Abstract
The first-line treatment of venous thromboembolisms (VTE) is anticoagulant therapy, and unfractionated heparin and warfarin are used in Japan. However, as both drugs require dosage adjustments that are difficult, VTE recurrences occur relatively frequently, and hemorrhagic complications are extremely common. The parenteral factor Xa inhibitor fondaparinux and the direct oral anticoagulants (DOACs) edoxaban, rivaroxaban, and apixaban have recently become available as treatments for VTE in Japan. These novel anticoagulants have more stable effects than traditional therapies and are thus considered safer and more effective than the traditional agents. Especially, DOACs offer improved long-term prevention of recurrence in patients with unprovoked VTE. The initiation of DOAC monotherapy soon after VTE onset leads to shorter hospital stays than required with the older therapies and allows for outpatient treatment. DOACs have additional benefits, such as safer anticoagulant therapy for cancer patients. These novel anticoagulants are extremely promising, but there is a current lack of evidence in areas such as dosing regimens for highly vulnerable patients and dosing for long-term use, and alternative regimens for each DOAC.
Keywords: apixaban, direct oral anticoagulant, edoxaban, fondaparinux, rivaroxaban
Introduction
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are considered as one combined condition that is referred to as venous thromboembolism (VTE), and both are diagnosed, treated, and prevented in much the same way. The treatment of VTE encourages dissolution of thromboemboli, inhibition of local thrombosis, and preventing the embolization of thrombi. Anticoagulant therapy plays a main role in treatment.1) In Japan, unfractionated heparin (UFH) and warfarin have traditionally been used; however, these drugs require monitored dose adjustments, and there is a lack evidence supporting their use in Japanese patients. Considering these circumstances, evidence-based guidelines for the safe use of anticoagulants that are simpler to use are desired. The clinical development of novel anticoagulants for treating VTE is ongoing, and the use of anticoagulants as a first-line treatment is becoming more common. This review summarizes anticoagulant therapy for VTE and discusses the features and possible uses of novel anticoagulants, including direct oral anticoagulants (DOACs).
Traditional Anticoagulant Therapy for VTE
A. Initial treatment with UFH
Anticoagulant therapy significantly reduces the mortality and recurrence rates associated with VTE and are becoming a standard first-line treatment. A randomized trial conducted in PE patients in the 1960s, confirmed the benefit of heparin therapy by demonstrating that mortality was significantly higher in a non-therapy than a heparin therapy group.2) Patients diagnosed with VTE are initially given UFH, which exerts an effect quickly. After prompt intravenous injection of 5,000 U heparin, the dose is adjusted during continuous intravenous infusion until the activated partial thromboplastin time (APTT) is 1.5–2.5 times the control value. Subcutaneous doses of UFH can also be administered twice daily in the place of an intravenous infusion.
B. Long-term treatment with warfarin
Following dosing with a parenteral anticoagulant such as UFH, treatment with oral warfarin is initiated. The use of warfarin is based on a randomized trial in which a 3-month administration of warfarin following heparin treatment markedly reduced the rate of out-of-hospital VTE recurrence.3,4) Warfarin can be started soon after administering parenteral anticoagulants. When the prothrombin time-international normalized ratio (PT-INR) has reached the optimum range, parenteral anticoagulant therapy is discontinued and oral warfarin monotherapy is begun. The optimum therapeutic range for warfarin therapy is internationally considered to be a PT-INR of 2.0–3.0. However, the range is often adjusted to 1.5–2.5 in Japan because of potential hemorrhagic complications. However, there are no data supporting this practice.
The dosing period for warfarin is 3 months if reversible risk factors were the cause of VTE. If the cause was a congenital coagulation disorder, or in cases of idiopathic VTE, warfarin is administered for at least 3 months, with subsequent continuation is determined by the risks and benefits of each individual patient. Dosing is extended in patients with a lifetime predisposition for diseases such as cancer and in patients who have experienced multiple relapses. In Japan, self-injection of UFH at home was approved for health insurance coverage in January 2012 for cases where warfarin cannot be used or when warfarin is ineffective for long-term treatment and prevention of VTE. Currently, long-term UFH is used for VTE prevention in pregnant women and cancer patients.
