Abstract
Objective: Endovascular repair has become the treatment of choice for ruptured abdominal aortic aneurysms (RAAAs). To improve surgical outcomes, preoperative management is important. In 2011, we introduced integrated management, which involves endovascular aneurysm repair, stabilization of hemodynamics by endovascular clamping, and open abdominal decompression to address abdominal compartment syndrome (ACS).
Methods: To evaluate the efficacy of this management strategy, 62 patients who had undergone emergency surgery for an RAAA were analyzed retrospectively: group A (n=39), where an old strategy was used, and group B (n=23), where integrated management was introduced. Patient characteristics and 30-day mortality rates were compared between the two groups.
Results: The average patient age was 67.7 years and 74.7 years for groups A and B, respectively (P=0.032). Group B patients required more frequent use of vasopressors (P=0.035). Other patient characteristics did not differ between the two groups. The duration of surgery was significantly shorter in group B than in group A (P=0.001). The total amount of transfused blood did not differ between the two groups. No patients showed symptoms of ACS. Early mortality rates were 12.8% and 8.7% in groups A and B, respectively. The number of wound infections was significantly fewer in group B than in group A.
Conclusion: Although group B patients were significantly older and had a higher rate of vasopressor use, early mortality was improved in both groups. Morbidity was significantly better in group B with respect to the duration of surgery and number of wound infections than in group A.
Keywords: ruptured abdominal aortic aneurysm, shock, EVAR, abdominal compartment syndrome, endovascular clamp
Introduction
Ruptured abdominal aortic aneurysms (RAAAs) can be fatal without prompt surgical intervention. Approximately 2% of men older than 65 years of age die from RAAAs.1) The prognosis of RAAAs is poor, with a mortality of 80% within several days of onset.2) One-third of all patients with an RAAA die before hospitalization and another one-third do not undergo surgical intervention.3) The estimated overall mortality rate of hospitalized RAAA patients approaches 48%.4)
The conventional surgical treatment for RAAAs is graft replacement with exclusion of the aneurysmal sac. The risks of open surgery include intraperitoneal rupture after laparotomy, massive bleeding due to coagulopathy, and abdominal compartment syndrome (ACS) with multiple organ failure after peritoneal closure. Martin and colleagues5) reported a successful endovascular replacement for an RAAA patient in 1995. Since that time, endovascular aneurysmal repair (EVAR) using stent grafts has gradually gained popularity as a useful tool for RAAA repair. The efficacy of EVAR for RAAAs was tested in four randomized trials,6–9) which demonstrated improved morbidity, but not superiority, to open repair in terms of 30-day mortality rates.
To improve surgical outcomes for RAAAs, preoperative management is an important factor. Ogino et al.10) reported improved mortality from 57% to 26% by introducing a protocol-based EVAR strategy for RAAAs. Immediate surgery improves mortality rates in critically ill RAAA patients, but is not applicable at every hospital because of overcrowded operating rooms, staff shortages, availability of stent grafts, etc. To avoid these challenges, an occlusion balloon is typically inserted into the abdominal aorta from the femoral artery in the emergency department.
The purpose of this study was to evaluate the efficacy of an integrated management system, involving endovascular clamping, EVAR, and open abdominal decompression, to address ACS after surgery.
Materials and Methods
Sixty-two patients who had undergone emergency surgery for an RAAA at Hirosaki University Hospital from January 2004 to December 2015 were analyzed retrospectively. During the same period, 365 patients had undergone surgical repair of infra- and juxtarenal abdominal aortic aneurysms by either open repair or EVAR. An RAAA was defined as an extravasation of blood outside of the abdominal aorta, confirmed by enhanced computed tomography (CT). Preoperative CT was performed in all patients.
Inclusion and exclusion criteria for surgery
All RAAA patients arriving at the emergency department were considered for surgical intervention. Four patients with cardiopulmonary arrest refractory to cardiopulmonary resuscitation; three patients with diffuse brain damage, such as deep coma with dilated pupils; and 19 patients from whom consent for surgery could not be obtained were excluded from surgery. Thus, 26 of 88 patients with RAAAs were excluded from surgery.
