Abstract
The aim of the present study was to assess the effectiveness of preemptive dexamethasone in surgery of the lower third molars and to compare it with other oral anti-inflammatories. An electronic search was conducted for preemptive effects related to lower third-molar surgery in 3 separate databases. The variables pain, swelling, and trismus were assessed. Meta-analysis was used to calculate the pooled effect measures for mean and standard deviation values (95% confidence interval [CI]). Seven split-mouth clinical trials were selected. Two studies were included in the meta-analysis. Three studies showed a low risk of bias; 2 studies exhibited a moderate risk and 2 a high risk of bias. Dexamethasone was better than nonsteroidal anti-inflammatories for preemptive effectiveness. Meta-analysis for swelling confirmed better results for dexamethasone than for methylprednisolone after 2 days (95% CI = −1.28 to −0.38), 4 days (95% CI = −1.65 to −0.71), 7 days (95% CI = −1.42 to −0.71), and overall (95% CI = −1.25 to −0.72). Dexamethasone was better than methylprednisolone for mouth opening after 4 days (95% CI = 0.18 to 1.07). There is insufficient evidence through meta-analysis to conclude that dexamethasone is better than other nonsteroidal anti-inflammatories or methylprednisolone as a preemptive analgesic. The results of this meta-analysis suggest that dexamethasone is more effective than methylprednisolone for swelling and trismus.
Key Words: Molar, third; Surgery, Oral; Dexamethasone; Meta-analysis
In the field of oral and maxillofacial surgery, the removal of impacted third molars is considered a routine procedure.1 This procedure can lead to painful symptoms, swelling, and disorders that may be transitory or permanent, including trismus and paresthesia. Pain is considered severe by 93% of patients in the first 24 to 48 hours after surgery.2 Therefore, preoperative intake of anti-inflammatories should be considered to minimize pain (preemptive analgesia), swelling, and trismus in the postoperative period.3
Preemptive analgesia involves treatment that prevents the establishment of central sensitization, which is caused by peripheral nociceptor activity secondary to surgical trauma. In the absence of local anesthesia, this process begins at the time of incision and continues during the intraoperative and postoperative periods.4,5 Preemptive analgesia has been studied since the beginning of the 20th century. In the field of dentistry, it is usually used in isolation or in combination with 4 groups of drugs: local anesthetics, steroidal anti-inflammatories (corticosteroids), nonsteroidal anti-inflammatories (NSAIDs), and opioid analgesics.6,7
Dexamethasone and methylprednisolone are the most commonly used corticosteroids for preemptive analgesia.7 NSAIDs, such as diclofenac and ibuprofen,8,9 and central-acting analgesics, such as tramadol,10,11 have also been studied when used preemptively in surgical procedures involving third molars.
The effectiveness of preemptive analgesia with the use of corticosteroids and NSAIDs has been demonstrated in previous studies that used either a placebo or different doses of the medication in question.12,13 Nevertheless, there is no consensus in the literature concerning the question of which medication is the most effective when used preemptively to decrease postoperative pain for surgical procedures involving lower third molars.
The aim of the present study was to assess the effectiveness of preemptive oral administration of dexamethasone in lower third-molar extractions, in terms of reducing pain, swelling, and/or trismus, when compared with other oral anti-inflammatories.
METHODS
The present study followed the guidelines of the PRISMA declaration.14 Ethical committee approval was not necessary as this article is a systematic review.
The PICO components were the following: Patient, patients with impacted lower third molars who were submitted to surgery; Intervention, oral administration of dexamethasone; Comparison, other oral anti-inflammatories; Outcome, effectiveness in terms of reducing pain, swelling, and trismus.
Search Strategy
The electronic search for articles took place in April 2015. No restrictions were used in relation to language or date of publication. The following databases were used: PubMed, Web of Science, and Cochrane Oral Health and Group Trial Register.
The following terms were used in the search strategy for the PubMed database, selecting Clinical Trial and Comparative Study in the “type of articles” filter: (Third molar AND dexamethasone OR preemptive OR pre-emptive) [Title/Abstract]. The following terms were used in the search strategy for the Web of Science database: third molar* AND dexamethasone* OR preemptive* [Title]. The following terms were used in the search strategy for the Cochrane Oral Health Group Trials Register: Third molar AND Dexamethasone.
