Abstract
The metabolic syndrome is highly prevalent in patients with schizophrenia, and is associated with a state of chronic, low-grade inflammation. Schizophrenia is also associated with increased inflammation, including aberrant blood levels of pro-inflammatory cytokines and high-sensitivity C-reactive protein (hsCRP). The purpose of this study is to investigate the relationship between total and differential white blood cell (WBC) counts, hsCRP, and the metabolic syndrome in patients with schizophrenia and related non-affective psychoses. 59 inpatients and outpatients age 18–70 with non-affective psychotic disorders and 22 controls participated in this cross-sectional study. Subjects had a fasting blood draw between 8 and 9 am for glucose, lipids, total and differential WBC counts, and hsCRP. Vital signs and anthropometric measures were obtained. Patients with non-affective psychosis and the metabolic syndrome had significantly higher total WBC counts, monocytes, and hsCRP levels than patients without the metabolic syndrome (p≤0.04 for each). In binary logistic regression analyses, after controlling for potential confounding effects of age, race, sex, age at first hospitalization for psychosis, parental history of diabetes, smoking, and psychotropic medications, total WBC count, monocytes, and hsCRP were significant predictors of metabolic syndrome in patients (p≤0.04 for each). hsCRP was also a significant predictor of increased waist circumference and triglycerides in patients (p≤0.05 for each). Our findings suggest that measurement of total and differential WBC counts and hsCRP blood levels may be germane to the clinical care of patients with schizophrenia and related disorders, and support an association between inflammation and metabolic disturbance in these patients.
Keywords: Schizophrenia, Nonaffective Psychosis, WBC, Monocytes, Lymphocytes, C-reactive protein, Inflammation, Metabolic Syndrome
1. Introduction
The metabolic syndrome is a constellation of metabolic risk factors associated with the development of atherosclerotic cardiovascular disease (Galassi et al., 2006; Grundy et al., 2005) and cardiovascular disease mortality (Galassi et al., 2006). The metabolic syndrome is common in patients with schizophrenia and related disorders, with a prevalence of 43%—based on American Heart Association criteria (Grundy et al., 2005)—in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE; McEvoy et al., 2005). Cardiovascular disease is also the leading cause of mortality in patients with schizophrenia and related disorders (Saha et al., 2007).
The metabolic syndrome syndrome is also associated with a state of chronic, low-grade inflammation (Devaraj et al., 2010). A recent meta-analysis found that high-sensitivity CRP (hsCRP) was an independent predictor of cardiovascular disease (Kaptoge et al., 2010). Schizophrenia is also associated with increased inflammation, including aberrant blood levels of pro-inflammatory cytokines (Miller et al., 2011) and CRP (Miller et al., in press), including studies in patients with first-episode psychosis and minimal exposure to antipsychotics. The adverse metabolic effects of atypical antipsychotics, which increase metabolic syndrome risk, may potentiate aberrant blood levels of inflammatory markers (Beumer et al., 2012). The efficacy of antipsychotic augmentation with non-steroidal anti-inflammatory agents (NSAIDs) also supports an association between inflammation and the pathophysiology of schizophrenia (Akhondzadeh et al., 2007; Laan et al., 2010; Muller et al., 2002; Muller et al., 2010).
Several large population-based samples found that total and differential white blood cell (WBC) counts were associated with metabolic syndrome risk and individual metabolic syndrome criteria (Kim et al., 2008; Lao et al., 2008). Within schizophrenia, one study found that higher total WBC counts are associated with increased risk of the metabolic syndrome and more severe psychopathology (Fan et al., 2010). Several studies have also reported significant associations between CRP levels and components of the metabolic syndrome in patients with schizophrenia. Fawzi et al. (2011) found higher mean CRP levels in males with waist circumference >94 cm (versus <94 cm) compared to age- and sex-matched patients. Another study found that CRP levels were significantly positively correlated with BMI and significantly negatively correlated with HDL levels (Carizzo et al., 2008). Vuksan-Cusa et al. (2010) found that subjects with CRP level >5 mg/L (versus <5 mg/L) were twice as likely to have the metabolic syndrome. Although previous studies have reported increased blood levels of total monocytes (Wilke et al., 1996; Zorrilla et al., 1996) and lymphocytes (Masserini et al., 1990; Sperner-Unterweger et al., 1999), differential WBC counts have not been explored in schizophrenia as a predictor of the metabolic syndrome.
The purpose of this study is to investigate the relationship between total and differential WBC counts, hsCRP, and the metabolic syndrome in patients with schizophrenia and related non-affective psychosis and in controls. We hypothesize that total WBC count, monocytes, lymphocytes, and hsCRP are associated with metabolic syndrome risk and individual metabolic syndrome criteria in these subjects.
