Abstract
Objective: To evaluate in vivo patency rates of silk fibroin (SF) vascular grafts and resulting histological reactions in a canine model.
Methods: To generate 3.5-mm inner diameter vessels, a combination of plaited silk fibers were wound with cocoon filaments and subsequently coated with an SF solution. The resulting SF grafts (n=35) were implanted into the carotid arteries of male beagles (age, 1–2 years; body weight: 9.0–10.5 kg). Expanded polytetrafluoroethylene (4-mm inner diameter, ePTFE) grafts (n=5) were used as controls. Graft patency was monitored via ultrasonography with histological changes analyzed via microscopic examination.
Results: Compared with animals that received the ePTFE grafts, animals that received SF grafts exhibited the same thickness of luminal layers and fibrin accumulation and collagen fiber replacement with endothelialization at 3 months post-implantation via histological examination. The patency rates of the SF and the ePTFE grafts at 6 months post-implantation were 7.8% and 0%, respectively.
Conclusion: This canine model study demonstrated that SF grafts induce unique histological reactions but fail to achieve long-term patency.
Keywords: silk graft, histology, graft patency
Introduction
The number of patients with atherosclerotic disease has been increasing with peripheral arterial disease currently affecting >5 million adults in the United States.1) Consequently, the need for small vascular grafts for surgical procedures, such as lower limb and coronary bypass, has been increasing. Implantation of autologous vessels is considered the gold-standard treatment for patients requiring such procedures. Unfortunately, atherosclerotic patients often have veins with small diameters and arterial calcification, which render their vessels unsuitable for these procedures. Accordingly, many vascular surgeons use small-diameter synthetic grafts composed of materials such as expanded polytetrafluoroethylene (ePTFE) and polyethylene terephthalate for lower limb bypass surgery, in the event that an autologous vessel is not available. Synthetic grafts with small internal diameters (<6 mm) have poor patency rates,2,3) which is associated with a compliance mismatch between the native artery and the prosthesis,4,5) in addition to low flow conditions within the resultant narrow conduits.6) Surface thrombogenicity and anastomotic intimal hyperplasia2,3) are additional factors that may result in poor performance of the synthetic grafts.
Numerous tissue engineering techniques have been reported to produce synthetic grafts that are comparable in quality to human vessels.7,8) Among these techniques, heparin bonded synthetic grafts are among the most famous in current clinical use.9) Despite the high volume of preliminary research in this area, these methods are still limited by the time requirements and complex procedures. To date, no alternative synthetic grafts have emerged that can fully replace autologous veins.
Remodeling ability and non-thrombogenicity are key factors in the development of a novel synthetic vascular graft. To address these challenges, we have suggested the use of silk, which has been used for surgical sutures for centuries, and has been proven as a safe biomaterial in humans.10) Silk fibers are composed of silk fibroin (SF) and sericin. SF is a core fiber, which is comprised of highly organized β-sheet crystal regions and it is responsible for the elasticity of silk. Sericin is an antigenic gum-like protein that surrounds the SF fibers.10) SF appears to possess three major biological characteristics: (1) biocompatibility with responses similar to those of other major biomaterials,11–13) (2) a high affinity for several types of cells,14,15) and (3) a susceptibility to proteolytic degradation in vivo.16,17) Accordingly, SF provides an antithrombotic surface with various types of scaffolds, rendering it useful for tissue engineering.18) Additionally, SF offers diversity with respect to matrix scaffolding, as it can be used for vascular grafts, as well as bone, ligaments, tendons, and cartilage.19,20)
Previous studies have described the benefits of SF vascular grafts in small rats.21–23) However, the long-term patency rates and histological effects of these grafts have not been clarified in larger animal models. It is key to test the SF grafts in larger animals, as the biological responses after bypass surgery with synthetic grafts, including patency, flow, coagulation, and conduit healing, vary among species.24) In the present study, we evaluated the clinical usefulness of SF vascular grafts by evaluating their in vivo patencies, as well as the associated histological changes following implantation in dogs.