A manual search of the most notable journals related to oral and maxillofacial surgery was conducted to find published articles that had not been indexed. The following journals were included in this manual search: Annals of Maxillofacial Surgery, British Journal of Oral and Maxillofacial Surgery, International Journal of Oral and Maxillofacial Surgery, Journal of Oral and Maxillofacial Surgery Medicine and Pathology, Journal of Oral and Maxillofacial Surgery, Journal of Craniofacial Surgery, Journal of Cranio-maxillofacial Surgery, Journal of Maxillofacial and Oral Surgery, and Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.
After the search had been conducted, the references in the articles that were included were also reviewed to include additional studies that were not found in the original electronic search. A number of websites that list ongoing clinical trials were also searched (http://clinicaltrials.gov, http://www.centerwatch.com/, and http://www.clinicalconnection.com).
Inclusion and Exclusion Criteria
The eligibility criteria included clinical studies of humans that compared the preemptive use of dexamethasone with other oral anti-inflammatories during lower third-molar surgery. At least 1 of the variables of pain, swelling, or trismus needed to be assessed in the postoperative period. The exclusion criteria included the following: case reports, technical notes, studies that compared dexamethasone with only a placebo, studies that assessed other nonoral administration routes, and in vitro studies and revision articles.
Study Selection
The articles underwent a rigorous, independent assessment by the 2 authors of this article (S.G.M.F. and T.C.L.). The selection process began with a reading of the titles and abstracts of all of the articles found in the above-mentioned databases. After reading the titles and abstracts, articles that did not fulfill the established inclusion criteria were excluded. When in doubt, the articles were read in full prior to the decision to include or exclude them from the final analysis. Differences of opinion related to inclusion or exclusion were resolved by discussions between the 2 authors. When an agreement could not be reached, a third author was consulted to arrive at a final decision (Figure 1).
Figure 1.
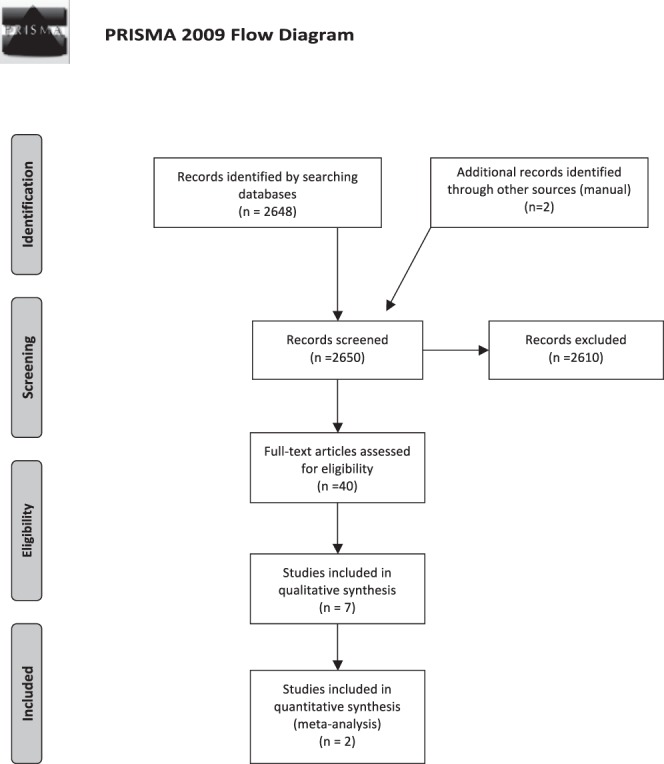
Study screening process.
Assessment of the Methodological Quality
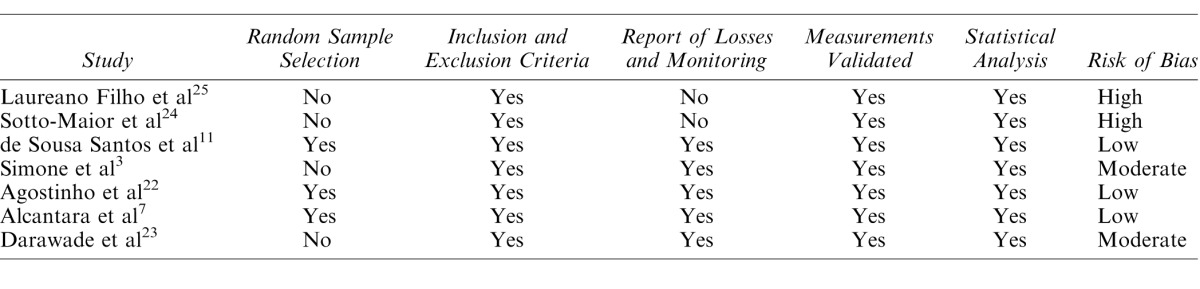
The recommendations of the Cochrane review were used to determine the quality of each study individually, in terms of the risk of bias.15 A combination of the following assessment methods was used: Meta-Analysis of Observational Studies in Epidemiology (MOOSE),16 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE),17 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).14 The potential risk of bias in each study was determined as follows: (1) random sample selection, (2) definition of the inclusion and exclusion criteria of the study, (3) report of losses and monitoring, (4) validated measurements, and (5) statistical analysis. Studies that satisfied all of these 5 criteria were considered to have a low risk of bias. Studies that did not satisfy 1 of these criteria were considered to exhibit a moderate risk of bias, and those that did not satisfy 2 or more of these criteria were considered to exhibit a high risk of bias (Table 1).
