molecular-mass flavin-containing TrxRs from several archaea and bacteria (Figure 5).12 Some of these enzymes do not utilize NADPH, even though they exhibit significant structural similarities to NTR, and thus far NADH, ferredoxin, and coenzyme F420H2 have been identified as alternate electron donors.10,7,12 In this context, the observed phylogenetic position of Ta-TrxR was intriguing. It belonged to the non-NADPH I group and showed a close relationship with an NTR group of methanogens from the class of Methanomicrobiales and certain bacteria and archaea (Figure 5). Included in this group were TrxRs of Thermotoga maritima and Desulfovibrio vulgaris (TrxR2).8,10 Both of these organisms are anaerobic bacteria, and T. maritima is a thermophile.19,20 The T. maritima enzyme prefers NADH over NADPH (Kmapp NADH = 7.3 μM; Kmapp NADPH = 780 μM), whereas TrxR2 of D. vulgaris exclusively uses NADH with an apparent Km of 5.6 μM.8,10 In the primary sequence, Ta-TrxR was found to be closely related to the Pyrococcus horikoshii enzyme, moderately related to the D. vulgaris enzyme and distant from E. coli NTR. Similar to the T. maritima enzyme, the TrxR of P. horikoshii, an anaerobic thermophilic archaeon, uses both NADH and NADPH, with a preference for NADH (Kmapp NADH = 26 μM; Kmapp NADPH = 2.7 μM) (Table 1).9,11 Taken together, the observations presented above suggested that Ta-TrxR, an enzyme from a facultative anaerobe, might use NADH as electron donor for Trx reduction.
Figure 5.
Maximum-likelihood phylogenetic analysis of noncanonical flavin-dependent thioredoxin reductases (TrxR) from archaea and bacteria and selected NTR. This figure has been reproduced from ref (12) with permission. A noncanonical TrxR is defined by the lack of the HRR motif, as shown in Figure 4. Taxa labels in bold, bona fide TrxR validated by direct activity assay. Black and white bullets near branches: bootstrap values >70 (calculated from 100 replicates). Each label appearing on the outer side of the tree: electron donor utilized by the members of the respective clade. Unknown: TrxR with unknown reductant. Non-NAD(P)H: inability to use NAD(P)H as electron donor as determined via direct activity assay. Symbols for the departures in the amino acid sequences at select conserved elements of TrxRs, as described in Figure 4: filled black and gray circles, unrecognizable HRR and GGG motifs, respectively; unfilled circle, absence of the GG motif. Ec-DLD, dihydrolipoamide dehydrogenase of E. coli used as an outgroup. Taxa abbreviations, A. fulgidus, Archaeoglobus fulgidus; A. pernix, Aeropyrum pernix K1; A. profundu, Archaeoglobus profundus; A. saccharo, Acidolobus saccharovorans; A. veneficu, Archaeoglobus veneficus; B. fragilis, Bacteroides fragilis; C. aceti, Clostridium acetibutylicum; C. pasteu, Clostridium pasteurianum; C. symbiosu, Cenarchaeum symbiosum A; D. desul, Desulfovirbrio desulfuricans; D. fermenta, Desulfurococcus fermentans; D. kamchat, Desulfurococcus kamchatkensis; D. vulg, Desulfovibrio vugaris str. Hildenborough; E. coli, Escherichia