Abstract
Opioid use disorder is a persistent problem in the United States and has become an important issue to medical and dental professionals. Americans are the largest users of opioids by a large margin. The importance of knowing how to identify, handle, refer, and treat patients with opioid use disorder cannot be understated. This article attempts to educate dental professionals on the current epidemiology of opioid use, explain the physiology of addiction, teach practitioners how to identify chronic opioid users, gives options for treating dental pain, establishes criteria for referring to an addiction specialist, and describes the laws, regulations, and resources available to practitioners. With this article, practitioners should have a greater understanding of the current problem of opioid use disorder and be able to develop a protocol for treating these patients.
Key Words: Opioids, Opioid use disorder, Prescription drugs, Analgesic, Addiction
The abuse of prescription and nonprescription drugs in the United States is omnipresent and continues to grow. In 2015, 20.8 million Americans qualified as having a substance use disorder.1 Of these, 2.0 million had a prescription pain reliever disorder,1 an increase from 1.935 million in 2013.2 3.8 million people, or 1.4% of the United States population ages 12 and older, misused prescription pain relievers in 2015, with the greatest consumption between the ages of 18 and 25.1
Americans continue to be the largest users of opioids. Despite comprising only 4% of the world's population, Americans consumed 80% of all opioids, and 99% of all hydrocodone.3 From 1997 to 2005, prescriptions for hydrocodone and oxycodone rose 198% and 588%, respectively.4 85% of the 79.5 million opioid prescriptions in 2009 contained either hydrocodone or oxycodone.4
Opioid overdoses have accounted for more deaths than cocaine and heroin.4 In 2015, 63.1% of the 52,404 drug overdose-related deaths involved an opioid.5 Since 2013, the incidence of drug-use-related deaths has surpassed motor vehicle collisions as the number one cause of accidental death in the United States,6 with prescription opioids being responsible for 16,000.2
Birnbaum et al7 estimated in 2001 that $55.7 billion dollars is spent each year in the United States on prescription drugs and treatment of their abuse. Chronic opioid users overburden emergency departments and mental health outpatient clinics, and have more inpatient hospital stays than acute opioid and nonopioid users.4
As oral healthcare practitioners, we are faced with the challenge to balance pain management with the clinical and economic burdens of prescription drugs. “Doctor shopping,” where patients visit multiple medical professionals to obtain prescriptions, remains a common portal for receiving prescriptions.3 Dental professionals are not immune to these phenomena. Despite this, misused prescription drugs are most often obtained from a friend or relative (60%), with prescription by a single doctor (17%) a distant second.3 In 2009, dentists prescribed 8% of all opioids,8 and more opioids to 10- to 19-year-olds than any other medical specialty.1 Approximately 12% of all opioid prescriptions in the United States are related to dental procedures.9 Wong et al10 reported that opioids were prescribed 27.5% of the time following dental procedures where analgesic use was recommended.
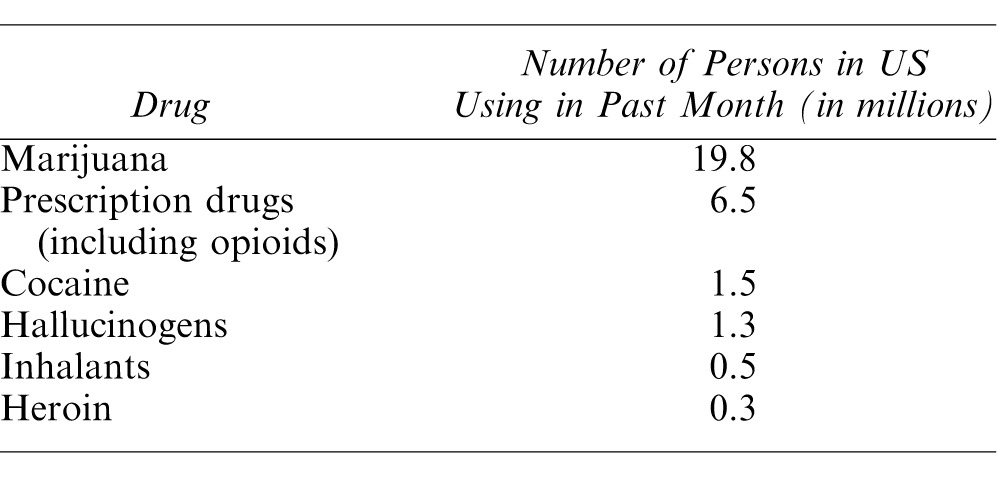
According to the Substance Abuse and Mental Health Services Administration, the most commonly misused prescription drugs include oxycodone, hydrocodone, hydromorphone, methadone, morphine, and codeine.4 These compounds are contained in various brand name formulations, including Tylenol #2-4, Vicodin, Norco, Percocet, Percodan, Oxycontin, and Roxicet. The most commonly misused drugs in the United States are listed in Table 1.