Table 1.
Assessment of the Methodological Quality of the Studies Included in Relation to the Risk of Bias
Data Extraction
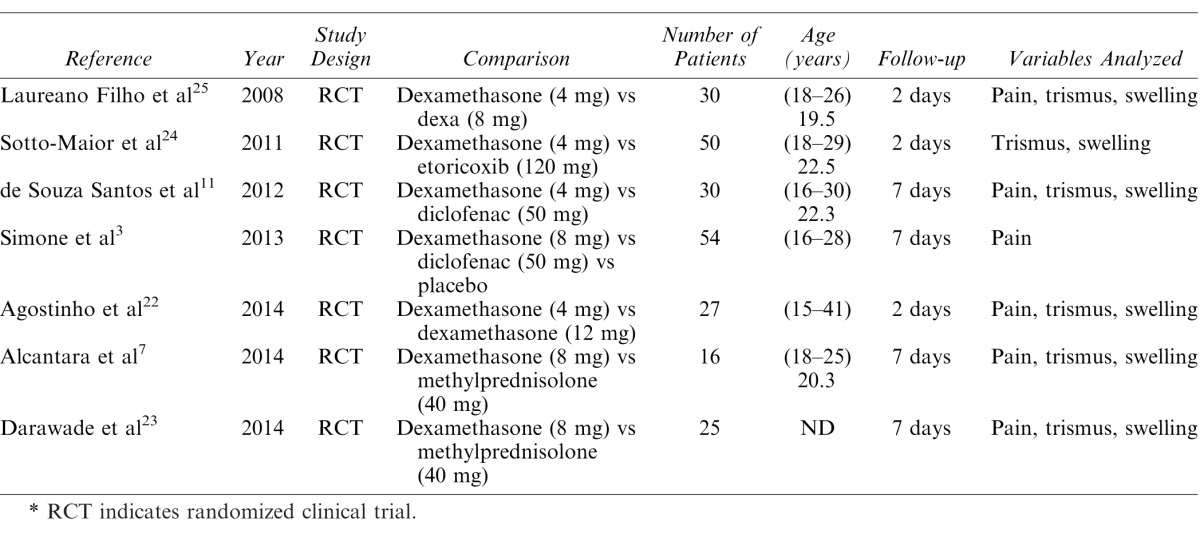
The following data (when available) were assessed and included in the final analysis: author, year of publication, study design, anti-inflammatories used, sample size, age of the patients, and monitoring procedures (Table 2). The mean values of the variables pain, swelling, and trismus were extracted from the articles when present. When these data were absent, the authors of the study were contacted. The data were tabulated independently using electronic formulae and then interpreted in order to conduct the statistical analysis.
Table 2.
Characteristics of the Studies Included in the Systematic Review*
Statistical Methods and Data Synthesis
Comprehensive meta-analysis software (version 2) was used for the meta-analysis.18 The heterogeneity of studies was assessed using the I2 test.19 A sensitivity test was conducted to test the consistency of data when heterogeneity was greater than 50%.18 When homogeneity was confirmed (I2 = 0.00), the fixed-effect model was used. For statistical heterogeneity, the random-effect model was used for meta-analysis.18,19 The summary effect measure was calculated using the standard difference in means, a 95% confidence interval (CI), and the p value described in the forest plot. Publication bias was not assessed as there were not enough studies to be grouped in a funnel plot.20,21
RESULTS
Figure 1 displays the selection process of the present study. The search in the databases analyzed resulted in a total of 2650 articles, after the removal of duplicates. Of this total, 2610 articles were excluded after a reading of the title and abstract as they did not satisfy the inclusion criteria. A total of 40 articles were read in full. Thirty-three of these 40 articles were excluded for not satisfying the inclusion criteria. Thus, a total of 7 studies3,7,11,22–25 satisfied the inclusion criteria and were included in the final analysis. Pain, swelling, and trismus were assessed in 5 of these 7 studies,7,11,22,23,25 while 1 study assessed only pain3 and another assessed only swelling and trismus.24
Table 1 displays the assessment of the quality of the studies, based on the risk of bias. Table 2 displays the data extracted from the studies. Among the 7 studies analyzed in the final analysis, 3 exhibited a low risk of bias,7,11,22 while 2 exhibited a moderate risk3,23 and 2 exhibited a high risk.24,25
Two studies compared pain, swelling, and trismus using 2 different doses of dexamethasone.22,25 One of these studies compared 4 mg versus 8 mg of dexamethasone,25 whereas the other compared 4 mg versus 12 mg.22 The former study demonstrated that a concentration of 8 mg of dexamethasone was more effective in controlling swelling and trismus in the postoperative period, without reporting differences in the control of pain in this period.25 The latter study reported no statistically significant differences between the 2 doses of dexamethasone (4 mg × 12 mg).22 Meta-analysis was not recommended for this assessment because of the different doses of the drug.