coli; F. acidar, Ferroplasma acidarmanus; F. placidus, Ferroglobus placidus; H. walsb, Haloquadratum walsbyi; H. borin, Halogeometricum borinquense DSM11551; H. butylicu, Hyperthermus butylicus; H. jeotgali, Halalkalicoccus jeotgali; H. lacus, Halorubrum lacusprofundi; H. marism, Haloarcula marismortui ATCC 43049; H. salinaru, Halobacterium salinarum; H. tiama, Halorhabdus tiamatea; H. turkm, Haloterrigena turkmenica; H. volcan, Haloferax volcanii; I. hospital, Ignicoccus hospitalis; L. casei, Lactobacillus casei; M. mazei, Methanosarcina mazei; M. thermoli, Methanothermococcus thermolithotrophicus; M. acetivor, Methanosarcina acetivorans C2A; M. aeolicus, Methanococcus aeolicus; M. arvoryza, Methanocella arvoryzae MRE50; M. barkeri, Methanosarcina barkeri; M. boonei, Methanoregula boonei 6A8; M. bourgens, Methanoculleus bourgensis; M. burtonii, Methanococcoides burtonii; M. concilii, Methanosaeta concilii; M. conradii, Methanocella conradii HZ254; M. evestiga, Methanohalobium evestigatum; M. fervens, Methanocaldococcus fervens; M. fervidus, Methanothermus fervidus; M. FS406-22, Methanocaldococcus sp. FS406-22; M. harundin, Methanosaeta harundinacea; M. hungatei, Methanospirillum hungatei JF-1; M. igneus, Methanotorris igneus; M. infernus, Methanocaldococcus infernus; M. jannasch, Methanocaldococcus jannaschii; M. kandleri, Methanopyrus kandleri; M. labreanu, Methanocorpusculum labreanum; M. mahii, Methanohalophilus mahii; M. marburge, Methanothermobacter marburgensis; M. maripalu, Methanococcus maripaludis S2; M. marisnig, Methanoculleus marisnigri JR1; M. okinawen, Methanococcus okinawensis; M. paludi, Methanocella paludicola SANAE; M. palustri, Methanosphaerula palustris; M. petrolea, Methanolacinia petrolearia DSM11571; M. psychrop, Methanolobus psychrophilus R15; M. ruminant, Methanobrevibacter ruminantium M1; M. sedula, Methanosphaera sedula; M. smithii, Methanobrevibacter smithii; M. stadtman, Methanosphaera stadtmanae; M. thermaut, Methanothermobacter thermautotrophicus strain Delta H; M. thermop, Methanosaeta thermophila; M. vanniell, Methanococcus vannielii; M. villosus, Methanocaldococcus villosus; M. voltae, Methanococcus voltae; M. vulvaniu, Methanocaldococcus vulcanius; M. zhilinae, Methanosalsum zhilinae; N. asia, Natrialba asiatica; N. equitans, Nanoarchaeum equitans; N. magadi, Natrialba magadii; N. pharao, Natromonas pharaonic; P. abyssi, Pyrococcus abyssi GE5; P. aero, Pyrobaculum aerophilum str. IM2; P. furiosus, Pyrococcus furiosus DSM 3638; P. island, Pyrobaculum islandium; P. torridus, Picrophilus torridus; S. acido, Sulfolobus acidocaldarius; S. marinus, Staphylothermus marinus; S. solfa, Sulfolobus solfataricus; S. tokodai, Sulfolobus tokodai str.7; T. acido, Thermoplasma acidophilum; T. aggregan, Thermosphaera aggragans; T. barophil, Thermococcus barophilus; T. kodakaren, Thermococcus kodakarensis; T. maritima, Thermotoga maritima; T. onnurine, Thermococcus onnurineus; T. pendens, Thermofilum pendens; T. tenax, Thermoproteus tenax; T. volvanic, Thermoplasma volcanicum.