2. Methods
2.1. Subjects
Fifty-nine inpatients and outpatients age 18–70 and diagnosed with schizophrenia (n=39), brief psychotic disorder (n=1), psychotic disorder not otherwise specified (n=4), or schizoaffective disorder (n=15), and twenty-two controls, who were part of a larger, ongoing study of immune function in schizophrenia and related disorders, were recruited in the Augusta, Georgia area between July 2010 and May 2012. Recruitment was non-randomized. Subjects were referred to the investigators by their inpatient or outpatient psychiatrist. Exclusion criteria for all subjects for the present study included alcohol withdrawal; pregnancy; current scheduled use of non-steroidal anti-inflammatory agents, corticosteroids, or other immunomodulatory agents; history of diabetes or current use of anti-diabetic therapy; history of hyperlipidemia or current lipid-lowering therapy; history of exposure to an antibiotic in the past 2 weeks (based on subject’s self-report plus a review of their electronic medical record); history of an immune disorder; and illicit drug use in the past 30 days. No subject had a history of recent trauma or surgical intervention. Additional exclusion criteria for controls were lifetime diagnosis of schizophrenia or related disorder; lifetime or current diagnosis of a manic, depressed, or mixed affective episode, or history of exposure to an antipsychotic, antidepressant, valproate, lithium or gabapentin.
2.2. Procedures
After providing written informed consent, subjects underwent a laboratory, physical, and psychiatric diagnostic evaluation. Subjects had a blood draw between 8 and 9 am after a ten-hour fast. Vital signs and anthropometric measures, including height, weight, waist circumference (at the level of the umbilicus) and hip circumference were obtained. Diagnosis (or absence of a diagnosis in the controls) was verified using the Structured Clinical Interview for DSM-IV disorders (SCID) psychosis and mood disorders modules. For patients, symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS). A total of four different raters performed the SCID and PANSS interviews for subjects in this study, although one rater (BJM) performed the majority (n=49, 61%) and together three raters performed 94% (n=76) of the interviews. All raters were trained to perform the SCID and PANSS and inter-rater reliability with the principal investigator (BJM) was well established, with 100% concordance on a series of ten training vignettes. Data on age at first hospitalization for psychosis and parental history of diabetes were also obtained by patient interview. Data on smoking (number of cigarettes per day) and other substance use was obtained using the Dartmouth Assessment of Lifestyle Inventory. The study was approved by the IRB’s of both Georgia Health Sciences University and the Georgia Department of Community Health.
2.3. Laboratory evaluation
Blood analyses were performed at Clinical Pathology Laboratories Southeast (Augusta, Georgia). Complete blood counts with differential, fasting serum glucose, and lipid panels were analyzed by standard clinical laboratory assays. Glucose and lipids were measured using an Olympus AU2700 Chemistry-Immuno Analyzer (Olympus America, Inc., Melville, NY). CBC with differential was analyzed using a COULTER LH 750 Hematology Analyzer (Beckman Coulter, Inc., Brea, CA). hsCRP levels were measured using an enzyme-linked immunosorbent assay.
2.4. Metabolic syndrome
Metabolic syndrome was defined as per the American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement (Grundy et al., 2005). Subjects meeting three or more of the following five criteria were defined as having the metabolic syndrome: 1) waist circumference ≥102 cm in males or ≥88 cm in females, 2) fasting triglycerides ≥150 mg/dL, 3) fasting HDL <40 mg/dL in males or <50 mg/dL in females, 4) blood pressure ≥130/85 mmHg or on antihypertensive drug treatment in a subject with a history of hypertension, 5) fasting glucose ≥ 100 mg/dL or on drug treatment for elevated glucose.
2.5. Statistical analysis
The data were analyzed using SPSS version 19 (SPSS, Inc.; Chicago, Illinois). Subjects were stratified based on the presence or absence of the metabolic syndrome. Patients with non-affective psychoses and controls were analyzed separately. Demographic and clinical characteristics, and blood analyses in subjects with and without the metabolic syndrome were analyzed using either Student’s t-test (2-sided), Mann-Whitney U, or Fisher’s exact test (2-sided). WBC, hsCRP, and triglycerides were not normally distributed, and were log transformed prior to the analyses. Regarding psychotropic medications, antipsychotics, mood stabilizers, antidepressants, benzodiazepines, and anti-EPS agents were modeled as separates categorical yes/no variables in the analyses, based on whether or not patients were currently taking these classes of agents. Binary (Pearson) correlation coefficients were calculated between WBC, hsCRP, monocytes, lymphocytes, metabolic syndrome variables, and other demographic and clinical variables. Partial correlation coefficients were calculated between WBC, hsCRP, monocytes, and lymphocytes and metabolic syndrome variables, after controlling for potential confounding effects of age, sex, race, age at first hospitalization for psychosis, smoking, parental history of diabetes, alcohol use (DALI alcohol subscore), and psychotropic medications. Binary logistic regression models were used to evaluate WBC, hsCRP, monocytes, and lymphocytes as predictors of the metabolic syndrome and individual metabolic syndrome criteria, after controlling for potential confounding effects of age, sex, race, age at first hospitalization for psychosis, smoking, parental history of diabetes and psychotropic medications. Due to the relatively small sample size, only potential confounding factors that were correlated with metabolic syndrome variables with a p-value <0.10 in binary correlation analyses were included in the corresponding logistic regression model. For all analyses, results were considered statistically significant at the α=0.05 level (two-sided).