C. Problems with UFH and warfarin therapy
If UFH is used to treat VTE, APTT needs to be measured every 6 hours after starting therapy to ensure that the therapeutic range is rapidly reached. However, as UFH and warfarin doses are not easy to adjust, they are associated with a significantly higher rate of VTE recurrence than therapy with low-molecular-weight heparin (LMWH), which does not require monitoring.5) In the Japan VTE Treatment Registry (JAVA) study, a retrospective registration study, APTT did not rapidly reach the therapeutic range in at least 30% of VTE patients,6) suggesting difficulties of dose adjustment even in clinical settings in Japan.
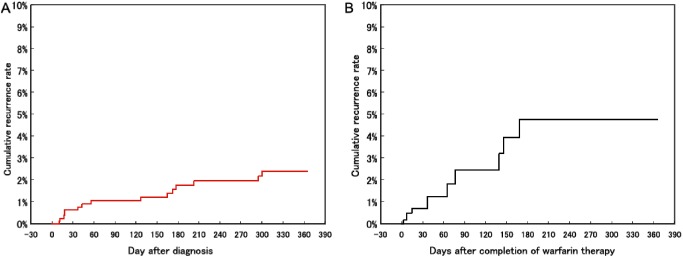
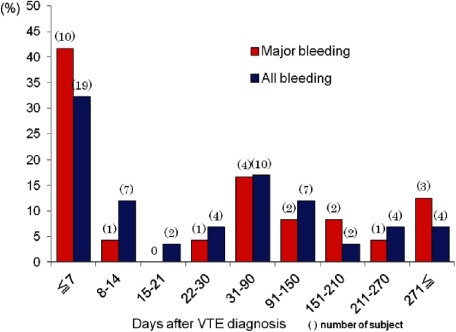
The JAVA study also found that symptomatic VTE recurrences during ongoing warfarin therapy occurred in 2.8% of patients annually and rose to 8.1% of patients annually after stopping warfarin therapy (Fig. 1).7) However, it is not easy to recommend simple long-term dosing of warfarin because of a constant occurrence of hemorrhage complications throughout the duration of warfarin administration (Fig. 2).6) The feasibility of continuous warfarin dosing can only be determined by careful consideration of the thrombotic and hemorrhagic risks in individual patients starting from 3 months after VTE onset.
Fig. 1 Cumulative incidence of recurrent venous thromboembolism with or without warfarin treatment in the JAVA study including 1,076 patients in Japan. (A) An incidence rate of 2.8 per 100 patient-years determined by Kaplan–Meier analysis of patients receiving warfarin therapy. (B) An incidence rate of 8.1 per 100 patient-years by Kaplan–Meier analysis following completion of warfarin therapy. JAVA: Japan VTE Treatment Registry. Reprinted with permission.7).
Fig. 2 Timing of major and all types of bleeding observed after the index event in the JAVA study of 1,076 patients in Japan. During the observation period, the incidence of all types bleeding was 8.2% per patient-year and the incidence of major bleeding was 3.3% per patient-year. The peak onset of bleeding events occurred during week 1 of the acute phase of VTE management. JAVA: Japan VTE Treatment Registry; VTE: venous thromboembolism. Reprinted with permission.6).
Novel Anticoagulant Therapy for VTE
A. Initial treatment with fondaparinux
Fondaparinux, a parenterally administered factor Xa inhibitor, became available for initial treatment of VTE in Japan in March 2011. It has more stable effects than UFH because of less variation in individual patient response. Fondaparinux is administered subcutaneously once daily and does not require monitoring.8) It has a low incidence of side effects, such as thrombocytopenia and osteopenia, and is considered safe with an equivalent or better effect than appropriately adjusted UFH. A dose of 7.5 mg is usually administered, but doses of 5 mg or 10 mg are administered to patients weighing less than 50 kg and more than 100 kg, respectively. As fondaparinux is primarily excreted by the kidneys, continuous assessment of renal function is important. Administration of fondaparinux is contraindicated in patients with a creatinine clearance of less than 30 mL/min.