Surgical procedure and preoperative management
From 2004 to 2010, open repair was used as the standard treatment for RAAAs. A custom-made stent graft was used for a patient with a history of laparotomy (n=1). EVAR using commercially available stent grafts was introduced in 2011. Since that time, all patients with RAAAs were considered candidates for EVAR, when preoperative CT showed suitable morphology and board-approved surgeons were available. Recently, EVAR has become the treatment of choice for RAAAs. Open repair is chosen as the alternative to EVAR for morphologically unsuitable cases, such as short landing zone for EVAR (<10 mm) or severe angulation of the aneurysm neck. The integrated management of RAAAs involves three components: EVAR, stabilization of hemodynamics by endovascular clamping, and abdominal decompression and monitoring of intra-abdominal pressure (IAP) to address ACS. Continuous renal replacement therapy (CRRT) was aggressively initiated in cases of oliguria or elevated serum creatinine levels after surgery. When board-approved surgeons for EVAR were unavailable, patients underwent open surgery but with other management protocols.
Endovascular clamping was used for patients in shock. An aortic occlusion balloon (AOB, Block Balloon™, Senko Medical Instrument Mfg. Co. Ltd., Tokyo, Japan) was inserted through the femoral artery into the suprarenal abdominal aorta under fluoroscopic monitoring in the emergency department. After transfer of the patient for surgery, the AOB was exchanged for a TMP lock balloon catheter (Tokai Medical Products Co., Aichi, Japan), which was inserted through the brachial artery into the descending aorta. For patients in whom endovascular clamping could not be performed, mild hypotension (a blood pressure between 70 and 90 mmHg) was maintained by fluid replacement and minimal doses of vasopressors. Thoracotomy and clamping of the descending aorta prior to laparotomy were performed in seven patients.
General anesthesia, using propofol, ketamine, and a muscle relaxant, was administered. Supraceliac manual aortic compression was performed in cases where there were difficulties exposing the aneurysmal neck during open repair. An albumin-coated, bifurcated Dacron graft was used during open repair. EVAR was performed with a Gore-Excluder™ bifurcated endoprosthesis (Gore Medical, Flagstaff, AZ, USA), as previously described.11) Patients with morphologically unsuitable AAAs (neck length <10 mm, severe atherosclerosis of access artery) underwent open repair.
IAP was monitored through the bladder using an IAP monitoring device R (C.R. Bard Medical, Covington, GA, USA) in patients who experienced massive retroperitoneal hematoma, deep shock, and/or intestinal swelling. We did not hesitate to apply delayed closure in hemodynamically unstable patients, such as those having a blood pressure less than 80 mmHg during open repair. Additional laparotomy was performed for patients undergoing EVAR who experienced massive retroperitoneal hematoma or severe abdominal distension in whom ACS was a concern.12)
Decompression laparotomy
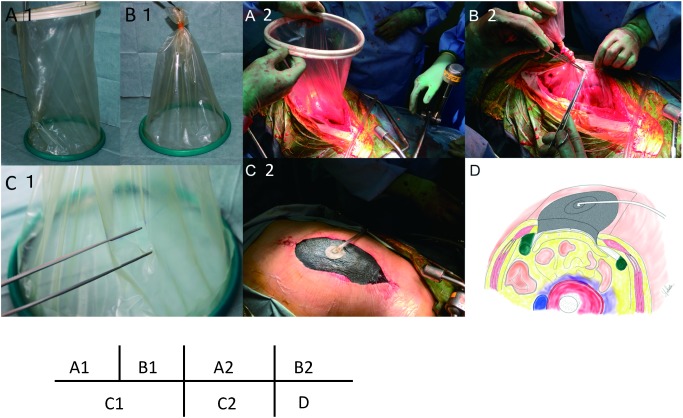
The abdominal wall was initially covered with a plastic sheet, and a peritoneal drain was placed underneath.13) However, the sucking of the omentum into drainage tube frequently frequently occluded the drains. Since 2010, an Alexis wound protector (Applied Medical, Rancho Santa Margarita, CA, USA) and a vacuum-assisted closure system (VAC Abdominal Dressing System; KCI, San Antonio, TX, USA) have been applied onto the wound (Fig. 1).