3. Results
A total of eighty-one subjects - fifty-nine patients and twenty-two controls - were included in the study. Data on total and differential WBC counts were missing for 2 patients, and parental history of diabetes were missing for 7 patients and 1 control; otherwise, complete data were available for all other variables. Table 1 presents the demographic and clinical characteristics of the study sample. 19 of the 59 patients (32%), and 5 of the 22 controls (23%) met the criteria for the metabolic syndrome. In both the patient and control groups, there was no difference in age, race, smoking, alcohol use, or parental history of diabetes based on the presence or absence of the metabolic syndrome. Patients, but not controls, with the metabolic syndrome were more likely to be female (p=0.02). Patients did not differ with regard to age at first hospitalization for psychosis or PANSS scores (total score and positive, negative, and general subscales) based on metabolic syndrome status
Table 1.
Demographic and Clinical Characteristics of the Study Sample
| Patients
|
Controls
|
|||||
|---|---|---|---|---|---|---|
| Metabolic Syndrome | p-value* | Metabolic Syndrome | p-value* | |||
| Yes (N=19) | No (N=40) | Yes (N=5) | No (N=17) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age | 42 (10) | 38 (12) | 0.20 | 45 (11) | 33 (15) | 0.13 |
| Age at first hospitalization for psychosis | 25 (8) | 25 (9) | 0.97 | |||
| Smoking (cigarettes/day) | 8.5 (9.4) | 10 (11.7) | 0.92 | 2.0 (4.5) | 1.2 (3.8) | 0.68 |
| Waist circumference (cm) | 110 (13) | 99 (16) | 0.01 | 104 (14) | 91 (14) | 0.07 |
| Fasting triglycerides (mg/dL) | 161 (55) | 98 (52) | <0.01 | 167 (34) | 75 (25) | <0.01 |
| Fasting HDL (mg/dL) | 44 (12) | 50 (10) | 0.05 | 43 (17) | 53 (11) | 0.12 |
| Systolic blood pressure (mmHg) | 130 (14) | 124 (16) | 0.15 | 135 (16) | 126 (18) | 0.36 |
| Diastolic blood pressure (mmgHg) | 83 (11) | 76 (13) | 0.05 | 83 (8) | 78 (8) | 0.30 |
| Fasting glucose (mg/dL) | 94 (16) | 80 (16) | <0.01 | 74 (22) | 84 (9) | 0.36 |
| WBC (×103/μL) | 7.7 (2.0) | 6.0 (2.1) | <0.01 | 5.8 (1.5) | 5.4 (1.2) | 0.46 |
| Monocytes (×103/μL) | 0.45 (0.12) | 0.35 (0.14) | 0.02 | 0.39 (0.13) | 0.27 (0.09) | 0.03 |
| Lymphocytes (×103/μL) | 2.1 (0.67) | 1.8 (0.57) | 0.07 | 1.8 (0.4) | 1.7 (0.5) | 0.81 |
| High-sensitivity CRP (mg/L) | 10.8 (17) | 5.6 (7.1) | 0.04 | 3.8 (1.2) | 3.9 (4.3) | 0.94 |
| DALI Alcohol | −0.88 (2.35) | −1.25 (2.26) | 0.57 | 0.832 (1.59) | −0.69 (2.13) | 0.34 |
| PANSS Positive | 18 (6) | 20 (6) | 0.19 | |||
| PANSS Negative | 17 (7) | 17 (6) | 0.77 | |||
| PANSS General | 38 (10) | 38 (10) | 0.94 | |||
| PANSS Total | 73 (17) | 74 (16) | 0.77 | |||
| n (%) | n (%) | p-value** | n (%) | n (%) | p-value** | |
| Gender (male) | 7 (37) | 28 (70) | 0.02 | 2 (40) | 7 (41) | 1.00 |
| Race | ||||||
| Caucasian | 8 (42) | 11 (28) | 0.26 | 2 (40) | 7 (41) | 0.28 |
| African Descent | 10 (53) | 28 (70) | 2 (40) | 9 (53) | ||
| Western Asian | 0 (0) | 1 (3) | 0 (0) | 0 (0) | ||
| East/Southeast Asian | 0 (0) | 0 (0) | 0 (0) | 1 (6) | ||
| Hispanic | 1 (5) | 0 (0) | 1 (20) | 0 (0) | ||
| Parental history of diabetes (yes)# | 9 (50) | 11 (33) | 0.37 | 1 (20) | 3 (19) | 1.00 |
| Mood stabilizer (yes) | 7 (37) | 11 (28) | 0.55 | |||
| Antidepressant (yes) | 10 (53) | 21 (53) | 1.00 | |||
| Benzodiazepine (yes) | 7 (37) | 5 (13) | 0.04 | |||
| Anti-EPS (yes) | 7 (37) | 16 (40) | 1.00 | |||
Student's t-test, 2-sided was used for all comparisons except for hsCRP (Mann-Whitney U)
Fisher's exact test, 2-sided was used for all comparisons
Data were unavailable for 7 patients and 1 control without metabolic syndrome
Table 2 lists the antipsychotic medications for the patients in the study sample. Subjects were most commonly treated with risperidone (n=16, 27%). Ten subjects (17%) were treated with two different antipsychotics, and 4 subjects (7%) were antipsychotic-free at the time of the laboratory evaluation. There was no difference in the prevalence of the metabolic syndrome in patients treated with one (31%) or two (40%) antipsychotics. Other concomitant medication use is described in Table 1. There was no difference in the prevalence of mood stabilizers, antidepressants, and anti-EPS medications based on metabolic syndrome status. However, subjects with the metabolic syndrome were more likely to be taking benzodiazepines (p=0.04).