B. Treatment with DOACs
Recent international clinical trials evaluated DOACs for treating VTE and preventing recurrences in the long-term as well as for preventing cardiogenic cerebral embolisms in patients with atrial fibrillation and postoperative primary VTE. The results were promising (Table 1).9–12) The oral factor Xa inhibitor edoxaban was approved in Japan for the treatment and secondary prevention of VTE (DVT and PE) in September 2014. Rivaroxaban was approved for the treatment and secondary prevention of DVT and PE in September 2015, and apixaban was approved for the treatment and secondary prevention of VTE (DVT and PE) in December 2015.
Table 1 Large clinical trials of non-vitamin K antagonist oral anticoagulant/direct oral anticoagulant for venous thromboembolism.
| Hokusai-VTE | EINSTEIN-DVT | EINSTEIN-PE | AMPLIFY | |
|---|---|---|---|---|
| Product name (Generic name) | Lixiana (edoxaban) | Xarelto (rivaroxaban) | Eliquis (apixaban) | |
| Trial method | Double-blind | Open-label | Double-blind | |
| Regimen | 60 mg (30 mg) once daily | 15 mg twice daily for 3 weeks, followed by 20 mg once daily | 10 mg twice daily for a week, followed by 5 mg twice daily | |
| Dose reduction | Body weight ≤60 kg | N/A | N/A | |
| Creatinine clearance: 30–50 mL/min | ||||
| Combination with P-glycoprotein inhibitor | ||||
| Initial heparin use | 5–12 days | 72% | 92% | 87% |
| Population | 8,292 | 3,449 | 4,832 | 4,816 |
| Warfarin TTR (%) | 63.5% | 57.7% | 62.7% | 60.9% |
| Treatment period | 3–12 months (Flexible) | 3, 6, and 12 months | 6 months | |
| Japanese patients | 209 patients | N/A | N/A | N/A |
| Primary efficacy outcome | Non-inferiority (VTE recurrence) | Non-inferiority (VTE recurrence) | Non-inferiority (VTE recurrence) | Non-inferiority (VTE recurrence or VTE-related death) |
| Primary safety outcome | Superiority (Major bleeding+CRNM) | Non-inferiority (Major bleeding+CRNM) | Non-inferiority (Major bleeding+CRNM) | Superiority (Major bleeding) |
CRNM: clinically relevant non-major bleeding; N/A: not available; TTR: target therapeutic range; VTE: venous thromboembolism. From ref. 9–12.
The efficacy and safety of edoxaban were demonstrated in the Hokusai-VTE study, an international clinical trial including Japanese patients9) and in a subsequent sub-analysis of an East Asian population, also including Japanese patients.13) The efficacy and safety of rivaroxaban and apixaban were demonstrated in international clinical studies, the EINSTEIN-PE/DVT10,11) and the AMPLIFY trials.12) The J-EINSTEIN14) and AMPLIFY-J15) trials also confirmed the safety of these drugs in Japanese people.
1) Edoxaban and the Hokusai-VTE study
The Hokusai-VTE study evaluated the efficacy and safety of edoxaban after initial treatment with a parenteral anticoagulant for at least 5 days. The efficacy of edoxaban was noninferior to warfarin therapy in an overall analysis, it significantly reduced recurrence of symptomatic VTE compared with warfarin in a subanalysis of PE patients with an N-terminal pro B-type natriuretic peptide (NT-proBNP) concentration≥500 pg/mL. The dose reduction criteria for edoxaban are determined based on detailed analyses, and a phase III trial has proven its benefits. Edoxaban is administered once daily at 60 mg; in patients <60 kg, with a creatinine clearance of ≤50 mL/min, or are also taking P-glycoprotein inhibitors, the daily dose is reduced to 30 mg. Administration of edoxaban is contraindicated in patients with a creatinine clearance <15 mL/min.