Methods
Preparation of vascular grafts
SF was obtained from cocoons of the mulberry silkworm Bombyx mori. Tubes with a 3.5-mm inner diameter were fabricated using a combination of plaited silk fibers and wound cocoon filaments on a computer controlled double Raschel knitting machine (Fukui Warp Knitting Co., Ltd., Fukui, Japan). The sericin component was completely removed from the silk via a degumming process. Each resulting tube was coated with an SF solution, according to previously described procedures.22,23,25,26) Each tube was subsequently dried, fixed in ethanol, and removed from the central core to yield a graft. Light microscopy and scanning electron microscopy (SEM; VE-7800, Keyence, Tokyo, Japan) were used to assess the internal and external morphologies of the grafts pre-implantation. For controls, 4.0-mm inner diameter ePTFE vascular grafts (EXXCEL SOFT®, MAQUET Cardiac Assist, Fairfield, NJ, USA) were used.
SF grafts were evaluated for water permeability, which provides an index of the interstitial leakage rate. Specifically, water permeability was calculated as the amount of water leaked per unit area and period under a physiologic pressure of 120 mmHg (mL/cm2/min at 120 mmHg). Structural property tests were performed on SF and ePTFE grafts, as previously described.23) Briefly, an EZ test (Shimadzu, Kyoto, Japan) with a 100-N capacity load cell and pneumatic clamps was used to test the sample grafts. The lengths of the samples were measured using a 20-nm caliper. A crosshead displacement rate of 3 mm/min was used, and the measured tensile stress was plotted against the tensile strain. The elastic modulus was calculated using the least squares method in the linear range of the stress-strain plot.
Implantation of SF grafts (surgical procedures)
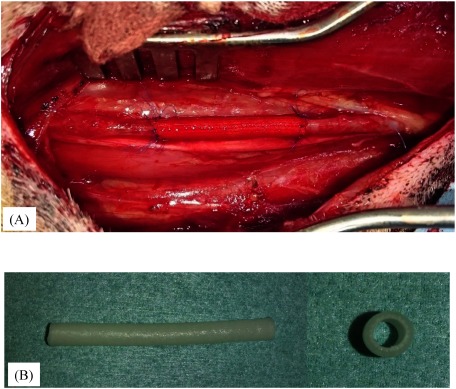
SF grafts (n=35) were implanted into the carotid arteries of male beagles (age, 1–2 years; body weight, 9.0–10.5 kg) using end-to-end anastomosis with 7-0 monofilament polypropylene sutures (Prolene, Ethicon, San Lorenzo, Puerto Rico). All implantation surgeries were performed by the same group of vascular surgeons at the same institution. The implanted SF grafts were 5 cm in length (Fig. 1A). Each dog was anesthetized with an intravenous injection of propofol (10 mg/kg). Respiration was maintained with a respirator via an orotracheal tube, and the dogs were ventilated with a mixture of oxygen and halothane. A 100-IU/kg dose of heparin was injected intravenously before implantation. Each dog was subcutaneously injected with 50 mg of orbifloxacin (DS Pharma Animal Health Co., Ltd., Osaka, Japan) after surgery. No anticoagulants or antiplatelet agents were administered postoperatively. The 4.0-mm inner diameter ePTFE vascular grafts (n=5) were implanted into the carotid arteries in the same manner in the control group.
Fig. 1 (A) Macroscopic image of the implanted fibroin graft. (B) Silk fibroin grafts were soft and flexible and did not present difficulties during handling and anastomosis suturing.
Graft patency was monitored in the anastomosis and midsections of the graft by measuring blood flow velocity using color Doppler ultrasonography (US; MicroMaxx®, SonoSite, Inc., Bothwell, WA, USA) at 1–3 days after surgery; at weeks 1, 2, 3, and 4, and finally every month. Subjects were carefully monitored for signs of aneurysm, stenosis, and thrombus occlusion via US. All grafts were retrieved for histological analysis; patent grafts were retrieved without damaging the sheathing tissues.