Table 2.
Antipsychotic Medications in the Study Sample
| Medication | N |
|---|---|
| Risperidone | 16 |
| Ziprasidone | 6 |
| Aripiprazole | 5 |
| Quetiapine | 5 |
| No antipsychotic | 4 |
| Haloperidol | 3 |
| Olanzapine | 3 |
| Clozapine | 2 |
| Paliperidone Palmitate | 1 |
| Perphenazine | 1 |
| Risperidone Consta | 1 |
| Thiothixene | 1 |
| Haloperidol + Thiothixene | 1 |
| Olanzapine + Fluphenazine Decanoate | 1 |
| Olanzapine + Haloperidol | 1 |
| Olanzapine + Haloperidol Decanoate | 1 |
| Olanzapine + Risperidone | 1 |
| Quetiapine + Haloperidol Decanoate | 1 |
| Risperidone + Asenapine | 1 |
| Risperidone + Haloperidol | 1 |
| Risperidone + Haloperidol Decanoate | 1 |
| Risperidone + Paliperidone Palmitate | 1 |
| Risperidone + Risperidone Consta | 1 |
|
|
|
| 59 |
Patients with the metabolic syndrome had significantly higher mean total WBC count (p<0.01), monocytes (p=0.02), and hsCRP levels (p=0.04), and trend for higher lymphocytes (p=0.07) than subjects without the metabolic syndrome. Controls with the metabolic syndrome had significantly higher monocytes (p=0.03) than those without the metabolic syndrome.
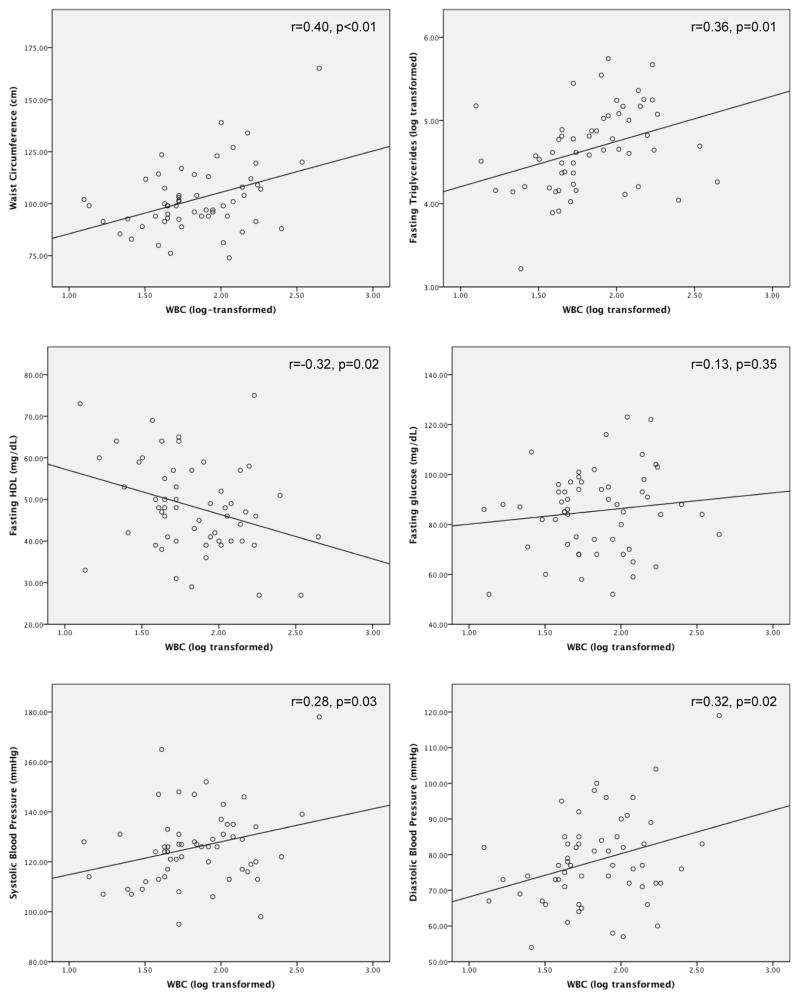
As shown in Figure 1, for patients there were significant binary positive correlations between total WBC count and waist circumference (r=0.41, p<0.01), fasting triglycerides (r=0.36, p<0.01), systolic blood pressure (r=0.28, p=0.03), and diastolic blood pressure (r=0.32, p=0.02), and a significant negative correlation with fasting HDL (r=−0.32, p=0.02). hsCRP was significantly positively correlated with waist circumference (r=0.44, p=0.01) and fasting triglycerides (r=0.30, p=0.02). There was a significant positive correlation between monocytes and waist circumference (r=0.39, p<0.01), fasting triglycerides (r=0.52, p<0.01), and diastolic blood pressure (r=0.34, p=0.01). Lymphocytes were significantly positively correlated with fasting triglycerides (r=0.33, p=0.01).
Figure 1.