Three studies compared dexamethasone with NSAIDs.3,11,24 Two of these compared dexamethasone with diclofenac, a traditional NSAID,11,24 while the third compared dexamethasone and etoricoxib, a cycloxygenase-2 inhibitor NSAID not available in the United States.3 The 2 studies that compared the preemptive effects of dexamethasone and diclofenac confirmed better results for dexamethasone, in relation to the variables studied.
When compared with etoricoxib, dexamethasone provided similar results in relation to the assessments of swelling and trismus. Meta-analysis was not recommended for this assessment because of the different properties of the drugs and the different assessment methods used.
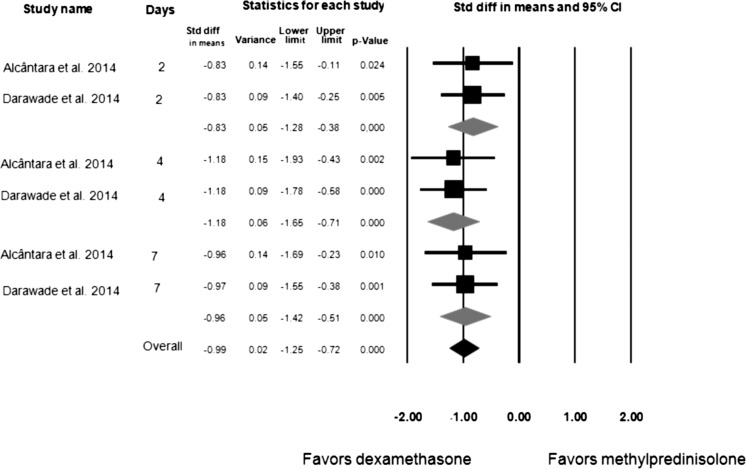
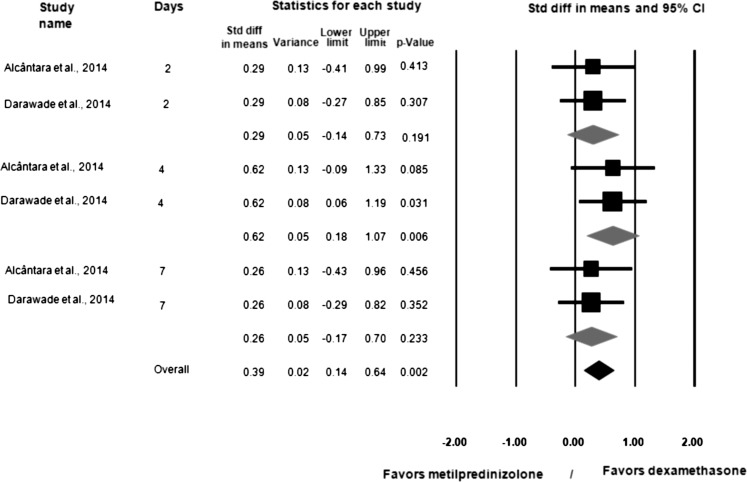
Dexamethasone 8 mg and methylprednisolone 40 mg were compared via meta-analysis in only 2 studies.7,23 Figure 2 displays the meta-analysis subgroups of the 2 studies that compared dexamethasone and methylprednisolone in terms of swelling assessments.7,23 Dexamethasone provided better results than methylprednisolone when swelling was assessed 2 days (95% CI = −1.28 to −0.38; p < .001), 4 days (95% CI = −1.65 to −0.71; p = .010), and 7 days (95% CI = −1.42 to −0.71; p < .001) after lower third-molar surgery, as well as overall (95% CI = −1.25 to −0.72; p < .001). Figure 3 displays the meta-analysis of the 2 studies that compared dexamethasone and methylprednisolone in terms of mouth opening.7,23 Dexamethasone provided better results than methylprednisolone for mouth opening only at the 4-day postoperative assessment (95% CI = 0.18 to 1.07; p = .006) after lower third-molar surgery (not 2 or 7 days after surgery).
