Abstract
We report two cases of persistent sciatic artery (PSA) aneurysm with limb ischemia. Physicians who treat peripheral artery disease should be aware that PSA is a very rare congenital malformation of the lower extremities that is potentially hazardous, and that revascularization should be performed when a PSA aneurysm is treated.
Keywords: persistent sciatic artery, limb ischemia, aneurysm
Introduction
Persistent sciatic artery (PSA) is a very rare congenital malformation1) that can cause complications including acute and chronic limb ischemia, aneurysm formation, and compression of adjacent tissues.2–5) PSA patients with leg ischemia should be treated by revascularization and management of PSA aneurysm. Although endovascular treatment is frequently chosen, open repair remains preferable in most cases. This report describes two PSA patients with different clinical presentations.
Case Reports
Case 1
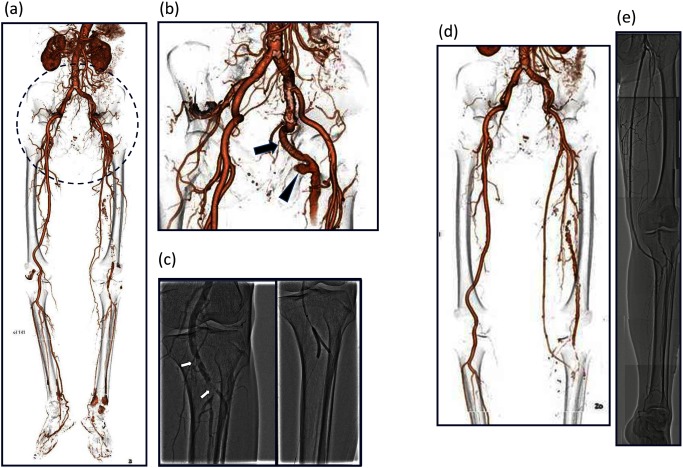
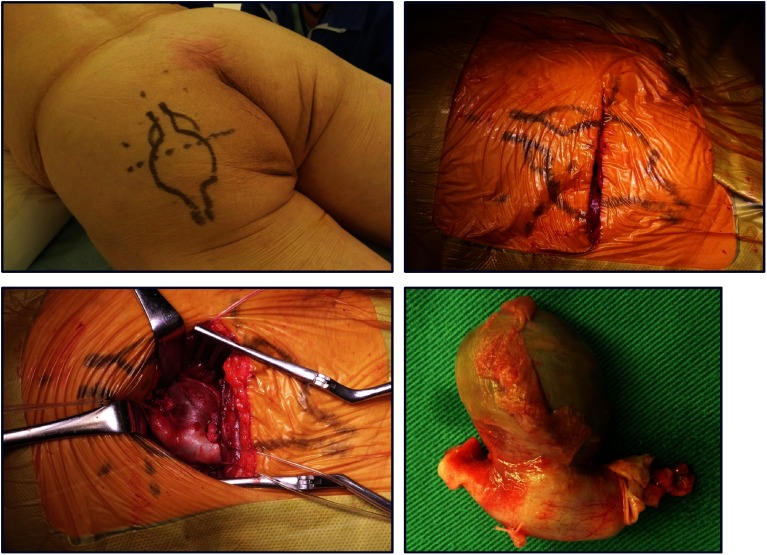
An 89-year-old man presented with a painful gluteal mass that had developed over several months and left calf claudication. Computed tomography (CT) showed a fusiform aneurysmal dilation and occlusion of a PSA (Figs. 1a and 1b). The popliteal artery was fed from the collateral circulation. Before presentation, endovascular treatment (EVT) was administered for embolization distal to a PSA aneurysm by cardiologists because of the patient’s advanced age. Filling defects of the popliteal artery were detected below the knee, and balloon angioplasty was performed three times (Fig. 1c). Symptoms spontaneously improved, but intermittent claudication recurred several months after the EVT and recurrent distal embolization was treated surgically. Under general anesthesia, with the patient in the prone position, a transverse incision was made on the buttock and the aneurysm was resected (Fig. 2). The patient was then placed in the supine position, and a left superficial femoro-posterior tibial artery bypass was performed in a non-reversed fashion using the ipsilateral great saphenous. The left ankle brachial index (ABI) increased to 0.83 from 0.64, and claudication was improved. Postoperative CT and angiography showed a patent bypass graft (Figs. 1d and 1e).