Fig. 1 Schematic of VAC system. (A1) Alexis wound protector, (B1) The ventral end of the Alexis wound protector is removed and tied. (C1) Small holes are made in the film of the wound protector to facilitate drainage and avoid sucking of the VAC system. Intestinal injuries may also be avoided by the filtering effect of this film. (A2) The Alexis wound protector is placed permanently into the peritoneal cavity to expose the abdominal cavity. (B2) The outer ring is removed from the plastic cylinder, which is then ligated. (C1) Small holes are cut in the plastic cylinder. The skin is partially closed and fitted to the protector. (C2) The VAC system is applied to the sponge to aspirate the peritoneal fluid and exudate under a continuous negative pressure of –50 mmHg. (D) A cross-sectional schematic diagram of a patient’s abdomen. VAC: vacuum assisted closure.
Hypothesis and statistics
We hypothesized that such an integrated management strategy improves mortality and morbidity for patients undergoing surgery for an RAAA. Risk factors for unfavorable outcomes, such as in-hospital death, prolonged hospital stay, and renal insufficiency, were also evaluated. To test our hypothesis, patients were divided into two groups: patients who had undergone surgery before 2011 without systematic integrated management (group A, n=39) and those who had undergone surgery with integrated management (group B, n=23).
The severity of RAAAs was evaluated by the Glasgow aneurysm score (GAS),14) Hardman index,15) and Rutherford classification.16) Details of these scoring systems are described in the Appendix.
To analyze the effects of preoperative hemodynamics stability, subgroups of patients from the two groups with preoperative unstable hemodynamics (blood pressure <80 mmHg for 10 min) were compared.17)
Statistical analyses were conducted using SPSS Software (release 11.5.0; SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as the means±standard error. The Mann–Whitney test was used for continuous variables and the chi-square analysis was used for categorical variables. A P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
The average age of all study patients was 70.3 years; for groups A and B, respectively, the average age was 67.7 and 74.7 years (P=0.032, group A vs. B). Group B patients required more frequent use of vasopressors (P=0.035). Other patient characteristics did not differ between the two groups (Table 1). Preoperative hemodynamic parameters (Rutherford type 3 or 4, GAS, Hardman index) did not differ between the two groups.
Table 1 Preoperative characteristics.
| Characteristics | A (n=39) | B (n=23) | P |
|---|---|---|---|
| Age (mean±SD), years | 67.7±11.7 | 74.7±9.7 | 0.032 |
| Sex: male | 33 (85%) | 19 (83%) | 0.836 |
| Serum creatinine (mg/dL) | 1.37±1.27 | 1.26±0.43 | 0.594 |
| Hemoglobin (g/dL) | 10.1±2.43 | 9.28±2.47 | 0.329 |
| Systolic blood pressure (mmHg) | 96±30.4 | 99.6±34.4 | 0.73 |
| Mean diameter of AAA | 8.57±10.6 | 7.00±1.97 | 0.698 |
| Total protein (g/dL) | 5.78±1.17 | 5.44±1.13 | 0.287 |
| T-bilirubin (mg/dL) | 0.66±0.31 | 0.55±0.39 | 0.099 |
| EVAR | 1 | 17 | <0.01 |
| Abdominal pain | 31 | 21 | 0.222 |
| Loss of consciousness | 6 | 5 | 0.527 |
| Rutherford classification 3 or 4 | 12 | 9 | 0.502 |
| Rutherford class grade | 2.03±1.16 | 2.26±1.18 | 0.407 |
| Shock | 19 | 11 | 0.946 |
| Preoperative intubation | 4 | 1 | 0.409 |
| Ischemic heart disease | 5 | 3 | 0.98 |
| Cerebrovascular disease | 5 | 4 | 0.622 |
| Diabetes mellitus | 7 | 1 | 0.123 |
| Hypertension | 33 | 21 | 0.448 |
| CRF on HD | 1 | 0 | 0.439 |
| COPD | 4 | 1 | 0.409 |
| Suitability for EVAR | 31 | 18 | 0.909 |
| CIAA | 7 | 6 | 0.447 |
| Use of vasopressor | 6 | 9 | 0.035 |
| Use of transfusion | 5 | 5 | 0.38 |
| Glasgow aneurysm score | 81.1±18.3 | 90.4±13.3 | 0.062 |
| Hardman index value | 1.08±1.09 | 1.57±0.99 | 0.074 |
AAA: abdominal aortic aneurysm; EVAR: endovascular aneurysm repair; CRF: chronic renal failure; HD: hemodialysis; COPD: chronic obstructive pulmonary disease; CIAA: common iliac artery aneurysm
Preoperative and postoperative outcomes (Table 2)
Table 2 Preoperative and postoperative characteristics.