Study protocols were conducted in accordance with the Guide for Animal Experimentation of The University of Tokyo and the Guide for Care and Use of Laboratory Animals of the US National Institute of Health (National Academies Press, revised 2011).
Histological analysis
After retrieving a graft, the microscopic examination was performed longitudinally at the anastomosed areas and cross-sectionally at the midsection. Each sample was formalin-fixed, paraffin-embedded, and stained with hematoxylin-eosin (H&E) and Elastica van Gieson. Immunostaining was also performed on the SF grafts using a polyclonal antibody against von Willebrand factor (Cat N: A0082, Dako, Santa Clara, CA, USA).
Differences in the changes in luminal layers (LLs) of the stained specimens were examined via light microscopy to determine the number of endothelial cells, the LL collagenization ratio, and the mean LL thickness. Each parameter was classified after receiving scores of − to ⧻, based upon our previously described procedure.26) The number of endothelial cells across the LL surface, which was determined based on the results of immunostaining with the polyclonal antibody against von Willebrand factor, was classified into the following four categories: ⧻, ≥100; ⧺, 50–99; +,<50; and −, 0 (Table 1). The LL collagenization ratio, defined as the ratio of the collagen fiber-occupied area to the whole LL area and based on the Elastica van Gieson staining, was classified into the following four categories: ⧻, 100%; ⧺, 50–99%; +,<50%; and −, 0% (Table 1). The mean LL thickness was calculated using Image J software (version 1.44; National Institute of Mental Health, Bethesda, MD, USA),27) as the LL area divided by the length of the inner graft circumference. The mean LL thickness was classified into the following four categories: ⧻, ≥ 400 µm; ⧺, 200–399 µm; +, 100–199 µm; and −,<100 µm (Table 1).
Table 1 Development of endothelial cells, collagenization ratio, and mean thickness of the LL.
| − | + | ⧺ | ⧻ | |
|---|---|---|---|---|
| Number of endothelial cells over the whole surface of the LL | 0 | <50 | 50–99 | ≥100 |
| Collagenization ratio of the LL (%) | 0 | <50 | 50–99 | 100 |
| Mean thickness of the LL (µm) | <100 | 100–199 | 200–399 | ≥400 |
| LL: luminal layer | ||||
| Mechanical properties of the SF/SF and the ePTFE grafts (±standard deviation) | ||||
| Water permeability (mL/min/cm2 120 mmHg) | Circumferential strength (N/mm2) | Longitudinal strength (N/mm2) | Compressive elastic modulus (N/mm2) | |
| SF/SF | 50.0±1.0 | 48.0±2.0 | 8.0±1.0 | 0.008±0.002 |
| ePTFE | 51.0±1.0 | 51.0±1.0 | 7.0±1.0 | 0.008±0.003 |
| SF/SF: silk fibroin-coated silk fibroin tubes; ePTFE: expanded polytetrafluoroethylene | ||||
| Histological appearance of luminal layers in the graft | ||||
| Length of time | Grafts | Luminal layer | ||
| Number of ECs | Collagenization | Thickness | ||
| 1 week | SF/SF | − | − | + |
| ePTFE | − | + | ||
| 3 weeks | SF/SF | ⧺ | + | + |
| ePTFE | + | + | ||
| 3 months | SF/SF | ⧻ | ⧻ | + |
| ePTFE | ⧺ | + | ||
| SF/SF: silk fibroin-coated silk fibroin tubes; ePTFE: expanded polytetrafluoroethylene; EC: endothelial cell | ||||
Statistical analysis
Graft patency in each group was determined using a Kaplan–Meier analysis. The log-rank test was used to test for differences between the groups. Statistical significance was defined as a P-value <0.05.