Fig. 1 (a) CT showing a fusiform aneurysmal dilation and occluded PSA. The popliteal artery was fed by collateral circulation. (b) PSA (arrow) and fusiform aneurysmal dilation of PSA (arrowhead). (c) Filling defects of the popliteal artery were detected below the knee. Endovascular treatment of distal embolization from a PSA aneurysm had been performed three times. (d) Postoperative computed tomography showing a patent bypass graft. (e) Postoperative angiography showing a patent bypass graft.
Fig. 2 Intraoperative findings following a transverse incision of the left buttock and resected persistent sciatic artery aneurysm.
Case 2
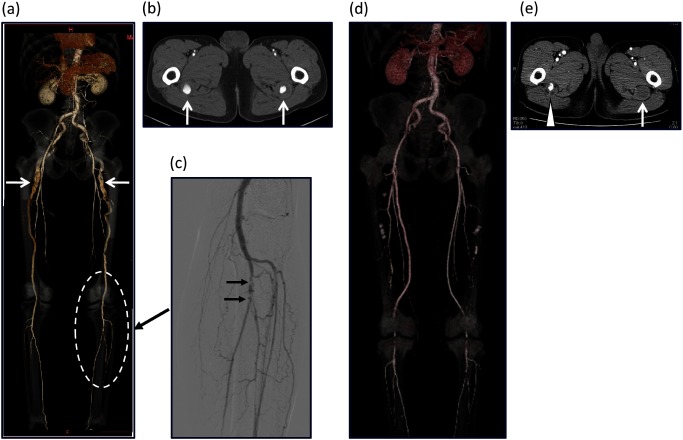
A 70-year-old man presented with bilateral sciatic neuralgia. Two years previously, he had experienced pain in the left leg when at rest, which was diagnosed elsewhere as a thrombotic event. The ischemia symptoms resolved spontaneously. The patient’s medical history is significant for hypertension. CT and angiography showed complete PSA with an aneurysm that was 30 mm long and an incomplete superficial femoral artery (SFA) that did not extend to popliteal artery (Figs. 3a and 3b). A filling defect was observed at left tibioperoneal trunk (Fig. 3c). A left femoro-popliteal (FP) bypass was performed under general anesthesia using the ipsilateral great saphenous vein in a non-reversed fashion. One week after the bypass procedure, exclusion of aneurysm was performed with a Amplatzer Vascular Plug II (St. Jude Medical, Inc., St. Paul, MN, USA) under local anesthesia. Three months after treatment of the left leg, a right femoral popliteal (FP) bypass using 7 mm PROPATEN (W. L. Gore & Associates, Flagstaff, AZ, USA), and exclusion of the aneurysm with a plug were performed. Postoperative CT showed retrograde flow to the right PSA aneurysm (Figs. 3d and 3e), but sciatic neuralgia was improved.
Fig. 3 (a) Preoperative CT showing a complete PSA with aneurysm formation and an incomplete superficial femoral artery that does not reach the popliteal artery. (b) CT showing an aneurysm that extends for 30 mm. (c) Angiography showing a filling defect of the left tibioperoneal trunk. (d) Postoperative CT showing a patent bilateral femoropopliteal bypass graft. (e) A left PSA aneurysm is completely excluded (arrow). Retrograde flow into right PSA aneurysm persists (arrowhead).
Discussion
PSA is a rare congenital anomaly, with an incidence ranging from 0.01% to 0.06% and associated with various complications2–5) including atherosclerotic changes or aneurysms that are often mechanically compressed because of their anatomical location. These changes can lead to limb ischemia, thrombosis of the PSA or distal embolization, rupture of the aneurysm and buttock pain or sciatic neuralgia because of compression of adjacent tissue. Symptoms of acute limb ischemia were not observed at case 1. In case 2, symptoms of acute limb ischemia were observed, pain while at rest resolved, and the thrombotic events that occurred did not lead to limb threatening events.
The treatment of PSA depends on the patient’s symptoms, aneurysm formation, and the type of PSA. Complete PSA has communicates directly with the popliteal artery with hypoplasia or deficit of the superficial femoral artery (SFA) (Types 1, 2). Incomplete PSA is characterized by a normal SFA and no direct communication of the PSA and popliteal artery (Types 3, 4).2) Approximately 80% of PSAs are of the complete type, which requires revascularization by surgical bypass because of hypoplasia of the SFA.6) Treatment of a PSA aneurysm, requires surgical or endovascular resection and exclusion by coiling or the placement of a stent-graft.7–10)
These two cases were of the complete type of PSA. For revascularization, EVT is less invasive than surgical treatment, but surgical treatment is preferred treatment in the majority of cases as the patency of the popliteal and infrapopliteal lesions after EVT may not be adequate.11) In case 1, resection of the aneurysm was performed with revascularization by surgical bypass. EVT had been performed three times by cardiovascular specialists because of the patient’s advanced age. However endovascular treatment appears to have been mistakenly chosen. First, the origin of the embolus was a PSA aneurysm. Therefore, the aneurism should have been treated initially. Furthermore, revascularization by surgical bypass is required for complete type PSA. Physicians who treat peripheral artery disease should be aware that of PSA and that revascularization should be performed whenever a PSA aneurysm is treated.