| Variable | A (n=39) | B (n=23) | P |
|---|---|---|---|
| Supraceliac clamp | 10 (25.6%) | 9 (39.1%) | 0.266 |
| AOB | 3 (7.7%) | 7 (30.4%) | 0.019 |
| CAC | 6 (15.4%) | 2 (8.7%) | 0.448 |
| Duration of surgery (min) | 334±103 | 246±114 | 0.001 |
| Bleeding (mL) | 3595±4360 | 1592±3322 | <0.001 |
| Blood transfused (mL) | 1175±1075 | 1088±763 | 0.809 |
| FFP (U) | 10.3±11.0 | 11.4±13.4 | 0.865 |
| PC (U) | 11.7±10.3 | 9.3±12.3 | 0.272 |
| 30-day mortality | 5 (12.8%) | 2 (8.7%) | 0.62 |
| CRE max (mg/dL) | 1.87±1.82 | 1.76±1.25 | 0.651 |
| Hospital stay (days) | 25.4±17.0 | 21.2±18.2 | 0.229 |
| ICU stay (days) | 4.28±3.51 | 5.78±6.20 | 0.883 |
| Intubation time (days) | 3.03±3.35 | 4.7±6.34 | 0.307 |
| Open abdominal management | 9 (23.1%) | 10 (43.5%) | 0.092 |
| ACS | 0 | 0 | — |
| Bleeding | 4 (10.3%) | 2 (8.7%) | 0.841 |
| Infectious AAA | 1 (2.6%) | 0 | 0.439 |
| Wound infection | 6 (15.4%) | 0 | 0.048 |
| Cardiac disease | 2 (5.2%) | 1 (4.3%) | 0.89 |
| Pulmonary disease | 6 (15.4%) | 5 (21.7%) | 0.527 |
| POHD | 8 (20.8%) | 7 (30.4%) | 0.378 |
| Sepsis | 0 | 0 | — |
| DVT | 2 (5.2%) | 0 | 0.27 |
| Additional surgery | 7 (17.9%) | 3 (13.0%) | 0.612 |
| Discharge to home | 13 (33.3%) | 11 (47.8%) | 0.258 |
AOB: aortic occlusion balloon; CAC: conventional aortic cross-clamping; FFP: fresh frozen plasma; PC: platelet rich plasma; CRE: serum creatinine; ACS: abdominal compartment syndrome; AAA: abdominal aortic aneurysm; POHD: postoperative hemodialysis; DVT: deep vein thrombosis
The incidences of EVAR and endovascular clamp use were 1/39 (2.6%) and 3/39 (7.7%) in group A and 17/23 (73.9%) and 7/23 (38.1%) in group B, respectively. In patients undergoing endovascular clamping, 3/3 patients in group A and 6/7 patients in group B acquired stable hemodynamics. One patient in group B could not obtain hemodynamic stabilization by endovascular clamping and died after EVAR. Early mortality did not differ between the two groups. The duration of surgery was significantly shorter in group B than in group A (P=0.001). Blood loss was significantly less in group B than in group A (P<0.001). Postoperative CRRT was introduced in 8/39 patients (20.5%) in group A and 7/23 patients (30.4%) in group B.
No patient showed evidence of ACS because open abdomen management was begun aggressively in cases where ACS was a concern. One patient undergoing EVAR in group A did not have a laparotomy. However, 10/18 patients in group B who underwent EVAR also underwent a laparotomy. No additional procedures were necessary to control bleeding in patients who underwent concomitant EVAR and laparotomy. The mean duration of abdominal closure was 5.2 days. No harmful side-effects, such as graft infection and colonic ischemia, were observed. The frequency of wound infections was significantly lower in group B than in group A.