The above hypothesis is consistent with the structural features of Ta-TrxR.16 The differences between the DBMFAD sequences of Ta-TrxR and E. coli NTR were minor16 (Figure 4). The notable one concerned the first residue of the ATG motif, which was Ala in NTR and Thr in Ta-TrxR. However, there exist major differences between the DBMNADPH features of E. coli NTR and Ta-TrxR. The HRR element of the 2′-phosphate-binding motif (VXXXHRRDXXRA) of E. coli NTR is absent in Ta-TrxR.15 In E. coli NTR, His175, Arg176, and Arg177 constitute the HRR motif, which is followed by Asp178 (Figure 4).16,18 In Ta-TrxR, the equivalent residues were Glu185, Tyr186, Met187, and Pro188, respectively. The change of His175 (E. coli NTR) to Glu185 (Ta-Trx) could create an unfavorable interaction of Ta-TrxR with 2′-phosphate of NADPH. Similarly, the presence of Pro, a nonpolar and structurally constrained residue, in place of Asp, a polar acidic residue, in the vicinity of the HRR element (Pro188 in Ta-TrxR vs Asp178 in E. coli NTR), could offer steric hindrance at the NADPH-binding site. In fact, docking studies with the three-dimensional structure of Ta-TrxR have shown that although Glu185 could repel 2′-phosphate of NADPH electrostatically, this unfavorable interaction would be removed with NADH.15 Additional suggestions along this line come from a comparative analysis of the DBMNADPH sequences of Ta-TrxR (Figure 4). There are subtle structural differences between E. coli NTR and Ta-TrxR in the vicinity of the HRR motif. The GXGXXG/A motif of the DBMNADPH of E. coli NTR was found in Ta-TrxR; however, in the latter, the terminal residue is Tyr and not Ala, and in other NADH-dependent TrxRs, the respective residue was either Tyr or Phe (Figure 4). This feature further suggested the use of NADH by Ta-TrxR.
Our biochemical characterizations of the enzyme showed that Ta-TrxR indeed used NADH for the reduction of Ta-Trx. The kinetic analysis of this reaction was impeded by the observation that although a rise of Ta-Trx concentration from 5 to 10 μM increased the activity 1.6-fold, any further increase in the concentration led to the precipitation of this substrate. For this reason, for further studies on the kinetic properties of Ta-TrxR, DTNB was used as the disulfide substrate in place of Ta-Trx. The Km values for NADH (3.12 μM) and DTNB (2.93 mM) obtained from these assays were typical of flavin-containing TrxRs.6,8−10 Thus, the thioredoxin reductase of T. acidophilum is a bona fide NADH-dependent enzyme. This property is fully supported by the redox potential value of the Ta-TrxR-bound flavin, which was determined to be −305 mV,21 as it makes NADH a thermodynamically accessible electron donor for this enzyme.
The prokaryotic TrxRs that use solely NADH as an electron donor thus far have been found in obligate and facultative anaerobic bacteria and archaea.8−10 However, TrxRs that can use NADH as well as NADPH have been reported in both anaerobes and aerobes (Table 1). The Deinococcus radiophilus TrxR is the sole example of such an enzyme from an aerobic bacterium. However, it shows preference for NADPH.11 Interestingly, a phylogenetic analysis suggested that D. radiophilus obtained the enzyme through horizontal gene transfer from anaerobic archaea.11 These observations suggest that the NADH-dependent TrxRs likely originated in anaerobes.
It is not clear why the anaerobes would prefer NADH to NADPH for Trx reduction. One of the possible reasons is to achieve energy efficiency. Generally, anaerobic metabolism generates less ATP than the aerobic counterparts and the use of NADH avoids investment in the conversion of NADH to NADPH by NAD kinase, which requires ATP.22 However, the T. acidophilum genome encodes both NAD+/NADH- and NADP+/NADPH-dependent enzymes, which makes the above-mentioned explanation less likely.
Our findings have shown that the investigations on the electron donors of yet-to-be characterized flavin-containing Ta-TrxRs that lack specific structural features for binding of the 2′-phosphate of NADPH could be guided by their relative phylogenetic positions. Our phylogenetic studies have also indicated that there exist at least four more groups of flavin-containing TrxRs that are likely to be non-NADPH types and need to be investigated for their reductants.
Materials and Methods
Materials
E. coli SHuffle T7 Express competent cells and restriction enzymes were obtained from the New England Biolabs (Ipswich, MA). Thermoplasma acidophilum genomic DNA and pTev5 were generous gifts from Dr. Dennis G. Searcy of University of Massachusetts Amherst and Dr. Jorge Escalante-Semerena of the University of Georgia, respectively. All chemicals were purchased from either Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Waltham, MA).