Figure 2.
Meta-analysis of dexamethasone versus methylpredinisolone for swelling, with statistical significance; I2 = 0.00, fixed-effect model used.
Figure 3.
Meta-analysis of dexamethasone versus methylpredinisolone for trismus, with significance; I2 = 0.00, fixed-effect model used.
DISCUSSION
Preemptive administration of drugs can be considered a beneficial option when dental surgeons seek to decrease a patient's pain during impacted third-molar surgery.2 The aims of this intervention are as follows: to reduce the pain caused by the surgery in both the intraoperative and postoperative periods; to prevent the establishment of plasticity mechanisms in the central nervous system, which are responsible for chronic pain; and to prevent pain perception during the postoperative period.26 NSAIDs, opioid analgesics, and corticosteroids are the most commonly used drugs to help control the pain caused by the extraction of third molars.27,28 Preemptive analgesia can involve several methods: local anesthesia for the prevention of nociceptor stimulation and neurogenic inflammation, the inhibition of the inflammatory process and peripheral sensitization using NSAIDs and corticosteroids, and the inhibition of central sensitization using opioid analgesics and NSAIDs. Thus, a combination of these methods could be capable of suppressing postoperative pain.29
The use of corticosteroids in preemptive analgesia is based on its mechanism of action, which affects the initial stage of the inflammatory response. The inhibition of phospholipase A2, which reduces the liberation of arachidonic acid, leads to a decrease in the production of numerous vasoactive substances, including prostaglandins and leukotrienes.30 Conversely, NSAIDs affect cyclooxygenases, preventing the formation of prostaglandins only, which can help reduce inflammation.31 Dexamethasone is a corticosteroid that has been shown to be effective in preemptive analgesia when compared with a placebo.32 However, this systematic review assessed the effectiveness of the oral administration of dexamethasone in comparison with other anti-inflammatories (steroidal and nonsteroidal) during lower third-molar surgery. Despite the scarcity of studies that assessed the preemptive analgesic effectiveness of dexamethasone in comparison with other types of anti-inflammatories, as well as the high level of heterogeneity among the studies, dexamethasone can be indicated as an effective anti-inflammatory, based on the results presented in the studies analyzed herein.
The split-mouth study model used by the researchers to study bilateral dentoalveolar surgery offers great credibility. The greatest advantage of this type of study is its control. In this study model, confusion variables, such as the pain threshold, anxiety, and different lifestyle habits found among the patients, minimally affect the final result because the patient is his or her own control.33,34 Therefore, since all of the studies included in this systematic review used the split-mouth model, these confusion variables were maximally controlled.
Of the 7 studies included in the analysis, 3 exhibited a low risk of bias.7,11,22 Two exhibited a moderate risk of bias because they did not adequately cite the randomization of the sample.3,23 Although these studies did not fulfill the randomization criteria, the authors believe that this is the most important domain because selection bias may be present. Therefore, the results of these studies must be interpreted with caution. The final 2 studies exhibited a high risk of bias as they did not adequately cite the randomization of the sample or describe the losses or monitoring protocols of the study.24,25 The studies that exhibited a high risk of bias compared 4 mg and 8 mg of dexamethasone25 and dexamethasone with etoricoxib.24 Thus, the results of these studies should be assessed with caution. The other study that compared 4 mg and 12 mg of dexamethasone found no differences in the results obtained for the 2 doses.22 Consequently, the ideal concentration of dexamethasone for preemptive analgesia remains unclear. Meta-analysis was not recommended for the assessment of these studies because of the different doses of the medication and the different methodologies used.