Causes of death were early bleeding in two cases, hypoxic brain damage in one patient, prolonged shock in two patients, and multiple organ failure in two patients who experienced shock.
Analysis of hemodynamically unstable patients
Twenty-one hemodynamically unstable patients were subcategorized into groups Au (n=12) and Bu (n=9). An unstable patient was defined as having a systolic blood pressure lower than 80 mmHg for at least 10 min.18) The average age of the patients in these subgroups was high: 72.8 years in group Au and 74.9 years in group Bu. Males outnumbered females in group Au (P=0.031). The severity index of RAAAs and the frequency of preoperative vasopressor use were high in both groups but did not differ between the groups. The incidences of EVAR (P=0.001), endovascular clamp use (P=0.005), and open abdominal management (P=0.092) were higher in group Bu than in group Au (Table 3). Overall, early mortality was 7/21 (33.3%). Early mortality was lower in group Bu than in group Au (22.2% vs. 41.7%) but did not statistically differ between the two groups (P=0.35) (Table 4). The duration of surgery did not differ between the groups. Blood loss was significantly less in group Bu than in group Au, but the total amount of transfused blood did not differ between the groups. Length of ICU stay and intubation time were long in both groups, reflecting the severe conditions of the patients in these subgroups. Postoperative maximal serum creatinine levels were high in both groups, with approximately 50% of the patients in each group undergoing hemodialysis.
Table 3 Preoperative characteristics in unstable patients.
| Characteristics | Au (n=12) | Bu (n=9) | P |
|---|---|---|---|
| Age (mean±SD), years | 72.8±8.16 | 74.9±9.82 | 0.602 |
| Sex: male | 12 (100%) | 6 (66.7%) | 0.031 |
| Serum creatinine (mg/dL) | 1.32±0.45 | 1.09±0.28 | 0.169 |
| Hemoglobin (g/dL) | 9.85±2.99 | 8.64±2.20 | 0.651 |
| Systolic blood pressure (mmHg) | 64.2±9.96 | 66.7±13.2 | 0.702 |
| Mean diameter of AAA | 7.55±1.70 | 7.29±2.15 | 0.862 |
| Total protein (g/dL) | 4.87±1.06 | 4.71±1.01 | 0.508 |
| T-bilirubin (mg/dL) | 0.63±0.31 | 0.5±0.32 | 0.345 |
| EVAR | 0 | 6 | 0.001 |
| Abdominal pain | 8 | 9 | 0.054 |
| Loss of consciousness | 5 | 5 | 0.528 |
| Rutherford classification 3 or 4 | 12 | 9 | — |
| Rutherford class grade | 3.58±0.51 | 3.56±0.53 | 0.971 |
| Shock | 12 | 9 | — |
| Preoperative intubation | 4 | 1 | 0.237 |
| Ischemic heart disease | 3 | 0 | 0.105 |
| Cerebrovascular disease | 2 | 1 | 0.719 |
| Diabetes mellitus | 3 | 0 | 0.105 |
| Hypertension | 12 | 7 | 0.086 |
| CRF on HD | 0 | 0 | — |
| COPD | 1 | 0 | 0.375 |
| Suitability for EVAR | 10 | 6 | 0.375 |
| CIAA | 1 | 3 | 0.149 |
| Use of vasopressor | 5 | 7 | 0.098 |
| Use of transfusion | 4 | 4 | 0.604 |
| Glasgow aneurysm score | 95.6±12.0 | 94.6±10.6 | 0.917 |
| Hardman index value | 1.58±1.08 | 1.67±0.71 | 0.972 |
AAA: abdominal aortic aneurysm; EVAR: endovascular aneurysm repair; CRF: chronic renal failure; HD: hemodialysis; COPD: chronic obstructive pulmonary disease; CIAA: common iliac artery aneurysm
Table 4 Preoperative and postoperative characteristics in unstable patients.