Heterologous Expression of TrxR and Trx of T. acidophilum (Ta-TrxR and Ta-Trx) in E. coli SHuffle T7 Express
Coding sequences for Ta-TrxR and Ta-Trx were amplified from T. acidophilum genomic DNA using Q5 DNA polymerase (NEB, Ipswich, MA), and the respective amplicons were cloned into NdeI and BamHI sites of pTev5, a T7-based expression vector,23 resulting pUL207 and pUL208, respectively. These plasmids were designed to express recombinant proteins with an NH2-terminal His6-tag, followed by a TEV protease recognition site. Both plasmids were transformed into E. coli SHuffle T7 Express competent cells (NEB, Ipswich, MA) containing pRIL plasmid (Stratagene, La Jolla, CA). The resulting strains were grown at 37 °C in Luria Bertani media containing 100 and 34 μg/mL ampicillin and chloramphenicol, respectively. For the expression of Ta-Trx, the LB media was supplemented with DTT at a final concentration of 0.2 mM. When a growing culture reached an optical density (OD600) of 0.8, as measured with a DU800 UV–visible spectrophotometer (Beckman Coulter, Inc., Brea, CA), IPTG was added to a final concentration of 0.4 mM and the cultivation was continued for additional 5 h at 37 °C for Ta-TrxR overexpression and for 12 h at 15 °C for Ta-Trx overexpression. The cells from these cultures were harvested by centrifugation at 10 000g for 10 min at 4 °C using a Sorvall LYNX 4000 centrifuge (Thermo Fisher Scientific, Waltham, MA), and the cell pellets were stored at −20 °C until used.
Purification of Recombinant Ta-TrxR and Ta-Trx
This task was performed under air and as previously described.12 Typically, 4 g of frozen cell pellets of E. coli SHuffle T7 Express harboring pRIL and either pUL207 or pUL208 were thawed on ice and resuspended in 4 mL of a lysis buffer containing 50 mM sodium phosphate buffer (pH 7.0), 300 mM NaCl, 10 mM imidazole, and a cOmplete EDTA-free Protease Inhibitor Cocktail tablet (Roche Life Sciences, Indianapolis, IN). The cells were then lysed by three passages of the suspension through a French pressure cell operating at a pressure of 1.28 × 108 Pa, and the lysate was centrifuged at 18 000g at 4 °C for 30 min. The resulting supernatant was subjected to heating at 80 °C for 10 min to precipitate most of E. coli proteins, which were then removed by centrifugation at 18 000g at 4 °C for 30 min. From the clarified supernatant, recombinant protein was purified via Ni–NTA chromatography.12 Both Ta-TrxR and Ta-Trx were eluted at an imidazole concentration range of 100–200 mM. The column fractions that contained homogeneous proteins were then pooled and concentrated using an Amicon stirred ultrafiltration cell fitted with a membrane with a desired molecular weight cutoff (MWCO) (EMD Millipore, Bedford, MA); the MWCO was 10 kDa for Ta-TrxR and 3 kDa for Ta-Trx. To cleave the NH2-terminal His6-tag, the purified protein was incubated overnight at 4 °C with recombinant TEV protease carrying a His6-tag (rHis6-TEV)24 at a protein-to-rHis6-TEV ratio of 20:1, following a protocol as described previously.12 From the resulting mixture, Ta-TrxR carrying intact His6-tag, cleaved His6-tag, and rHis6-TEV were removed by passing through a Ni–NTA column. The purities of as-purified and digested proteins were analyzed by SDS-PAGE, and the protein concentrations were determined via Bradford assay using a protein assay kit (Bio-Rad Laboratories, Hercules, CA) following the manufacturer’s protocol.25
Insulin Disulfide Reductase Assay for Ta-Trx and Ta-TrxR
Disulfide reductase activity of Ta-Trx was measured by insulin reduction assay, as described previously, using DTT as an artificial electron donor.26,27 A standard assay mixture contained 50 μM Ta-Trx, 0.8 mg/mL insulin, 2 mM EDTA, 100 mM potassium phosphate buffer (pH 7.0), and 1 mM DTT. This assay was also used to test the reactivity of Ta-TrxR toward Ta-Trx, where it was performed under anaerobic conditions,12 DTT was replaced by NADH (0.1 mM), and Ta-TrxR was added to a final concentration of 100 nM. The assay temperature was 25 °C. The reaction was initiated by the addition of the reductant, DTT or NADH, and the increase of absorbance at 650 nm indicating insulin reduction was monitored using a Beckman Coulter DU800 spectrophotometer (Brea, CA).