2) Rivaroxaban in the EINSTEIN-PE/DVT trials
In the EINSTEIN-PE/DVT trials, rivaroxaban was administered orally soon after onset by at 1.5 times the usual dose for the first 3 weeks. In Japan, the utility of rivaroxaban was demonstrated in the J-EINSTEIN trial, in which the usual dose, starting from 3 weeks after onset, was reduced to 75% of that used in the EINSTEIN-PE/DVT trials. A dose of 15 mg twice daily was administered continuously for 3 weeks soon after VTE onset. Therapy was then continued at the usual dose of 15 mg once daily. It should be noted that different dosages were used in Japan and the international countries. There were no specific dose reduction criteria, but administration of rivaroxaban is contraindicated in patients with a creatinine clearance of <30 mL/min.
3) Apixaban in the AMPLIFY trial
In the AMPLIFY trial, apixaban was administered orally soon after onset at twice the usual dose, for the first week. In the AMPLIFY-EXT trial, the efficacy of apixaban for continuous therapy was demonstrated at a decreased dose of 2.5 mg twice daily starting from 6 months after VTE onset. In Japan, the utility of apixaban was demonstrated in the AMPLIFY-J trial, which used the same dosages as in the AMPLIFY trial.16) A dose of 10 mg twice daily was administered for 1 week soon after VTE onset, after which therapy was continued with the usual dose of 5 mg twice daily. There were no specific dose reduction criteria, but the administration of apixaban is contraindicated in patients with a creatinine clearance of <30 mL/min.
4) Challenges of VTE treatment using DOACs
As the effects of DOACs cannot be monitored, sufficient caution must be used when administering them to patients at a high risk of bleeding, such as the elderly, those with low body weight or renal dysfunction, and those taking a combination of antiplatelet agents. In addition, as DOACs are not easily neutralized, the use of drugs with a short half-life, such as UFH, is considered if invasive treatment is anticipated. While three DOACs are currently available to treat VTE in Japan, each differs greatly in the initial dosing regimen, which makes it extremely difficult to decide which DOAC should be used in a specific case of VTE. Further clinical trials are needed to investigate the different uses for each DOAC. This is discussed in the following section.
Development of VTE Treatment in the Age of DOACs
A. Long-term prevention of VTE recurrence
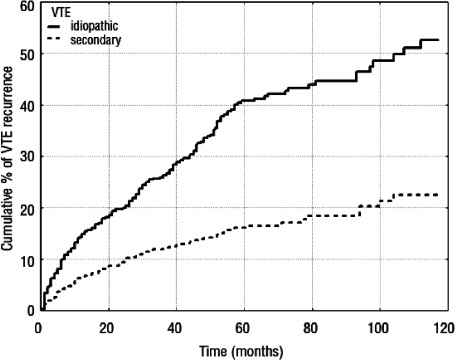
At least 3 months of anticoagulant therapy is recommended for long-term VTE treatment. However, the evidence applies only to therapy with warfarin. Because the risk of VTE recurrence continues at a constant rate even after 3 months of treatment, longer-term anticoagulant therapy should be considered. This is particularly desirable for idiopathic or unprovoked VTE, which accounts for the majority of symptomatic cases in Japan,6) because it has the highest frequency of recurrence (Fig. 3).17) Drugs that are safe for long-term administration for this condition are highly desirable. DOACs were developed with that in mind, and are highly likely to be used for longer-term VTE treatment. Although they are viewed as safe anticoagulants with less bleeding risk than warfarin, no guidelines for proceeding with long-term treatment are available, and development depends on collecting evidence with future studies. The need for dose reduction during long-term treatment must also be examined.
Fig. 3 Cumulative incidence of recurrent thromboembolism in patients with idiopathic (unprovoked) and secondary VTE. The adjusted hazard ratio for recurrent VTE after withdrawal of vitamin K antagonists was the highest in patients whose first VTE was unprovoked, i.e., with no obvious cause. VTE: venous thromboembolism. Reprinted with permission.17).