Correlations between WBC and individual metabolic syndrome criteria in patients with non-affective psychoses
Table 3 shows the partial correlations between total WBC count, monocytes, lymphocytes, hsCRP and individual metabolic syndrome criteria for patients and controls, controlling for potential confounding effects of age, race, sex, age at first hospitalization for psychosis, parental history of diabetes, smoking, alcohol use, and psychotropic medications. In patients, total WBC count was significantly positively correlated with waist circumference (p<0.01) and significantly negatively correlated with fasting HDL (p=0.04). hsCRP was significantly positively correlated with waist circumference (p<0.01) and fasting triglycerides (p=0.01). Monocytes were also significantly positively correlated with waist circumference (p<0.01) and fasting triglycerides (p<0.01).
Table 3.
Partial correlations between immune parameters and individual metabolic syndrome criteria
| Subject | WBC (log transformed) | hsCRP (log transformed) | Monocytes | Lymphocytes | |||||
|---|---|---|---|---|---|---|---|---|---|
| Group | r | p-value | r | p-value | r | p-value | r | p-value | |
| Patients | Waist circumference (cm) | 0.45 | <0.01 | 0.45 | <0.01 | 0.46 | <0.01 | 0.24 | 0.18 |
| Fasting triglycerides (log transformed) | 0.30 | 0.09 | 0.44 | 0.01 | 0.58 | <0.01 | 0.30 | 0.09 | |
| Fasting HDL (mg/dL) | −0.36 | 0.04 | −0.23 | 0.21 | −0.32 | 0.07 | −0.18 | 0.33 | |
| Systolic blood pressure (mmHg) | 0.29 | 0.10 | 0.19 | 0.29 | 0.17 | 0.35 | 0.18 | 0.32 | |
| Diastolic blood pressure (mmgHg) | 0.28 | 0.12 | 0.14 | 0.44 | 0.30 | 0.09 | 0.07 | 0.69 | |
| Fasting glucose (mg/dL) | 0.06 | 0.73 | 0.11 | 0.55 | 0.18 | 0.92 | −0.14 | 0.44 | |
| Controls | Waist circumference (cm) | 0.69 | <0.01 | 0.44 | 0.09 | 0.43 | 0.09 | 0.73 | <0.01 |
| Fasting triglycerides (log transformed) | 0.58 | 0.02 | 0.28 | 0.29 | 0.68 | <0.01 | 0.22 | 0.42 | |
| Fasting HDL (mg/dL) | −0.48 | 0.06 | −0.37 | 0.16 | −0.52 | 0.04 | −0.32 | 0.23 | |
| Systolic blood pressure (mmHg) | 0.56 | 0.03 | −0.06 | 0.83 | 0.16 | 0.56 | −0.04 | 0.89 | |
| Diastolic blood pressure (mmgHg) | 0.25 | 0.35 | −0.14 | 0.59 | 0.26 | 0.33 | −0.28 | 0.30 | |
| Fasting glucose (mg/dL) | −0.03 | 0.90 | 0.16 | 0.57 | 0.13 | 0.63 | 0.03 | 0.93 | |
In controls, total WBC count was significantly positively correlated with waist circumference, fasting triglycerides, and systolic blood pressure (p<0.03 for each). Monocytes were significantly positively correlated with fasting triglycerides (p<0.01), and significantly negatively correlated with fasting HDL (p=0.04). Lymphocytes were significantly positively correlated with waist circumference (p<0.01).
In binary logistic regression analyses, after controlling for potential confounding factors, in patients total WBC count was a significant predictor of metabolic syndrome (p=0.01). hsCRP was a significant predictor of metabolic syndrome (p=0.04), increased waist circumference (p=0.03) and triglycerides (p=0.05), and a predictor of fasting glucose at the trend level (p=0.10). Monocytes were also a significant predictor of the metabolic syndrome (p=0.02) and increased triglycerides (p=0.02). Lymphocytes were a significant predictor of increased triglycerides (p=0.02).
Post-hoc analyses were also performed to evaluate the potential influence of outlying values of WBC and hsCRP on results. Two patients had elevated WBC counts (12.6 and 14.1 ×103/μL). After excluding these subjects, WBC remained a significant predictor of the metabolic syndrome, and was also a significant predictor of fasting triglycerides. The pattern of the results did not change for any other metabolic syndrome criteria. CRP levels >10 mg/L may suggest the presence of an underlying inflammatory disease, although levels in this range can be seen on a genetic basis in ~6% of apparently healthy individuals (Kushner et al., 2006). Twelve patients (20%) in our sample had a hsCRP >10 mg/L, in the absence of any evidence of intercurrent bacterial infection. After excluding these subjects, hsCRP remained a significant predictor of the metabolic syndrome, waist circumference, and fasting triglycerides, and was also a significant predictor of fasting HDL. The pattern of the results did not change for any blood pressure or fasting glucose.
We were unable to perform logistic regression analyses in the controls, as the estimates were unstable due to the small sample size.
4. Discussion
Non-diabetic subjects with non-affective psychoses and the metabolic syndrome had significantly higher total WBC count, monocytes, and hsCRP levels than patients without the metabolic syndrome. After controlling for potential confounding effects of age, race, sex, age at first hospitalization for psychosis, parental history of diabetes, smoking, alcohol use, and psychotropic medications, total WBC count, monocytes, and hsCRP levels were significant predictors of the metabolic syndrome. Furthermore, hsCRP levels were significant predictors of two individual criteria for the metabolic syndrome (waist circumference and fasting triglycerides). Outlying values for total WBC count and hsCRP did not account for these associations.