In all of the studies, paracetamol (acetaminophen in the United States) was used as an escape analgesic to control pain in the postoperative period. This is understandable as it is a safe drug that does not affect peripheral inflammation, coagulation time, platelet aggregation, or neutrophil defense.22 Thus, bias related to the analgesic was controlled in these studies. The methods used to collect data related to pain and the monitoring protocols were similar in all studies, with data collected 24 and 48 hours after the extraction, when pain scores are generally higher. In one of the studies analyzed in this systematic review, postoperative pain was more easily controlled with a dose of 8 mg of dexamethasone than with 4 mg of the same drug.25 However, this study exhibited a high risk of bias. When the effects of dexamethasone and methylprednisolone on postoperative pain were analyzed, the results obtained in the dexamethasone group were better.7 No significant differences were found between these corticosteroids in another study.23 Meta-analysis for the variable pain was not possible, given that 1 of the articles did not provide the raw mean and standard deviation data for this variable.23
Upon comparison of the effects of dexamethasone and NSAIDs, pain was shown to be lower in the dexamethasone group.3,11,24 Recent meta-analysis has shown that the preemptive use of NSAIDs during mandibular third-molar surgery does not produce a significant reduction in the variable postoperative pain, which could explain the better results obtained with dexamethasone in this systematic review. According to the authors, these results could be due to the NSAID-selective inhibition of cycloxygenase-2 as an active mechanism.35 The better results recorded with dexamethasone may have been achieved as a result of the active mechanism of corticosteroids,3 which inhibit the beginning of the inflammatory process (inhibition of phospholipase A2 early in the inflammation cascade), as well as the greater half-life of dexamethasone.11 These findings corroborate the results of an earlier meta-analysis, in which NSAIDs did not demonstrate a preemptive analgesic effect.35
Among the variables included in this review, swelling and trismus were assessed using 2 different methodologies,28,29 both described in the literature, which hindered the meta-analysis. The difference between the assessment methods for swelling involved the number of facial points assessed, which resulted in disparate differences in the mean values of swelling in the studies.13,36 It is important to highlight that the measurement of facial swelling is difficult to accurately quantify, since the facial surface is irregular and convex. In addition, the same quantity of swelling may occur internally or externally, depending on the facial area involved, and can be more or less visible,37 which hinders comparisons. Therefore, swelling and trismus were assessed using meta-analysis only in the 2 studies that had identical methodological characteristics.7,23 For these variables, dexamethasone 8 mg provided better results than methylprednisolone 40 mg, thereby confirming its superiority in the control of swelling and trismus. These 2 studies exhibited a low7 and moderate23 risk of bias, respectively. Swelling begins with surgical stimuli and reaches its peak approximately 48 hours after the operation.13 Thus, the half-life of the drug could be a fundamental factor in the more satisfactory performance of dexamethasone, when compared with other medications, given that it is a long-acting steroid and can be effective for between 36 and 54 hours. Conversely, methylprednisolone is effective for between 18 and 36 hours.7 The other reason for the difference in swelling may be the doses used, as the study design is not clear. Although the doses of 8 mg of dexamethasone and 40 mg of methylprednisolone are generally considered equivalent corticosteroid doses7 and seem to be a good comparison, the difference between the results in swelling could be attributed to dosing differences.
Similar to the variable pain,3 swelling and trismus were more easily controlled with dexamethasone than with NSAIDs.11 No statistical differences were recorded between dexamethasone and etoricoxib in 1 study24 when assessing pain and swelling. Etoricoxib is recognized as a highly analgesic NSAID.38 However, this result must be analyzed with caution as the study in question exhibited a high risk of bias. Furthermore, the dose of dexamethasone used in this study was 4 mg, while most other studies used a dose of 8 mg.3,7,23 Although there is no direct scientific evidence, a dose of 8 mg is commonly used preemptive analgesia in lower third-molar surgery. To obtain the anti-inflammatory effect, the dose should be equal to or greater than the physiological quantity liberated by the organism (300 mg of cortisol). A dose of 9 mg of dexamethasone would theoretically promote the maximum inflammatory effect, which explains why a dosage of 8 mg (4-mg tablets commonly available) is more generally used.1
The results of this systematic review suggest that dexamethasone may be the most effective anti-inflammatory in terms of controlling pain, swelling, and trismus (preemptive analgesia) during lower third-molar surgery, performing better than other NSAIDs or methylprednisolone at what is considered a comparable dose. The results are only suggestive as they must be assessed with caution because of weaknesses in the methodology and heterogeneity among the studies. In addition, only 7 articles were included in the overall analysis, and only 2 fulfilled the full criteria and were analyzed in the meta-analysis. It was not possible to determine the best dose of dexamethasone. Further split-mouth clinical trials are required to confirm with certainty the suggestion of this meta-analysis.
CONCLUSIONS
There is insufficient evidence through meta-analysis to conclude that dexamethasone is better than NSAIDs or methylprednisolone as a preemptive analgesic. The results of this meta-analysis suggest that dexamethasone may be more effective than methylprednisolone when administered preoperatively at comparable doses in lower third-molar surgery for swelling and trismus. Because of the limited number of studies, this result should be interpreted with caution. More clinical trials with a split-mouth design are required to answer this question.