| Variable | Au (n=12) | Bu (n=9) | P |
|---|---|---|---|
| Supraceliac clamp | 4 (33.3%) | 8 (88.8%) | 0.011 |
| AOB | 2 (16.7%) | 7 (77.7%) | 0.005 |
| CAC | 2 (16.7%) | 1 (11.1%) | 0.719 |
| Duration of surgery (minutes) | 350±121.7 | 261.6±64.4 | 0.111 |
| Bleeding (mL) | 6252±5725 | 1739±2149 | 0.009 |
| Blood transfused (mL) | 1857±1259 | 1739±556 | 0.917 |
| FFP (U) | 16.6±12.8 | 15.8±10.8 | 0.808 |
| PC (U) | 15±11.1 | 16.7±13.2 | 0.808 |
| 30-day mortality | 5 (41.7%) | 2 (22.2%) | 0.35 |
| CRE max (mg/dL) | 2.27±1.56 | 2.29±1.83 | 1 |
| Hospital stay (days) | 22.9±19.5 | 31±24.8 | 0.702 |
| ICU stay (days) | 5.42±4.54 | 10.56±6.84 | 0.082 |
| Intubation time (days) | 4.52±3.52 | 9.44±7.78 | 0.041 |
| Open abdominal management | 8 (66.6%) | 9 (100%) | 0.054 |
| ACS | 0 | 0 | — |
| Bleeding | 4 (33.3%) | 2 (22.2%) | 0.577 |
| Infectious AAA | 0 | 0 | — |
| Wound infection | 5 (41.7%) | 0 | 0.027 |
| Cardiac disease | 0 | 0 | — |
| Pulmonary disease | 3 (25%) | 3 (33.3%) | 0.676 |
| POHD | 5 (41.7%) | 5 (55.5%) | 0.528 |
| Sepsis | 0 | 0 | — |
| DVT | 1 (8.3%) | 0 | 0.375 |
| Additional surgery | 5 (41.7%) | 1 (11.1%) | 0.125 |
| Discharge to home | 3 (25%) | 4 (44.4%) | 0.35 |
AOB: aortic occlusion balloon; CAC: conventional aortic cross-clamping; FFP: fresh frozen plasma; PC: platelet rich plasma; CRE: serum creatinine; ACS: abdominal compartment syndrome; AAA: abdominal aortic aneurysm; POHD: postoperative hemodialysis; DVT: deep vein thrombosis
Discussion
To date, four randomized trials for open surgery versus EVAR for RAAAs have been published.6–9) These investigations demonstrated that the 30-day mortality rate was 24%–53% in the open surgery group and 18%–53% in the EVAR group; EVAR was thus not associated with a significant reduction in 30-day mortality rates. Gupta et al.17) reported that for unstable RAAA patients, the 30-day mortality rate was 52.8% in the open surgery group and 35.6% in the EVAR group. However, these rates did not differ significantly between the two groups; for stable RAAAs, the mortality rate was 22.4%. Mehta et al.18) reported that for RAAA patients undergoing EVAR, the 30-day mortality rate was 18% in hemodynamically stable patients and 33% in unstable patients. In the present study, 30-day mortality rates were better than those reported previously: 11.3%, 12.3%, and 8.7% in all, group A, and group B patients, respectively. In the present study, all seven patients who died were hemodynamically unstable, whereas no patient in the hemodynamically stable group died. In unstable cases, early mortality rates improved from 41.7% (5/12) in group Au to 22.2% (2/9) in group Bu, but these rates are not statistically different due to the small sample size of each group.
Raux et al.19) reported that, compared to conventional aortic cross-clamping, the use of an AOB is a valuable strategy that is associated with the reduced intraoperative mortality of unstable RAAA patients; unfortunately, its use does not reduce in-hospital mortality. In the present study, 16% (10/62) of RAAA patients (3/39 group A and 7/23 group B) required an AOB. For stabilization of hemodynamics, the need for an AOB was significantly higher in unstable patients. The present study demonstrated the efficacy of endovascular clamping using an AOB to provide immediate hemodynamic stabilization. In most cases, the AOB was placed into the supraceliac abdominal aorta, potentially prolonging renal and leg ischemia. Thus, we consider CRRT to be crucial. After surgery, CRRT was begun in 24.2% (15/62) of RAAA patients (20.5% group A and 30.4% group B) with oliguria or elevated serum creatinine levels. Postoperative maximal serum creatinine levels increased to 2.3 mg/dL in unstable patients, even though approximately 50% of these patients underwent hemodialysis after surgery. All surviving cases were weaned from CRRT.