We observed a similar pattern of results healthy controls, although our small sample size limited statistical power. However, several large population-based samples found that total and differential white blood cell (WBC) counts were associated with metabolic syndrome risk and individual metabolic syndrome criteria (Kim et al., 2008; Lao et al., 2008). In the general population, elevated CRP levels in the metabolic syndrome are a well-replicated finding (Ridker et al., 2008). Taken, together, these findings suggesting the observed relationships are not specific to subjects with non-affective psychoses.
The strengths of the present study include consideration of multiple potential confounding factors, including psychotropic medications, as classes other than antipsychotics are also associated with weight gain and other metabolic side effects. To our knowledge, ours is the first study to consider differential WBC counts, and one of a small number of studies to consider hsCRP, as a predictor of metabolic syndrome and its individual criteria in patients with schizophrenia and related disorders. Several potential limitations of the present study are the heterogeneity of the sample with respect to clinical status (i.e., inpatients and outpatients), and the non-standardized antipsychotic treatment. Another limitation is the relatively small sample size, as evidenced by the wide confidence intervals in the logistic regression analyses, which limited statistical power. Additional studies in both patients and controls are needed to evaluate whether or not the observed relationships are specific to subjects with non-affective psychoses. Due to the cross-sectional design, our study does not permit inferences regarding the ability of baseline total and differential WBC counts or hsCRP to predict incident cases of metabolic syndrome. A large general population study, however, found that total WBC count predicted future development of the metabolic syndrome over a 7year follow-up period (Odagiri et al., 2011).
In a sample of 199 stable outpatients with schizophrenia or schizoaffective disorder treated with olanzapine, risperidone, or typical antipsychotics, Fan et al. (2010) found that total WBC count was a significant predictor of the metabolic syndrome, as well as the individual metabolic syndrome criteria for waist circumference, fasting triglycerides, and blood pressure. We have replicated the finding of total WBC count as a significant predictor of metabolic syndrome. We also observed a very similar pattern of results in the present study for total WBC counts as a predictor of individual criteria for the metabolic syndrome, although none of the findings reached statistical significance, likely due to a lack of statistical power in our study. Our study also replicates previous findings of associations between hsCRP and the metabolic syndrome (Vuksan-Cusa et al., 2010) and increased waist circumference (Fawzi et al., 2011). Nurjono and Lee (2012) found that BMI, compared to waist circumference and blood pressure, was the most accurate predictor of the metabolic syndrome in patients with schizophrenia from Singapore. The present study extends these findings in patients with non-affective psychoses examining the relationship between differential WBC counts, hsCRP, and all individual criteria for the metabolic syndrome.
The mechanisms that underlie these associations remain unclear. In the general population, hsCRP levels have been proposed as an additional individual criterion for the metabolic syndrome. (Ridker et al., 2008) Visceral abdominal adiposity is associated with increased triglycerides and fasting glucose (Depres 2007), and one study found a significant correlation between hsCRP and visceral fat in Japanese subjects with mild obesity or impaired glucose tolerance (Tsuriya et al., 2011). Endothelial dysfunction, which is induced by CRP, plays a central role in atherosclerotic cardiovascular disease and is associated with all individual criteria for the metabolic syndrome (Devaraj et al., 2010). Thus, our findings of significant associations between hsCRP and increased waist circumference and triglycerides, and metabolic syndrome risk, are broadly consistent with an important role for inflammation in mediating the metabolic syndrome.
Our findings also suggest an association between monocytes and the metabolic syndrome in patients with non-affective psychoses. This finding is consistent with evidence that monocytes play a key role in metabolic homeostasis, and produce inflammatory mediators in response to cues that modulate metabolism (Bhargava and Lee, 2012). Subjects with metabolic syndrome have significantly higher monocytic tissue factor pro-coagulant activity, and up-regulation of monocyte tissue factor is associated with insulin resistance, higher hsCRP, and carotid intima-media thickness, a significant predictor of cardiovascular events (Nakagomi et al., 2010; Nakagomi et al., 2011). Subjects with the metabolic syndrome also have up-regulation of monocytic CD40/CD40 ligand expression (Natal et al., 2008).
Future studies in larger sample are needed to replicate findings of an association between total and differential WBC counts, hsCRP, and the metabolic syndrome in patients. Cytokines, particularly interleukin-6 (IL-6), are the primary inducers of CRP, and monocytes are a rich source of pro-inflammatory cytokines. Thus, future studies could investigate the relationship between individual cytokines and cytokine networks and the metabolic syndrome in non-affective psychosis. There is a need for prospective, longitudinal studies to examine the capacity of these parameters to predict future development of the metabolic syndrome. Another potentially important issue is whether polymorphisms in the gene for CRP or other immune-related genes moderate metabolic syndrome risk in patients with non-affective psychosis.