REFERENCES
- 1. Kim K, Brar P, Jakubowski J, Kaltman S, Lopez E. . The use of corticosteroids and nonsteroidal antiinflammatory medication for the management of pain and inflammation after third molar surgery: a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107: 630– 640. [DOI] [PubMed] [Google Scholar]
- 2. Coulthard P, Haywood D, Tai MA, Jackson-Leech D, Pleuvry BJ, Macfarlane TV. . Treatment of postoperative pain in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 2000; 38: 588– 592. [DOI] [PubMed] [Google Scholar]
- 3. Simone JL, Jorge WA, Horliana AC, Canaval TG, Tortamano IP. . Comparative analysis of preemptive analgesic effect of dexamethasone and diclofenac following third molar surgery. Braz Oral Res. 2013; 27: 266– 271. [DOI] [PubMed] [Google Scholar]
- 4. Jung YS, Kim MK, Um YJ, Park HS, Lee EW, Kang JW. . The effects on postoperative oral surgery pain by varying NSAID administration times: comparison on effect of preemptive analgesia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005; 100: 559– 563. [DOI] [PubMed] [Google Scholar]
- 5. Moiniche S, Kehlet H, Dahl JB. . A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology. 2002; 96: 725– 741. [DOI] [PubMed] [Google Scholar]
- 6. Markiewicz MR, Brady MF, Ding EL, Dodson TB. . Corticosteroids reduce postoperative morbidity after third molar surgery: a systematic review and metaanalysis. J Oral Maxillofac Surg. 2008; 66: 1881– 1894. [DOI] [PubMed] [Google Scholar]
- 7. Alcantara CE, Falci SG, Oliveira-Ferreira F, Santos CR, Pinheiro ML. . Preemptive effect of dexamethasone and methylprednisolone on pain, swelling, and trismus after third molar surgery: a split-mouth randomized triple-blind clinical trial. Int J Oral Maxillofac Surg. 2014; 43: 93– 98. [DOI] [PubMed] [Google Scholar]
- 8. Velásquez GC, Santa Cruz LA, Espinoza MA. . Ketoprofen is more effective than diclofenac after oral surgery when used as a preemptive analgesic: a pilot study. J Oral Facial Pain Headache. 2014; 28: 153– 158. [DOI] [PubMed] [Google Scholar]
- 9. Shah R, Mahajan A, Shah N, Dadhania AP. . Preemptive analgesia in third molar impaction surgery. Natl J Maxillofac Surg. 2012; 3: 144– 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pandit MK, Godhi S, Lall AB. . Preoperative intravenous tramadol versus diclofenac for preventing postoperative pain after third molar surgery: a comparative study. J Maxillofac Oral Surg. 2011; 10: 306– 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Sousa Santos JA, da Silva LC, de Santana Santos T, Menezes Junior LR, de Assunção Oliveira AC, Brandão JR. . Comparative study of tramadol combined with dexamethasone and diclofenac sodium in third-molar surgery. J Craniomaxillofac Surg. 2012; 40: 694– 700. [DOI] [PubMed] [Google Scholar]
- 12. Boonsiriseth K, Klongnoi B, Sirintawat N, Saengsirinavin C, Wongsirichat N. . Comparative study of the effect of dexamethasone injection and consumption in lower third molar surgery. Int J Oral Maxillofac Surg. 2012; 41: 244– 247. [DOI] [PubMed] [Google Scholar]
- 13. UStün Y, Erdogan O, Esen E, Karsli ED. . Comparison of the effects of 2 doses of methylprednisolone on pain, swelling, and trismus after third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 96: 535– 539. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; . PRISMA Group. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009; 89: 873– 880. [PubMed] [Google Scholar]
- 15. Higgins JPT, Green S, . eds Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011. Available at: www.cochrane-handbook.org. [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283: 2008– 2012. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. STROBE. Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 18. Borensterin M, Hedges L, Higgins J, Rothstein H. . Introduction to Meta-Analysis. 1st ed. Chichester, UK: John Wiley & Sons; 2009. [Google Scholar]
- 19. Higgins JP, Thompson SG. . Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21: 1539– 1558. [DOI] [PubMed] [Google Scholar]