Results
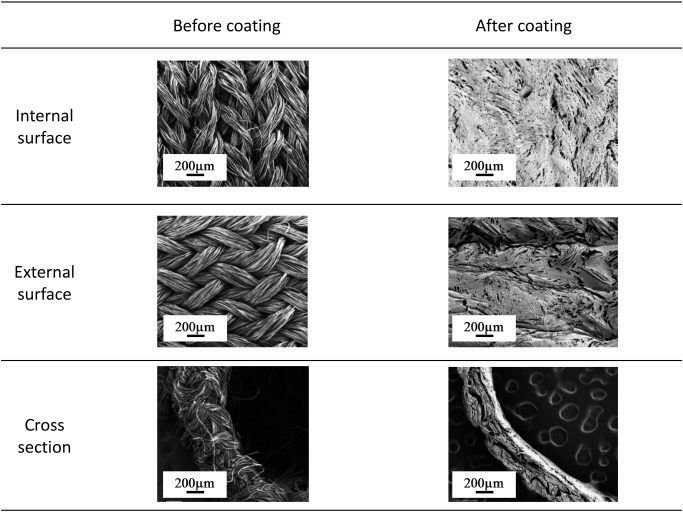
SF grafts were found to be softer and more flexible than the ePTFE grafts, and there were no difficulties with handling or anastomosis suturing (Fig. 1B). After implantation, no noticeable differences between the SF and the ePTFE grafts were visible to the naked eye. As visualized using SEM, the internal and external surfaces of the SF grafts, both before and after SF coating, effectively impregnated the wall and were spread in a thin layer (Fig. 2). Water permeability and tensile strength of the SF grafts (50.0±1.0 mL/cm2/min at 120 mmHg; longitudinal, 8.0±1.0 N/mm2; circumferential, 48.0±2.0 N/mm2; and compressive elastic modulus, 0.008±0.002 N/mm2) were equivalent to those of the ePTFE grafts (51.0±1.0 mL/cm2/min at 120 mmHg; longitudinal, 7.0±1.0 N/mm2; circumferential, 51.0±1.0 N/mm2; and compressive elastic modulus, 0.008±0.003 N/mm2) (Table 1). Mechanical properties did not differ significantly between the SF and ePTFE grafts. All implantations were performed successfully without infection or other major complications, and all dogs survived until the scheduled date of harvesting.
Fig. 2 Appearance of the internal and external surfaces of silk fibroin grafts via scanning electron microscopy before and after coating.
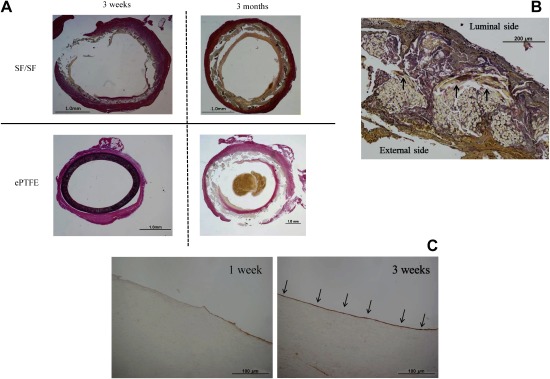
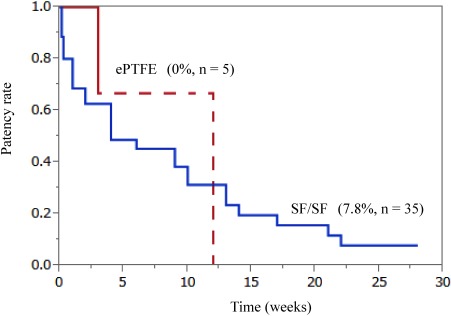
Small-diameters of SF grafts were associated with the same LL thickness at all time points post-implantation. At 3 weeks post-implantation, less fibrin accumulation and collagen fiber replacement with endothelialization were observed in the intraluminal layer of the SF grafts (Table 1, Figs. 3A and 3B). The LLs of SF grafts were aligned with endothelial cells in the midsections of the grafts (Fig. 3C). In addition, SF fiber degradation was observed and the external SF graft surface was surrounded by a thin connective tissue with collagen fiber replacement in the intraluminal layer, without macrophage infiltration or phagocytic phenomena around the SF grafts, indicating the lack of a foreign body reaction (Fig. 3B). At 3 months post-implantation, adequate collagenization and endothelialization were observed in the intraluminal layers of SF grafts (Table 1 and Fig. 3A). Patencies of 35 SF grafts and five ePTFE grafts were compared at intervals ranging from 1–28 weeks (Fig. 4). All ePTFE grafts became occluded within 12 weeks, whereas only nine of the 35 SF grafts became occluded. Moreover, the overall 6-month patency rate of the SF grafts was 7.8% compared to 0% for the ePTFE grafts (P=0.11).