A recent meta-analysis of 77 publications found that approximately one-third of patients with schizophrenia meet criteria for the metabolic syndrome (Mitchell et al., 2011). Despite the American Diabetes Association/American Psychiatric Association consensus guidelines for metabolic monitoring of patients on antipsychotic drugs (2004), monitoring rates in clinical practice remain alarmingly low (Mitchell et al., 2012). Our findings suggest that measurement of blood total and differential WBC counts and hsCRP levels may be germane to the clinical care of patients with schizophrenia and related disorders. Consistent replications of these associations in well-controlled studies would suggest that treatment with statins or other anti-inflammatory medications for some patients with schizophrenia may result in decreased risk of cardiovascular morbidity and mortality (Ridker et al., 2008). Additionally, the signal-to-noise ratio of treatment trials of adjunctive anti-inflammatory agents in schizophrenia may be increased by stratifying patients based on the presence or absence of the metabolic syndrome. Taken together, our results provide contribute to a growing body of evidence for an association between inflammation and the metabolic syndrome in non-affective psychoses.
Table 4.
Binary logistic regression of immune parameters as a predictor of individual metabolic syndrome criteria in patients with non-affective psychoses
| Parameter | Metabolic Syndrome (≥3 individual criteria) | Waist circumference (Male≥102; Female≥88 cm) | Fasting triglycerides (≥150 mg/dL) | Fasting HDL (Male<40; Female<50 mg/dL) | Blood pressure (≥130/85 mmHg or on meds) | Fasting gluose (≥100 mg/dL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| WBC (log transformed) | 16.3 (1.7–152) | 0.01 | 5.0 (0.5–56.4) | 0.19 | 9.4 (0.8–104) | 0.07 | 2.8 (0.4–19.8) | 0.30 | 4.5 (0.6–33.4) | 0.14 | 2.4 (0.2–35.6) | 0.52 |
| hsCRP (log transformed) | 1.9 (1.0–3.4) | 0.04 | 2.3 (1.1–5.1) | 0.03 | 2.0 (1.0–4.0) | 0.05 | 1.4 (0.9–2.3) | 0.15 | 1.2 (0.8–2.0) | 0.37 | 1.6 (0.8–3.4) | 0.21 |
| Monocytes | 404 (2.4–67357) | 0.02 | 81.4 (0.2–34581) | 0.15 | 689 (2.7–17551) | 0.02 | 1.8 (0.0–106) | 0.79 | 19.5 (0.2–1595) | 0.19 | 0.6 (0.0–274) | 0.85 |
| Lymphocytes | 1.7 (0.6–4.9) | 0.30 | 1.1 (0.3–3.9) | 0.85 | 5.5 (1.3–22.8) | 0.02 | 1.3 (0.5–3.5) | 0.59 | 1.7 (0.6–5.0) | 0.3 | 0.5 (0.1–2.7) | 0.45 |
Highlights.
Blood WBC count, monocytes, & hsCRP predicted the metabolic syndrome in patients with nonaffective psychoses, and may be germane to clinical care.
Footnotes
Disclosures
Dr. Miller is a recipient of the U.S. National Institutes of Health Clinical Loan Repayment Program. In the past 3 years, he has received: Grant support from the GHSU Intramural Scientist Training Program, the GHSU Brain & Behavior and Immunotherapy Discovery Institutes, the University of Oulu (Finland), the Thule Institute of the University of Oulu, and Oy H. Lundbeck Ab; Consultancy fees for surveys from Medefied Europe and Plaza Research, on behalf of Genetech/Roche; Speaker fees for grand rounds lectures from the Maryland Psychiatric Research Center and the Texas A&M University and Scott and White Hospital Department of Psychiatry; Travel/accommodations/meeting expenses from the National Institute of Mental Health New Clinical Drug Evaluation Unit New Investigator Award, and the American College of Psychiatrists Laughlin Fellowship; Payment for a survey from e-Rewards Medical Market Research and an award from the Georgia Psychiatric Physicians Association Resident Research Competition.
Dr. Mellor received funding support from the NIH (AI083005, AI075165), the Juvenile Diabetes Research Foundation, and the Carlos and Marguerite Mason Trust. Dr. Mellor is a member of the Scientific Advisory Board of NewLink Genetics Inc., and receives compensation for this service.