ACS is a life-threatening complication of RAAA repair and is associated with increased mortality. Rubenstein et al.20) reported that mortality was significantly higher in patients with ACS (63%) than in patients without ACS (33%). Open abdomen management is considered a lifesaving procedure in patients with ACS after EVAR or open repair for an RAAA. Sörelius et al.21) reported that the most feared complications of open abdomen management in vascular patients are graft infections, intestinal fistula, and late incisional herniation. Abdominal wall herniation is frequently observed, with a reported incidence of 60%. In the present study, no patients showed evidence of ACS because of the aggressive application of open abdomen management under IAP monitoring. In 2010, we introduced a sutureless, wound management technique combined with a VAC system. This technique is simple, easy, and feasible to manage ACS following RAAA repair. The mean duration of abdominal closure was 5.2 days. No wound or graft infections occurred in patients using VAC system, and no patients had an abdominal wall hernia postoperatively.
Conclusion
Although patients who underwent integrated management were significantly older and required more frequent use of vasopressors, early mortality did not differ from that of the conventionally managed group. Morbidity was significantly improved in the group that underwent integrated management in terms of the duration of surgery and frequency of wound infections. Integrated management, which includes EVAR as a first-line strategy, with endovascular aortic clamping and prevention of ACS with a VAC system improved the surgical outcomes. To reduce mortality rates of RAAA further, a systematic approach, including care before hospital admission, may be necessary.
Acknowledgments
Authors appreciate special support of operation and patient care from the following surgeons: Prof. Yasuyuki Suzuki, Prof. Masahito Minakawa, Dr. Tomonori Kawamura, Dr. Ryosuke Kowatari, Dr. Zaiqiang Yu, and Dr. Mari Chiyoya.
Disclosure Statement
The authors have no conflicts of interest.
Author Contributions
Study conception: IF, CA
Data collection: CA, ST, YS
Analysis: CA
Investigation: CA, KD, NK
Writing: CA, WF
Funding acquisition: IF
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Appendix
GAS14): (age in years)+(17×shock)+(7×myocardialdisease)+(10×cerebrovascular disease)+(14×renal disease). Shock was defined as hypotension (systolic blood pressure <90 mmHg) with signs of impaired perfusion, such as tachycardia, reduced level of consciousness, and cardiopulmonary arrest. Myocardial disease was defined as a previously documented myocardial infarction, ongoing angina pectoris, or both. Cerebrovascular disease was defined as all grades of stroke, including a transient ischemic attack. Renal disease was defined as a serumcreatinine level greater than 150 μmol/L (1.70 mg/dL) or creatinine clearance less than 50 mL/min, a history of acute or chronic renal failure, or both.
Hardman Index15): A single point was awarded for an age greater than 76 years, a hemoglobin level less than 9.0 g/dL, a serum creatinine level greater than 190 μmol/L (2.15 mg/dL), ischemic changes on an electrocardiogram, and a history of loss of consciousness. A Hardman Index of 3 or higher indicates a patient with a high mortality risk.
References
- 1).Office of National Statistics. Mortality statistics, cause, England and Wales 1995. London: TSO, 1997. (Series DH2, No. 22.)