B. Shortened hospital stays for VTE patients
With traditional VTE treatment, a parenteral anticoagulant is used first, followed by warfarin. As a result, several days are needed to adjust the PT-INR prior to switching to warfarin. Previously, the dosing period for parenteral anticoagulants was influenced more strongly by the number of days required to adjust the PT-INR than the need for that specific medication. DOACs begin to act immediately after administration, which avoids any delay until the appearance of effects. That allows a shorter duration of hospitalization and treatment in the acute phase, facilitates early discharge and outpatient treatment of stable patients who are at a low risk for VTE recurrence and bleeding.
C. DOAC monotherapy soon after VTE onset
If it is possible to use a single drug throughout the entire treatment period, treatment plans could be considerably simplified. Treatment with a DOAC from the onset of VTE is not seen as problematic in mild-to-moderate DVT. On the other hand, reliability of treatment is the priority in PE, and further studies are needed to determine whether oral monotherapy from the time of onset is adequate in patients with proven right ventricular overload.6)
In Western countries, LMWH and fondaparinux tend to be administered from early onset to outpatient treatment in DVT patients who can self-inject these medications. The available evidence on the efficacy of outpatient treatment has recently been reviewed.18) Because DOACs take effect rapidly and require no monitoring, they can be safely administered even when treatment is started on an outpatient basis.
D. DOACs in cancer patients
Cancer patients are prone to developing VTE, and in Japan, cancer is the greatest risk factor for VTE. Anticoagulant therapy with LMWH has been found to improve the prognosis of VTE in cancer patients,19) and Western guidelines recommend LMWH self-injection for cancer patients with VTE from early onset to the long-term. In Japan, only warfarin has been used to prevent the recurrence of VTE in cancer patients, but the dosage can be difficult to adjust because of fluctuations in combination drug doses and platelet numbers during chemotherapy. It remains unclear if DOACs are as effective as LMWH in cancer patients, but it is certain that DOACs are easy to adjust even in cancer patients. Going forward, there are plans to continue investigating the effects of DOACs on prognosis of cancer patients with VTE.
Selection of DOACs by Purpose
A. Basic approach
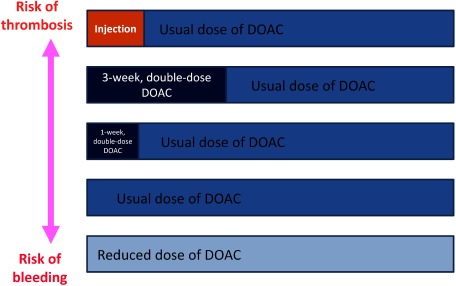
DOAC dosages after completion of initial VTE treatment are the same as those for atrial fibrillation, i.e., the “usual doses.” The possible options for initial VTE treatment are parenteral anticoagulants such as fondaparinux, continuous treatment with double the usual DOAC dose for 3 weeks, continuous treatment with a double the usual DOAC dose for 1 week, DOAC at the usual dose, and a reduced DOAC dose. The rationale of each approach is to achieve a balance between the benefit of treatment and the risks of thrombosis and bleeding. Measures to minimize the risks of hemorrhage and recurrence of VTE both need to be considered (Fig. 4).
Fig. 4 Regimens for direct oral administration of anticoagulants for the treatment of venous thromboembolism in Japan. Patients at increased risk of thrombosis are more suited for the dosing regimens in the upper row; patients at increased risk of bleeding are more suited to the dosing regimens in the lower row. DOAC: direct oral anticoagulant.
B. DOACs in PE
Because of the high rate of early recurrence in PE,6) the treatment effectiveness is very important in this population. If the risk of bleeding is low, then administration of a parenteral anticoagulant or a double DOAC dose might be considered upon hospitalization. A double dose of rivaroxaban and apixaban can be given, both of which have demonstrated non-inferiority to standard therapy, and are the available options when choosing initial oral therapy. However, the regimen including edoxaban begins with parenteral anticoagulants because that sequence enhances initial treatment and has been acknowledged to significantly improve secondary prevention in patients with right ventricular overload, such as those with an NT-proBNP ≥500 pg/mL.20) In severe PE, there is strong evidence in favor of a parenteral anticoagulant in combination with edoxaban. Initial treatment with the usual doses of edoxaban, rivaroxaban, and apixaban can also be considered in patients with asymptomatic PE detected by chance via contrast computed tomography or other means and in patients at high risk of hemorrhage.