Kinetic Assays of Ta-TrxR
The assay was performed anaerobically at 60 °C as described previously26 using an assay mixture (total volume, 800 μL) that contained 100 mM potassium phosphate buffer (pH 7.0), 2 mM EDTA, and desired levels of NADH and Ta-Trx or an alternate electron acceptor, such as 5′-5′-dithiobis(2-nitrobenzoate) or DTNB. For assays with Ta-Trx, the concentration of this electron acceptor was maintained constant by supplementing the assay mixture with oxidized glutathione to a final concentration of 1 mM.17 In each case, the reaction was initiated by the addition of the enzyme, and in all cases except with DTNB as electron acceptor, it was monitored at 340 nm for the consumption of NADH; with DTNB, the monitoring was at 412 nm. The initial velocity values were calculated using an extinction coefficient value of 6.2 mM–1 cm–1 for NADH at 340 nm or 14.15 mM–1 cm–1 for 5-thio-2-nitrobenzate or TNB at 412 nm.28 For pH studies, the potassium phosphate buffer was replaced by constant ionic strength buffers composed of 60 mM MES, 120 mM Tris, and 60 mM glacial acetic acid and adjusted to the desired pH values (4–9.5) with HCl or NaOH.29 To determine the activities of Ta-TrxR toward alternate substrates, Trx was replaced by one of the following (entity, (final concentration)): DTNB (varied), Na2SeO3 (50 μM), and oxidized l-glutathione (1 mM) and lipoate (1 mM). The apparent kinetic constants were calculated by fitting the initial velocity data to the Henri–Michaelis–Menten equation, v = Vm [S]/(Km + [S]), where S is the substrate for which the concentration is varied and Vm and Km are maximum velocity and Michaelis constant, respectively; KaleidaGraph 4.5 (Synergy Software Inc., Reading, PA) was used for data fitting.
Bioinformatic Analysis
Primary and secondary structure alignments of Ta-TrxR and its close homologues were performed using MUSCLE30 and PROMALS3D,31 respectively. For the latter, three-dimensional structures of E. coli NTR and T. acidophilum TrxR (pdb id: 1CL0 and 3CTY, respectively) served as references.16,18
Acknowledgments
This work was supported by the National Aeronautics and Space Administration Astrobiology: Exobiology and Evolutionary Biology Grant NNX13AI05G to B.M. and the Virginia Tech Agricultural Experiment Station Hatch Program (CRIS project VA-160021). The publication cost was partly defrayed by the Virginia Tech Open Access Subvention Fund.
Glossary
Abbreviations
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- Ta
Thermoplasma acidophilum
- NTR
NADPH-thioredoxin reductase
- FAD
flavin adenine dinucleotide
- DBM
dinucleotide-binding domain
- DTNB
5,5-dithio-bis-2-nitrobenzoic acid
Author Contributions
D.S. and B.M. designed the research; D.S., U.L., and A.C. performed the research; D.S., U.L., and B.M. analyzed the data; and D.S. and B.M. wrote the article.
The authors declare no competing financial interest.