Fig. 3 (A) Histological cross-section image of Elastica van Gieson (EG) staining showing the midsection of each graft at 3 weeks and 3 months post-implantation. (B) Histological cross-section image of EG staining at 3 weeks post-implantation in the silk fibroin (SF) grafts shows SF fiber degradation, and the external SF graft surface was surrounded by a thin connective tissue with collagen fiber replacement in the intraluminal layer (arrows). (C) Histological cross-section image of immunostaining with polyclonal antibody against von Willebrand factor at 3 weeks post-implantation. The luminal layers of the SF grafts were lined with endothelial cells in the midsections of the grafts (arrows).
Fig. 4 A Kaplan–Meier curve shows the graft patency rates for 35 silk fibroin grafts and 5 expanded polytetrafluoroethylene grafts implanted into the carotid arteries of dogs.
Discussion
In the present study, we evaluated the long-term patency rates and histological responses of small-diameter SF grafts in a canine model, in which grafts were implanted into the carotid arteries of beagles via end-to-end anastomosis. This study demonstrated that the SF grafts induced unique histological responses, such as maintaining the thickness of the LLs with SF fiber degradation, and exhibited fibrin accumulation and collagen fiber replacement with endothelialization at 3 months post-implantation compared with the ePTFE grafts. However, we observed no significant differences in the 6-month patency rates between the SF grafts and the ePTFE grafts.
Our results were consistent with those of a previous study, which showed that SF grafts and ePTFE grafts had similar water permeabilities.22,26) Water permeability should be maintained at <50 mL/cm2/min to avoid unacceptable bleeding in fully heparinized patients.28) Our results showed that both types of grafts were essentially impermeable to water. Generally, tensile strength and compressive elastic modulus showed little difference between the two types of grafts, indicating that SF grafts are as strong and stiff as the ePTFE grafts. Additionally, mechanical properties of SF grafts closely match those of the rat abdominal aorta.29) This indicates the possibility that by more closely matching the compliance properties of small vessels, thrombogenicity can be minimized.
In the present study, we observed endothelialization in the SF grafts, as previously reported.21,23,26) As ePTFE grafts have a closed structure to prevent bleeding, tissue infiltration is generally prevented, and the number of endothelial cells in the LLs is difficult to examine. As a result, we did not perform immunostaining on the ePTFE grafts. However, it is known that polyethylene terephthalate vascular grafts exhibit less complete endothelialization than SF grafts.23)
Endothelialization and tissue infiltration are closely related.3) Two types of endothelialization are known to occur: transanastomotic ingrowth from the adjacent artery, and transmural ingrowth through the graft wall.3,30) Transanastomotic endothelialization ingrowth does not exceed 1–2 cm, and a 56-week period is required for complete endothelialization in humans,30) in contrast to just 3.5 weeks in dogs.3,31,32) This suggests that in dogs, endothelialization is pronounced enough at 3.5 weeks that tissue infiltration into the LLs should be complete. Based on these results, we expected tissue infiltration into the LLs in the SF grafts. Although we did observe endothelialization in the SF grafts, we did not observe substantial layered formations of fibrin and collagen into the LLs at 3 weeks post-implantation, which does not support the observed phenomena in SF grafts implanted into rat abdominal aortas.21,23)
However, adequate collagenization and endothelialization were observed in the intraluminal layer of SF grafts at the final evaluation at 3 months post-implantation.