C. DOACs in DVT
Because the rate of recurrence in the initial stages of DVT is not as high as in PE, oral outpatient monotherapy can be chosen from an early stage. In addition, treatment focused more on reducing the risk of hemorrhagic complications than reduction of thrombus is a valid option from a risk–benefit standpoint. There is strong evidence from international large-scale clinical trials for administering an initial double DOAC dose in patients with femoral vein thrombosis and with a body weight of approximately 80 kg. A previous clinical trial found that initial DVT treatment starting with the usual DOAC dose resulted in the same rate of recurrence as standard therapy using an enhanced initial dose of LMWH.21) The need for a double DOAC dose is decreased in patients with a low body weight, high bleeding risk, or if the volume of thrombi is not significant. Therefore, administering DOAC at the usual dose is an option from the start of therapy, particularly in populations where the risk of bleeding is considerable. In such patients, the effects of usual doses of edoxaban, rivaroxaban, or apixaban are not expected to differ greatly.
D. DOACs in fragile patients
Dose reduction criteria have been established for each DOAC when administered for atrial fibrillation in fragile patient populations, including those with decreased renal function, low body weight, and the elderly. For the treatment of VTE, dose reduction criteria have been established for edoxaban based on the same criteria as for atrial fibrillation; no dose reduction criteria have been established for rivaroxaban or apixaban. Careful consideration needs to be given to the benefits and risks of long-term use of DOACs for which dose reduction criteria have not been established. If there are concerns regarding the risk of bleeding, edoxaban, for which dose reduction criteria have been established, is the preferred treatment.
E. DOACs for long-term treatment
Large international trials have presented evidence in support of 6- to 12-month DOAC therapy. However, patients with idiopathic VTE and ongoing risk of recurrence need longer-term anticoagulant therapy. All three DOACs have a lower risk of hemorrhage than warfarin and are safer when used for long-term treatment. The administration of reduced doses of DOACs to further reduce the risk of hemorrhage in long-term treatment is being investigated. As discussed earlier, evidence that administering reduced doses of apixaban inhibits recurrence without increasing bleeding risk has emerged from international trials,16) with similar benefits expected for the other DOACs. If dose reduction is needed, half the usual dose of edoxaban or apixaban and two-thirds the usual dose of rivaroxaban can be administered, with the choice of therapy based on expected risks and benefits.
Conclusion
Novel anticoagulant therapy offer numerous benefits for VTE treatment in the same way as prevention of cardiogenic cerebral embolism in patients with atrial fibrillation. As the underlying diseases and circumstances of patients with atrial fibrillation and VTE differ, it is highly likely that the effects of novel anticoagulants and the level of bleeding risk also differ. More dosing guidance needs to be obtained from clinical experience in various populations generally excluded from large clinical trials, to optimize the safety and ease of use of novel anticoagulants.
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Number JP26461067.
Disclosure Statement
Mashio Nakamura has received remuneration from DAIICHI SANKYO COMPANY, LIMITED, Bayer Yakuhin, Ltd., Pfizer Japan Inc. and Bristol-Myers Squibb K.K. Norikazu Yamada has received honoraria from Bayer Yakuhin, Ltd. and Bristol-Myers Squibb K.K. Masaaki Ito has received Scholarship from Bayer Yakuhin, Ltd., Pfizer Japan Inc., Bristol-Myers Squibb K.K., DAIICHI SANKYO COMPANY, LIMITED and Nippon Boehringer Ingelheim Co., Ltd.