References
- Arnér E. S.; Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 1989, 264, 13963–13966. [PubMed] [Google Scholar]
- Lu J.; Holmgren A. The thioredoxin antioxidant system. Free Radical Biol. Med. 2014, 66, 75–87. 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- Hirt R. P.; Muller S.; Embley T. M.; Coombs G. H. The diversity and evolution of thioredoxin reductase: new perspectives. Trends Parasitol. 2002, 18, 302–308. 10.1016/S1471-4922(02)02293-6. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B.; Schurmann P.; Wolosiuk R. A.; Jacquot J. P. The ferredoxin/thioredoxin system: from discovery to molecular structures and beyond. Photosynth. Res. 2002, 73, 215–222. 10.1023/A:1020407432008. [DOI] [PubMed] [Google Scholar]
- Thelander L. Studies on Thioredoxin Reductase from Escherichia coli B - Relation of Structure and Function. Eur. J. Biochem. 1968, 4, 407. 10.1111/j.1432-1033.1968.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Hammel K. E.; Cornwell K. L.; Buchanan B. B. Ferredoxin/flavoprotein-linked pathway for the reduction of thioredoxin. Proc. Natl. Acad. Sci. U.S.A. 1983, 80, 3681–3685. 10.1073/pnas.80.12.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Ma K. Characterization of a thioredoxin-thioredoxin reductase system from the hyperthermophilic bacterium Thermotoga maritima. J. Bacteriol. 2010, 192, 1370–1376. 10.1128/JB.01035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima Y.; Ishikawa K. A hyperthermostable novel protein-disulfide oxidoreductase is reduced by thioredoxin reductase from hyperthermophilic archaeon Pyrococcus horikoshii. Arch. Biochem. Biophys. 2003, 418, 179–185. 10.1016/j.abb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Pieulle L.; Stocker P.; Vinay M.; Nouailler M.; Vita N.; Brasseur G.; Garcin E.; Sebban-Kreuzer C.; Dolla A. Study of the Thiol/Disulfide Redox Systems of the Anaerobe Desulfovibrio vulgaris Points Out Pyruvate:Ferredoxin Oxidoreductase as a New Target for Thioredoxin 1. J. Biol. Chem. 2011, 286, 7812–7821. 10.1074/jbc.M110.197988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H. J.; Lee Y. N. Characterization of Deinococcus radiophilus thioredoxin reductase active with both NADH and NADPH. J. Microbiol. 2010, 48, 637–643. 10.1007/s12275-010-0283-7. [DOI] [PubMed] [Google Scholar]
- Susanti D.; Loganathan U.; Mukhopadhyay B. A Novel F420-dependent Thioredoxin Reductase Gated by Low Potential FAD: A Tool for REdox Regulation in an Anaerobe. J. Biol. Chem. 2016, 291, 23084–23100. 10.1074/jbc.M116.750208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerer A.; Langworthy T. A.; Stetter K. O. Thermoplasma acidophilum and Thermoplasma volcanium Sp. nov. from Solfatara Fields. Syst. Appl. Microbiol. 1988, 10, 161–171. 10.1016/S0723-2020(88)80031-6. [DOI] [Google Scholar]
- Darland G.; Brock T. D.; Samsonoff W.; Conti S. F. Thermophilic, Acidophilic Mycoplasma Isolated from a Coal Refuse Pile. Science 1970, 170, 1416. 10.1126/science.170.3965.1416. [DOI] [PubMed] [Google Scholar]
- Moore E. C.; Reichard P.; Thelander L. Enzymatic Synthesis of Deoxyribonucleotides.V. Purification and Properties of Thioredoxin Reductase from Escherichia Coli B. J. Biol. Chem. 1964, 239, 3445–3452. [PubMed] [Google Scholar]
- Hernandez H. H.; Jaquez O. A.; Hamill M. J.; Elliott S. J.; Drennan C. L. Thioredoxin reductase from Thermoplasma acidophilum: a new twist on redox regulation. Biochemistry 2008, 47, 9728–9737. 10.1021/bi8006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzok S. M.; Rahlfs S.; Becker K.; Schirmer R. H. Thioredoxin, thioredoxin reductase, and thioredoxin peroxidase of malaria parasite Plasmodium falciparum. Methods Enzymol. 2002, 347, 370–381. 10.1016/S0076-6879(02)47037-1. [DOI] [PubMed] [Google Scholar]