Normally, vascular grafts are thought to undergo the following processes: initially, the inner layer fills with blood cells and fibrin, then collagen fibers appear and the luminal surface is covered with collagen.33) In the current study, endothelial cells were observed on the LLs involving the SF-coated vascular grafts, with adequate fibrin accumulation and collagen fiber replacement. Additionally, all areas of the external SF graft surface were surrounded by thin connective tissue, which was considered to mimic host tissue. This phenomenon may be related to the high cellular affinity of the SF. Several studies have shown that nitric oxide produced during endothelialization confers antithrombogenic and antiatherogenic properties.34–36) The amount of nitric oxide production has correlated strongly with the patency rates of various vessel conduits used in bypass surgeries.37) In the present study, endothelial layer formation was observed, indicating highly effective antithrombogenicity after implantation with SF grafts.
Previously, Enomoto et al. reported a 1-year patency rate of 85.1% for SF grafts implanted into rat abdominal aortas.21) However, in the present study, no significant differences in patency were observed between the SF and ePTFE grafts. These results indicate the limits of the B. mori-derived SF based on strength and degradability. In addition, the biological responses in dogs after bypass surgery with SF grafts differed considerably from our expectations, which were based on previous results from rats. Although we observed biodegradation within 3 weeks, further evaluations to improve tube strength and degradability as well as the SF graft coating are warranted to confirm our findings. The mechanism underlying the unique histological changes in SF grafts has not been fully clarified, and their clinical feasibility should be further evaluated.
The present study has some limitations. Previous studies have shown that SF grafts implanted into rat abdominal aortas exhibited complete remodeling with native tissues,21) whereas complete remodeling was not observed in the SF grafts implanted into the carotid arteries of dogs in this study. Degradation was observed within 3 weeks in SF grafts, along with occlusion in some cases. This may have been due to graft fabrication conditions and the coating techniques used. Generally, the SF loses its tensile strength within 1 year after implantation due to slow proteolytic degradation.13,21) Degradation of SF grafts may have been the main cause of the low patency rate. However, this level of degradability is changing owing to progress in recent studies on SF. As new sources of silk fibers become available, including from spiders via genetic engineering and modification of the native silk sequence chemistry, a wider range of material properties can be generated and utilized for biomedical applications.13) Because all areas of the external SF graft surface were surrounded by a thin connective tissue with collagen fiber replacement within 3 weeks, the prevention of SF fiber degradation indicates the possibility of complete remodeling in LLs. Second, vascular biology and coagulation activity differ between humans and dogs. The latter have been described as relatively hypercoagulable,38) which would be associated with reduced patency compared to that in humans. Third, all five ePTFE grafts became occluded within 12 weeks, in contrast to the results by Wilson et al., showing an overall 3-month patency rate of 77% across 48 ePTFE conduits (4 mm) implanted into dogs as carotid or femoral interposition grafts.39) The number of implanted ePTFE grafts was small, and implanting a larger number may have influenced the patency rate obtained.
Conclusion
In conclusion, we have demonstrated that, although SF vascular grafts induce unique histological reactions, the patency rate in our study was not considerably better than that of the ePTFE grafts. Further studies are warranted to confirm our results. Furthermore, new and promising materials should be tested using similar larger animal models.
Acknowledgments
Funding: This study was supported by the Ministry of Agriculture, Forestry, and Fisheries of Japan (Agri-Health Translational Research Project 2010–2015 [Grant number: 3300]).
Disclosure Statement
The authors declare that they have no conflicts of interest in relation to the contents of this article.