Author Contributions
Writing: MN
Critical review and revision: all authors
References
- 1).JCS Joint Working Group. Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J 2011; 75: 1258-81. [DOI] [PubMed] [Google Scholar]
- 2).Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet 1960; 275: 1309-12. [DOI] [PubMed] [Google Scholar]
- 3).Hull R, Delmore T, Genton E, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med 1979; 301: 855-8. [DOI] [PubMed] [Google Scholar]
- 4).Lagerstedt CI, Fagher BO, Olsson CG et al. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet 1985; 326: 515-8. [DOI] [PubMed] [Google Scholar]
- 5).Breddin HK, Hach-Wunderle V, Nakov R, et al; CORTES Investigators. Clivarin: Assessment of Regression of Thrombosis, Efficacy, and Safety. Effects of a low-molecular-weight heparin on thrombus regression and recurrent thromboembolism in patients with deep-vein thrombosis. N Engl J Med 2001; 344: 626-31. [DOI] [PubMed] [Google Scholar]
- 6).Nakamura M, Miyata T, Ozeki Y, et al. Current venous thromboembolism management and outcomes in Japan. Circ J 2014; 78: 708-17. [DOI] [PubMed] [Google Scholar]
- 7).Nakamura M, Yamada N, Ito M. Current management of venous thromboembolism in Japan: current epidemiology and advances in anticoagulant therapy. J Cardiol 2015; 66: 451-9. [DOI] [PubMed] [Google Scholar]
- 8).Nakamura M, Okano Y, Minamiguchi H, et al. Multidetector-row computed tomography-based clinical assessment of fondaparinux for treatment of acute pulmonary embolism and acute deep vein thrombosis in Japanese patients. Circ J 2011; 75: 1424-32. [DOI] [PubMed] [Google Scholar]
- 9).Büller HR, Décousus H, Grosso MA, et al.; Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406-15. [DOI] [PubMed] [Google Scholar]
- 10).Bauersachs R, Berkowitz SD, Brenner B, et al.; EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363: 2499-510. [DOI] [PubMed] [Google Scholar]
- 11).Büller HR, Prins MH, Lensin AW, et al.; EINSTEIN-PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287-97. [DOI] [PubMed] [Google Scholar]
- 12).Agnelli G, Buller HR, Cohen A, et al.; AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369: 799-808. [DOI] [PubMed] [Google Scholar]
- 13).Nakamura M, Wang YQ, Wang C, et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: a subanalysis of East Asian patients in the Hokusai-VTE trial. J Thromb Haemost 2015; 13: 1606-14. [DOI] [PubMed] [Google Scholar]
- 14).Yamada N, Hirayama A, Maeda H, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism—the J-EINSTEIN DVT and PE program. Thromb J 2015; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Nakamura M, Nishikawa M, Komuro I, et al. Apixaban for the treatment of Japanese subjects with acute venous thromboembolism (AMPLIFY-J Study). Circ J 2015; 79: 1230-6. [DOI] [PubMed] [Google Scholar]
- 16).Agnelli G, Buller HR, Cohen A, et al.; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013; 368: 699-708. [DOI] [PubMed] [Google Scholar]
- 17).Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007; 92: 199-205. [DOI] [PubMed] [Google Scholar]
- 18).Kearon C, Akl EA, Anthony J, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e419S-94S. [DOI] [PMC free article] [PubMed]
- 19).Lee AY, Rickles FR, Julian JA, et al. Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol 2005; 23: 2123-9. [DOI] [PubMed] [Google Scholar]
- 20).Brekelmans MP, Ageno W, Beenen LF, et al. Recurrent venous thromboembolism in patients with pulmonary embolism and right ventricular dysfunction: a post-hoc analysis of the Hokusai-VTE study. Lancet Haematol 2016; 3: e437-45. [DOI] [PubMed] [Google Scholar]
- 21).Buller HR, Cohen AT, Davidson B, et al.; van Gogh Investigators. Idraparinux versus standard therapy for venous thromboembolic disease. N Engl J Med 2007; 357: 1094-104. [DOI] [PubMed] [Google Scholar]