- Lennon B. W.; Williams C. H.; Ludwig M. L. Crystal structure of reduced thioredoxin reductase from Escherichia coli: Structural flexibility in the isoalloxazine ring of the flavin adenine dinucleotide cofactor. Protein Sci. 1999, 8, 2366–2379. 10.1110/ps.8.11.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R.; Langworthy T. A.; Konig H.; Thomm M.; Woese C. R.; Sleytr U. B.; Stetter K. O. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90-Degrees-C. Arch. Microbiol. 1986, 144, 324–333. 10.1007/BF00409880. [DOI] [Google Scholar]
- Heidelberg J. F.; Seshadri R.; Haveman S. A.; Hemme C. L.; Paulsen I. T.; Kolonay J. F.; Eisen J. A.; Ward N.; Methe B.; Brinkac L. M.; Daugherty S. C.; Deboy R. T.; Dodson R. J.; Durkin A. S.; Madupu R.; Nelson W. C.; Sullivan S. A.; Fouts D.; Haft D. H.; Selengut J.; Peterson J. D.; Davidsen T. M.; Zafar N.; Zhou L.; Radune D.; Dimitrov G.; Hance M.; Tran K.; Khouri H.; Gill J.; Utterback T. R.; Feldblyum T. V.; Wall J. D.; Voordouw G.; Fraser C. M. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat. Biotechnol. 2004, 22, 554–559. 10.1038/nbt959. [DOI] [PubMed] [Google Scholar]
- Hamill M. J.; Chobot S. E.; Hernandez H. H.; Drennan C. L.; Elliott S. J. Direct electrochemical analyses of a thermophilic thioredoxin reductase: Interplay between conformational change and redox chemistry. Biochemistry 2008, 47, 9738–9746. 10.1021/bi800676g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni G.; Orsomando G.; Raffaelli N. Structural and functional properties of NAD kinase, a key enzyme in NADP biosynthesis. Mini-Rev. Med. Chem. 2006, 6, 739–746. 10.2174/138955706777698688. [DOI] [PubMed] [Google Scholar]
- Rocco C. J.; Dennison K. L.; Klenchin V. A.; Rayment I.; Escalante-Semerena J. C. Construction and use of new cloning vectors for the rapid isolation of recombinant proteins from Escherichia coli. Plasmid 2008, 59, 231–237. 10.1016/j.plasmid.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blommel P. G.; Fox B. G. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expression Purif. 2007, 55, 53–68. 10.1016/j.pep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Susanti D.; Wong J. H.; Vensel W. H.; Loganathan U.; DeSantis R.; Schmitz R. A.; Balsera M.; Buchanan B. B.; Mukhopadhyay B. Thioredoxin targets fundamental processes in a methane-producing archaeon Methanocaldococcus jannaschii. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 2608–2613. 10.1073/pnas.1324240111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J. Biol. Chem. 1977, 252, 4600–4606. [PubMed] [Google Scholar]
- Riddles P. W.; Blakeley R. L.; Zerner B. Ellman’s reagent: 5,5′-dithiobis(2-nitrobenzoic acid)-A reexamination. Anal. Biochem. 1979, 94, 75–81. 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- Ellis K. J.; Morrison J. F. Buffers of Constant Ionic-Strength for Studying Ph-Dependent Processes. Methods Enzymol. 1982, 87, 405–426. 10.1016/S0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Edgar R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J.; Grishin N. V. PROMALS: towards accurate multiple sequence alignments of distantly related proteins. Bioinformatics 2007, 23, 802–808. 10.1093/bioinformatics/btm017. [DOI] [PubMed] [Google Scholar]
- Choi A. R.; Kim M. S.; Kang S. G.; Lee H. S. Dimethyl sulfoxide reduction by a hyperhermophilic archaeon Thermococcus onnurineus NA1 via a cysteine-cystine redox shuttle. J. Microbiol. 2016, 54, 31–38. 10.1007/s12275-016-5574-1. [DOI] [PubMed] [Google Scholar]