- 2).Reimerink JJ, Hoornweg LL, Vahl AC, et al.; Amsterdam Acute Aneurysm Trial Collaborators. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg 2013; 258: 248-56. [DOI] [PubMed] [Google Scholar]
- 3).van Beek SC, Vahl AC, Wisselink W, et al.; Amsterdam Acute Aneurysm Trial Collaborators. Fate of patients unwilling or unsuitable to undergo surgical intervention for a ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 2015; 49: 163-5. [DOI] [PubMed] [Google Scholar]
- 4).Brown MJ, Sutton AJ, Bell PRF, et al. A meta-analysis of 50 years of ruptured aortic aneurysm repair. Br J Surg 2002; 89: 714-30. [DOI] [PubMed] [Google Scholar]
- 5).Marin ML, Veith FJ, Cynamon J, et al. Initial experience with transluminally placed endovascular grafts for the treatment of complex vascular lesions. Ann Surg 1995; 222: 449-65; discussion, 465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Powell JT, Sweeting MJ, Thompson MM, et al. IMPROVE Trial Investigators. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm; 30 day outcomes from IMPROVE randomized trail. BMJ 2014; 348 jan13 2: f7661. [DOI] [PubMed] [Google Scholar]
- 7).Desqranges P, Kobeiter H, Katsahian S, et al. Editor’s Choice—ECAR: A French randomized controlled trial of endovascular versus open surgical repari of ruptured aorto-iliac aneurysms. Eur J Vasc Endovasc Surg 2015; 50: 303-10. [DOI] [PubMed] [Google Scholar]
- 8).Reimerink JJ, Hoornweq LL, Vahl AC, et al. Endovascular repair versus open repair of ruptured abdominal aortic aneurysms: a multicenter randomized controlled trial. Ann Surg 2013; 258: 248-56. [DOI] [PubMed] [Google Scholar]
- 9).Hinchliffe RJ, Bruijstens L, MacSweeney ST, et al. A randomized trail of endovascular and open surgery for ruptured abdominal aortic aneurysm—results of a pilot study and lessons learned for future studies. Eur J Vasc Endovasc Surg 2006; 32: 506-13; discussion, 514-5. [DOI] [PubMed] [Google Scholar]
- 10).Ogino H, Watanabe K, Ikegaya Y, et al. Protocol-based strategy for endovascular repair of ruptured abdominal aortic aneurysms. Ann Vasc Dis 2013; 6: 169-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Eckroth-Bernard K, Garvin R, Ryer E. Current status of endovascular devices to treat abdominal aortic aneurysms. Biomed Eng Comput Biol 2013; 5: 25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Taniguchi S, Watanabe K, Fukuda W, et al. Effect of endovascular treatment for ruptured abdominal aortic aneurysm. Jpn J Vasc Surg. 2014; 23: 89-95. [Google Scholar]
- 13).Mayer D, Rancic Z, Meier C, et al. Open abdomen treatment following endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2009; 50: 1-7. [DOI] [PubMed] [Google Scholar]
- 14).Samy AK, Murray G, MacBain G. Glasgow aneurysm score. Cardiovasc Surg 1994; 2: 41-4. [PubMed] [Google Scholar]
- 15).Hardman DT, Fisher CM, Patel MI, et al. Ruptured abdominal aortic aneurysms: who should be offered surgery. J Vasc Surg 1996; 23: 123-9. [DOI] [PubMed] [Google Scholar]
- 16).Rutherford RB. Classification of ruptured aortic aneurysms aids comparison of results. Vasc Surg Outlook 1992; 4: 1-2. [Google Scholar]
- 17).Gupta PK, Ramanan B, Engelbert TL, et al. A comparison of open surgery versus endovascular repair of unstable ruptured abdominal aortic aneurysms. J Vasc Surg 2014; 60: 1439-45. [DOI] [PubMed] [Google Scholar]
- 18).Mehta M, Paty PS, Byrne J, et al. The impact of hemodynamic status on outcomes of endovascular abdominal aortic aneurysm repair for rupture. J Vasc Surg 2013; 57: 1255-60. [DOI] [PubMed] [Google Scholar]
- 19).Raux M, Marzelle J, Kobeiter H, et al. Endovascular balloon occlusion is associated with reduced intraoperative mortality of unstable patients with ruptured abdominal aortic aneurysm but fails to improve other outcomes. J Vasc Surg 2015; 61: 304-8. [DOI] [PubMed] [Google Scholar]
- 20).Rubenstein C, Bietz G, Davenport DL, et al. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2015; 61: 648-54. [DOI] [PubMed] [Google Scholar]
- 21).Sörelius K, Wanhainen A, Acosta S, et al. Open abdomen treatment after aortic aneurysm repair with vacuum-assisted wound closure and mesh-mediated fascial traction. Eur J Vasc Endovasc Surg 2013; 45: 588-94. [DOI] [PubMed] [Google Scholar]