- 20. Biljana M, Jelena M, Branislav J, Milorad R. . Bias in meta-analysis and funnel plot asymmetry. Stud Health Technol Inform. 1999; 68: 323– 328. [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. . Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315: 629– 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agostinho CN, da Silva VC, Maia Filho EM, Cruz ML, Bastos EG. . The efficacy of 2 different doses of dexamethasone to control postoperative swelling, trismus, and pain after third molar extractions. Gen Dent. 2014; 62: e1– e5. [PubMed] [Google Scholar]
- 23. Darawade DA, Kumar S, Mehta R, Sharma AR, Reddy GS. . In search of a better option: dexamethasone versus methylprednisolone in third molar impaction surgery. J Int Oral Health. 2014; 6: 14– 17. [PMC free article] [PubMed] [Google Scholar]
- 24. Sotto-Maior BS, Senna PM, de Souza Picorelli Assis NM. . . Corticosteroids or cyclooxygenase 2-selective inhibitor medication for the management of pain and swelling after third-molar surgery. J Craniofac Surg. 2011; 22: 758– 762. [DOI] [PubMed] [Google Scholar]
- 25. Laureano Filho JR, Maurette PE, Allais M, Cotinho M, Fernandes C. . Clinical comparative study of the effectiveness of two dosages of dexamethasone to control postoperative swelling, trismus and pain after the surgical extraction of mandibular impacted third molars. Med Oral Patol Oral Cir Bucal. 2008; 13: E129– E132. [PubMed] [Google Scholar]
- 26. Grape S, Tramèr MR. . Do we need preemptive analgesia for the treatment of postoperative pain? Best Pract Res Clin Anaesthesiol. 2007; 21: 51– 63. [DOI] [PubMed] [Google Scholar]
- 27. Liporaci JL Junior. . Assessment of preemptive analgesia efficacy in surgical extraction of third molars. Rev Bras Anestesiol. 2012; 62: 502– 510. [DOI] [PubMed] [Google Scholar]
- 28. Mehrabi M, Allen JM, Roser SM. . Therapeutic agents in perioperative third molar surgical procedures. Oral Maxillofac Surg Clin North Am. 2007; 19: 69– 84, vi. [DOI] [PubMed] [Google Scholar]
- 29. Yamaguchi A, Sano K. . Effectiveness of preemptive analgesia on postoperative pain following third molar surgery: review of literatures. Japanese Dental Science Review. 2013; 49: 131– 138. [Google Scholar]
- 30. Vegas-Bustamante E, Mico-Llorens J, Gargallo-Albiol J, Satorres-Nieto M, Berini-Aytes L, Gay-Escoda C. . Efficacy of methylprednisolone injected into the masseter muscle following the surgical extraction of impacted lower third molars. Int J Oral Maxillofac Surg. 2008; 37: 260– 263. [DOI] [PubMed] [Google Scholar]
- 31. Ilhan O, Agacayak KS, Gulsun B, Koparal M, Gunes N. . A comparison of the effects of methylprednisolone and tenoxicam on pain, edema, and trismus after impacted lower third molar extraction. Med Sci Monit. 2014; 20: 147– 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baxendale BR, Vater M, Lavery KM. . Dexamethasone reduces pain and swelling following extraction of third molar teeth. Anaesthesia. 1993; 48: 961– 964. [DOI] [PubMed] [Google Scholar]
- 33. Fletcher MC, Spera JF. . Pre-emptive and postoperative analgesia for dentoalveolar surgery. Oral Maxillofac Surg Clin North Am. 2002; 14: 137– 151. [DOI] [PubMed] [Google Scholar]
- 34. Kaufman E, Epstein JB, Gorsky M, Jackson DL, Kadari A. . Preemptive analgesia and local anesthesia as a supplement to general anesthesia: a review. Anesth Prog. 2005; 52: 29– 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Costa FW, Esses DF, de Barros Silva PG, et al. Does the preemptive use of oral nonsteroidal anti-inflammatory drugs reduce postoperative pain in surgical removal of third molars? A meta-analysis of randomized clinical trials. Anesth Prog. 2015; 62: 57– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neupert EA III, Lee JW, Philput CB, Gordon JR. . Evaluation of dexamethasone for reduction of postsurgical sequelae of third molar removal. J Oral Maxillofac Surg. 1992; 50: 1177– 1182. [DOI] [PubMed] [Google Scholar]
- 37. Bamgbose BO, Akinwande JA, Adeyemo WL, Ladeinde AL, Arotiba GT, Ogunlewe MO. . Effects of co-administered dexamethasone and diclofenac potassium on pain, swelling and trismus following third molar surgery. Head Face Med. 2005; 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moore RA, Derry S, McQuay HJ, Wiffen PJ. . Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database Syst Rev. 2011; 7:CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]