Surgery and EVT are both treatment options for PSA aneurysms. In case 1, surgical resection was performed without complications. However, surgical resection of a PSA aneurysm may be complicated by sciatic nerve injury. EVT may avoid surgical complications because it is a less invasive treatment. Therefore, endovascular approach was selected in case 2, and exclusion of the aneurysm was performed with an Amplatzer Vascular Plug (St. Jude Medical, Inc., St. Paul, MN, USA). This treatment may not decompress the aneurysm because of retrograde flow from collateral vessels. Even though retrograde flow to right PSA aneurysm persisted in case 2, the sciatic neuralgia has been improved. Open conversion should be considered when aneurysm sac enlargement is present or with recurrence of sciatic neuralgia.
A previous report demonstrated the effectiveness of stent-grafting to treat PSA aneurysms.10) In Japan, stent-grafts are an off-label treatment for PSA aneurysms. Patient may be at risk of stent-graft occlusion, and the long-term results are unknown. At present, aneurysm exclusion or resection are considered better treatment strategies.
Conclusion
Physicians who treat PAD should be aware of PSA is a congenital anomaly that poses a risk of lower limb amputation. Treatment of PSA aneurysm requires revascularization of the lower limb, and surgical bypass remains the preferable option in most cases.
Disclosure Statement
The authors have no conflicts of interest to declare.
Author Contributions
Study conception: KM
Study conception: KM
Analysis: KM, TY, JO
Investigation: KM
Writing: KM, TY, JO
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
References
- 1).Yang S, Ranum K, Malone M, et al. Bilateral persistent sciatic artery with aneurysm formation and review of the literature. Ann Vasc Surg 2014; 28: 264.e1-7. [DOI] [PubMed] [Google Scholar]
- 2).Patel MV, Patel NH, Schneider JR, et al. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57: 225-9. [DOI] [PubMed] [Google Scholar]
- 3).Bito Y, Sakaki M, Iida O, et al. Clinical management of lower limb ischemia secondary to a persistent sciatic artery aneurysm: report of a case. Surg Today 2011; 41: 402-5. [DOI] [PubMed] [Google Scholar]
- 4).Santaolalla V, Bernabe MH, Hipola Ulecia JM, et al. Persistent sciatic artery. Ann Vasc Surg 2010; 24: 691.e7-10. [DOI] [PubMed] [Google Scholar]
- 5).Ishida K, Imamaki M, Ishida A, et al. A ruptured aneurysm in persistent sciatic artery: a case report. J Vasc Surg 2005; 42: 556-8. [DOI] [PubMed] [Google Scholar]
- 6).van Hooft IM, Zeebregts CJ, van Sterkenburg SM, et al. The persistent sciatic artery. Eur J Vasc Endovasc Surg 2009; 37: 585-91. [DOI] [PubMed] [Google Scholar]
- 7).Chen B, Shi Z, Wang Y, et al. The management of persistent sciatic artery aneurysm with lower extremity ischemia: a case report. Ann Vasc Dis 2011; 4: 332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Ooka T, Murakami T, Makino Y. Coil embolization of symptomatic persistent sciatic artery aneurysm: a case report. Ann Vasc Surg 2009; 23: 411.e1-4. [DOI] [PubMed] [Google Scholar]
- 9).Ahmadi F, Zabihiyeganeh M, Abdollahi M. Coil embolization of persistent sciatic artery pseudoaneurysm presenting as blue toe syndrome, a rare case. Indian J Surg 2013; 75 Suppl. 1: 316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Fearing NM, Ammar AD, Hutchinson SA, et al. Endovascular stent graft repair of a persistent sciatic artery aneurysm. Ann Vasc Surg 2005; 19: 438-41. [DOI] [PubMed] [Google Scholar]
- 11).Iida O, Soga Y, Yamauchi Y, et al. Clinical efficacy of endovascular therapy for patients with critical limb ischemia attributable to pure isolated infrapopliteal lesions. J Vasc Surg 2013; 57: 974-81.e1. [DOI] [PubMed] [Google Scholar]