Dr. Buckley received Grant/Research Support from the National Institute of Mental Health, Janssen Pharmaceutica, Pfizer, and Sunovion, and is a Consultant (Honorarium/Expenses) for the National Institute of Mental Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhair SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. doi: 10.1016/j.schres.2006.11.016. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- Beumer W, Drexhage RC, De Wit H, Versnel MA, Drexhage HA, Cohen D. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology. 2012 Apr 26; doi: 10.1016/j.psyneuen.2012.04.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Lee CH. Role and function of macrophages in the metabolic syndrome. Biochem J. 2012;442:253–262. doi: 10.1042/BJ20111708. [DOI] [PubMed] [Google Scholar]
- Carrizo E, Fernández V, Quintero J, Connell L, Rodríguez Z, Mosquera M. Coagulation and inflammation markers during atypical or typical antipsychotic treatment in schizophrenia patients and drug-free first-degree relatives. Schizophr Res. 2008;103:83–93. doi: 10.1016/j.schres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Després JP. Cardiovascular disease under the influence of excess visceral fat. Crit Pathw Cardiol. 2007;6:51–59. doi: 10.1097/HPC.0b013e318057d4c9. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Valleggi S, Siegel D, Jialal I. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010;12:110–118. doi: 10.1007/s11883-010-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liu EY, Freudenreich O, Park JH, Liu D, Wang J, Yi Z, Goff D, Henderson DC. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res. 2010;118:211–217. doi: 10.1016/j.schres.2010.02.1028. [DOI] [PubMed] [Google Scholar]
- Fawzi MH, Fawzi MM, Fawzi MM, Said NS. C-reactive protein serum level in drug-free male Egyptian patients with schizophrenia. Psychiatry Res. 2011;90:91–97. doi: 10.1016/j.psychres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Noh JH, Lee BW, Choi YH, Chung JH, Min YK, Lee MS, Lee MK, Kim KW. The associations of total and differential white blood cell counts with obesity, hypertension, dyslipidemia and glucose intolerance in a Korean population. J Korean Med Sci. 2008;23:193–198. doi: 10.3346/jkms.2008.23.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:e17–28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71:520–527. doi: 10.4088/JCP.09m05117yel. [DOI] [PubMed] [Google Scholar]
- Lao XQ, Neil Thomas G, Jiang C, Zhang W, Adab P, Lam TH, Cheng KK. White blood cell count and the metabolic syndrome in older Chinese: the Guangzhou Biobank Cohort Study. Atherosclerosis. 2008;201:418–424. doi: 10.1016/j.atherosclerosis.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Masserini C, Vita A, Basile R, et al. Lymphocyte subsets in schizophrenic disorders. Relationship with clinical, neuromorphological and treatment variables. Schizophr Res. 1990;3:269–275. doi: 10.1016/0920-9964(90)90008-u. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80m:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Miller B, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH. C-Reactive Protein in Schizophrenia: A Review and Meta-Analysis. Clinical Schizophrenia and Related Psychoses. 2012 doi: 10.3371/CSRP.MICU.020813. In press. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of Metabolic Syndrome and Metabolic Abnormalities in Schizophrenia and Related Disorders--A Systematic Review and Meta-Analysis. Schizophr Bull. 2011 Dec 29; doi: 10.1093/schbul/sbr148. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42:125–147. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- Müller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, et al. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Nakagomi A, Sasaki M, Ishikawa Y, Shibui T, Kosugi M, Endoh Y, Morikawa M, Kusama Y, Atarashi H, Mizuno K. Upregulation of monocyte tissue factor activity is significantly associated with carotid intima-media thickness in patients with metabolic syndrome. J Atheroscler Thromb. 2011;18:475–486. doi: 10.5551/jat.6874. [DOI] [PubMed] [Google Scholar]
- Nakagomi A, Sasaki M, Ishikawa Y, Morikawa M, Shibui T, Kusama Y, Atarashi H, Mizuno K. Upregulation of monocyte tissue factor activity is significantly associated with low-grade chronic inflammation and insulin resistance in patients with metabolic syndrome. Circ J. 2010;74:572–577. doi: 10.1253/circj.cj-09-0835. [DOI] [PubMed] [Google Scholar]
- Natal C, Restituto P, Iñigo C, Colina I, Díez J, Varo N. The proinflammatory mediator CD40 ligand is increased in the metabolic syndrome and modulated by adiponectin. J Clin Endocrinol Metab. 2008;93:2319–2327. doi: 10.1210/jc.2007-2491. [DOI] [PubMed] [Google Scholar]
- Nurjono M, Lee J. Predictive utility of blood pressure, waist circumference and body mass index for metabolic syndrome in patients with schizophrenia in Singapore. Early Interv Psychiatry. 2012 doi: 10.1111/j.1751-7893.2012.00384.x. [DOI] [PubMed] [Google Scholar]
- Odagiri K, Uehara A, Mizuta I, Yamamoto M, Kurata C. Longitudinal study on white blood cell count and the incidence of metabolic syndrome. Intern Med. 2011;50:2491–2498. doi: 10.2169/internalmedicine.50.5877. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Jr, Kastelein JJP, et al. for the JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Sperner-Unterweger B, Whitworth A, Kemmler G, et al. T-cell subsets in schizophrenia: a comparison between drug-naïve first episode patients and chronic schizophrenic patients. Schizophr Res. 1999;38:61–70. doi: 10.1016/s0920-9964(98)00175-3. [DOI] [PubMed] [Google Scholar]
- Tsuriya D, Morita H, Morioka T, Takahashi N, Ito T, Oki Y, Nakamura H. Significant correlation between visceral adiposity and high-sensitivity C-reactive protein (hs-CRP) in Japanese subjects. Intern Med. 2011;50:2767–2773. doi: 10.2169/internalmedicine.50.5908. [DOI] [PubMed] [Google Scholar]
- Vuksan-Cusa B, Sagud M, Jakovljević M. C-reactive protein and metabolic syndrome in patients with bipolar disorder compared to patients with schizophrenia. Psychiatr Danub. 2010;22:275–277. [PubMed] [Google Scholar]
- Wilke I, Arolt V, Rothermundt M, et al. Investigations of cytokine production in whole blood cultures of paranoid and residual schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 1996;246:279–284. doi: 10.1007/BF02190280. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Cannnon TD, Gur RE, et al. Leukocytes and Organ-Nonspecific Autoantibodies in Schizophrenics and Their Siblings: Markers of Vulnerability of Disease? Biol Psychiatry. 1996;40:825–33. doi: 10.1016/0006-3223(95)00598-6. [DOI] [PubMed] [Google Scholar]