Author Contributions
Conception and design of the study: MH, HO and TA
Analysis and interpretation of data: MH, SY and OH
Collection and assembly of data: MH and SY
Drafting of the article: MH and KH
Critical revision of the article for important intellectual
content: KH
Final approval of the article: TW
References
- 1).Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation 2004; 110: 738-43. [DOI] [PubMed] [Google Scholar]
- 2).Baguneid MS, Seifalian AM, Salacinski HJ, et al. Tissue engineering of blood vessels. Br J Surg 2006; 93: 282-90. [DOI] [PubMed] [Google Scholar]
- 3).Zilla P, Bezuidenhout D, Human P. Prosthetic vascular grafts: wrong models, wrong questions and no healing. Biomaterials 2007; 28: 5009-27. [DOI] [PubMed] [Google Scholar]
- 4).Baguneid MS, Goldner S, Fulford PE, et al. A comparison of para-anastomotic compliance profiles after vascular anastomosis: nonpenetrating clips versus standard sutures. J Vasc Surg 2001; 33: 812-20. [DOI] [PubMed] [Google Scholar]
- 5).Tai NR, Giudiceandrea A, Salacinski HJ, et al. In vivo femoropopliteal arterial wall compliance in subjects with and without lower limb vascular disease. J Vasc Surg 1999; 30: 936-45. [DOI] [PubMed] [Google Scholar]
- 6).Salacinski HJ, Tiwari A, Hamilton G, et al. Cellular engineering of vascular bypass grafts: role of chemical coatings for enhancing endothelial cell attachment. Med Biol Eng Comput 2001; 39: 609-18. [DOI] [PubMed] [Google Scholar]
- 7).L’Heureux N, Dusserre N, Konig G, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 2006; 12: 361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).McAllister TN, Maruszewski M, Garrido SA, et al. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 2009; 373: 1440-6. [DOI] [PubMed] [Google Scholar]
- 9).Bosiers M, Deloose K, Verbist J, et al. Heparin-bonded expanded polytetrafluoroethylene vascular graft for femoropopliteal and femorocrural bypass grafting: 1-year results. J Vasc Surg 2006; 43: 313-8; discussion, 318-9. [DOI] [PubMed] [Google Scholar]
- 10).Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials 2003; 24: 401-16. [DOI] [PubMed] [Google Scholar]
- 11).Meinel L, Hofmann S, Karageorgiou V, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials 2005; 26: 147-55. [DOI] [PubMed] [Google Scholar]
- 12).Panilaitis B, Altman GH, Chen J, et al. Macrophage responses to silk. Biomaterials 2003; 24: 3079-85. [DOI] [PubMed] [Google Scholar]
- 13).Wang Y, Kim HJ, Vunjak-Novakovic G, et al. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006; 27: 6064-82. [DOI] [PubMed] [Google Scholar]
- 14).Chiarini A, Petrini P, Bozzini S, et al. Silk fibroin/poly(carbonate)-urethane as a substrate for cell growth: in vitro interactions with human cells. Biomaterials 2003; 24: 789-99. [DOI] [PubMed] [Google Scholar]
- 15).Unger RE, Wolf M, Peters K, et al. Growth of human cells on a non-woven silk fibroin net: a potential for use in tissue engineering. Biomaterials 2004; 25: 1069-75. [DOI] [PubMed] [Google Scholar]
- 16).Lam KH, Nijenhuis AJ, Bartels H, et al. Reinforced poly(L-lactic acid) fibres as suture material. J Appl Biomater 1995; 6: 191-7. [DOI] [PubMed] [Google Scholar]
- 17).Soong HK, Kenyon KR. Adverse reactions to virgin silk sutures in cataract surgery. Ophthalmology 1984; 91: 479-83. [DOI] [PubMed] [Google Scholar]
- 18).Kuboyama N, Kiba H, Arai K, et al. Silk fibroin-based scaffolds for bone regeneration. J Biomed Mater Res B Appl Biomater 2013; 101B: 295-302. [DOI] [PubMed] [Google Scholar]
- 19).Inouye K, Kurokawa M, Nishikawa S, et al. Use of Bombyx mori silk fibroin as a substratum for cultivation of animal cells. J Biochem Biophys Methods 1998; 37: 159-64. [DOI] [PubMed] [Google Scholar]
- 20).Sofia S, McCarthy MB, Gronowicz G, et al. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res 2001; 54: 139-48. [DOI] [PubMed] [Google Scholar]
- 21).Enomoto S, Sumi M, Kajimoto K, et al. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J Vasc Surg 2010; 51: 155-64. [DOI] [PubMed] [Google Scholar]
- 22).Yagi T, Sato M, Nakazawa Y, et al. Preparation of double-raschel knitted silk vascular grafts and evaluation of short-term function in a rat abdominal aorta. J Artif Organs 2011; 14: 89-99. [DOI] [PubMed] [Google Scholar]
- 23).Fukayama T, Takagi K, Tanaka R, et al. Biological reaction to small-diameter vascular grafts made of silk fibroin implanted in the abdominal aortae of rats. Ann Vasc Surg 2015; 29: 341-52. [DOI] [PubMed] [Google Scholar]
- 24).Byrom MJ, Bannon PG, White GH, et al. Animal models for the assessment of novel vascular conduits. J Vasc Surg 2010; 52: 176-95. [DOI] [PubMed] [Google Scholar]
- 25).Aytemiz D, Sakiyama W, Suzuki Y, et al. Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge. Adv Healthc Mater 2013; 2: 361-8. [DOI] [PubMed] [Google Scholar]
- 26).Yamamoto S, Okamoto H, Haga M, et al. Rapid endothelialization and thin luminal layers in vascular grafts using silk fibroin. J Mater Chem B Mater Biol Med 2016; 4: 938-46. [DOI] [PubMed] [Google Scholar]
- 27).Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Jonas RA, Schoen FJ, Levy RJ, et al. Biological sealants and knitted Dacron: porosity and histological comparisons of vascular graft materials with and without collagen and fibrin glue pretreatments. Ann Thorac Surg 1986; 41: 657-63. [DOI] [PubMed] [Google Scholar]
- 29).Lovett M, Eng G, Kluge JA, et al. Tubular silk scaffolds for small diameter vascular grafts. Organogenesis 2010; 6: 217-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Berger K, Sauvage LR, Rao AM, et al. Healing of arterial prostheses in man: its incompleteness. Ann Surg 1972; 175: 118-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Graham LM, Burkel WE, Ford JW, et al. Expanded polytetrafluoroethylene vascular prostheses seeded with enzymatically derived and cultured canine endothelial cells. Surgery 1982; 91: 550-9. [PubMed] [Google Scholar]
- 32).Herring M, Baughman S, Glover J, et al. Endothelial seeding of Dacron and polytetrafluoroethylene grafts: the cellular events of healing. Surgery 1984; 96: 745-55. [PubMed] [Google Scholar]
- 33).Sato O, Tada Y, Takagi A. The biologic fate of Dacron double velour vascular prostheses—a clinicopathological study. Jpn J Surg 1989; 19: 301-11. [DOI] [PubMed] [Google Scholar]
- 34).Kaushal S, Amiel GE, Guleserian KJ, et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 2001; 7: 1035-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Keaney JF Jr, Vita JA. Atherosclerosis, oxidative stress, and antioxidant protection in endothelium-derived relaxing factor action. Prog Cardiovasc Dis 1995; 38: 129-54. [DOI] [PubMed] [Google Scholar]
- 26).Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun 1987; 148: 1482-9. [DOI] [PubMed] [Google Scholar]
- 37).Shapira OM, Xu A, Aldea GS, et al. Enhanced nitric oxide-mediated vascular relaxation in radial artery compared with internal mammary artery or saphenous vein. Circulation 1999; 100 Suppl 2: II322-7. [DOI] [PubMed] [Google Scholar]
- 38).Sato M, Harasaki H. Evaluation of platelet and coagulation function in different animal species using the xylum clot signature analyzer. ASAIO J 2002; 48: 360-4. [DOI] [PubMed] [Google Scholar]
- 39).Wilson GJ, MacGregor DC, Klement P, et al. A compliant Corethane/Dacron composite vascular prosthesis. Comparison with 4-mm ePTFE grafts in a canine model. ASAIO J 1993; 39: M526-31. [PubMed] [Google Scholar]