Table 1.
Most Commonly Used Drugs for Nonmedical Purposes1,15
PAIN, ADDICTION, AND OPIOID USE DISORDER
The International Association for the Study of Pain11 defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage.” Besides biologic causes of dental pain (such as a neglected tooth infection), anxiety, depression, sleep disruption, and substance use disorders can contribute to the perception of pain.12 It is commonplace for those with substance-use disorders to have a lower pain threshold, and therefore, to experience more pain regardless of objective nociceptive input than nonusers.12 Table 2 defines the different types of pain.
Table 2.
Definitions Differentiating Types of Pain, Abuse, Addiction, and Substance Use Disorder

The Liaison Committee on Pain and Addiction13 defines addiction as “a primary, chronic disease of brain reward, motivation, memory and related circuitry. Dysfunction in these circuits leads to characteristic biological, psychological, social and spiritual manifestations.” Hallmark signs of addiction include relapse, multiple attempts at quitting, prolonged withdrawal symptoms, and re-entry into treatment facilities.14
With the release of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition,15 the terms substance dependence and substance abuse have been replaced by substance use disorders. Opioid Use Disorder15 is defined as “signs and symptoms that reflect compulsive, prolonged self-administration of opioid substances that are used for no legitimate medical purpose or, if another medical condition is present that requires opioid treatment, that are used in doses greatly in excess of the amount needed for that medical condition.” Table 3 shows the diagnostic criteria established by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition for Opioid Use Disorder.
Table 3. .
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition Criteria for Opioid Use Disorder15*

PHYSIOLOGY OF ADDICTION
Addiction has a multifactorial etiology16 and 40 to 60% of the vulnerability may be genetic.17 The progression from “I like the drug” to “I want the drug” to “I need the drug” occurs with repeated exposure and changes to the normal reward and adaptive behavior centers.18 Hyman19 proposed that drug addiction results in a “pathological hijacking” of the reward-related learning and memory areas of the brain.
Opioids act by binding to their receptors, which indirectly stimulate the release of dopamine. In addition, they also stimulate nicotinic cholinergic receptors.18 With continuous use, a permanent change in the dopamine receptor occurs, preventing reaction to normally pleasurable stimuli, such as food, water, and sex.20
The dysregulation of hormones and the brain's stress system is responsible for the relapse in patients who are trying to abstain from drug use.14 In the initial hours of abstinence, acute withdrawal sets in, leading to unpleasant effects, such as nausea, cramping, and mydriasis, and promoting relapse through negative reinforcement. During acute withdrawal, hormones such as adrenocorticotropic hormone, corticotropin-releasing factor, beta-endorphin, cortisol, vasopressin, and dynorphin are released causing states of irritability, anxiety, dysphoria, and craving.14 These hormones are being targeted in the development of new antiaddiction treatments.
IDENTIFYING A POTENTIAL DRUG ABUSER
A detailed and accurate medical and psychosocial history is a critical first step to identifying a potential chronic opioid user. Schwartz et al20 reported that 31% of physicians did not ask about recent alcohol or drug use before prescribing a course of opioids. A medical history should always include medications, smoking, alcohol, and illicit drug use, including types, dose, and frequency. The history can provide clues suggesting the patient's drug use habits. For example, risk factors for prescription opioid misuse include age less than 45 years, criminal history, personal and family history of substance use disorder, and mental health problems.1 The most common comorbidities related to addiction are acute and chronic pain, anxiety disorders, and attention-deficit disorder/attention-deficit/hyperactivity disorder.21
Longo et al21 described 4 common characteristics of patients with drug use disorders:
-
1.
Escalating use: the continuous increase in frequency or dose needed to treat the patient's pain.
-
2.
Drug-seeking behavior: relentless quest to obtain medication in order to feed cravings.
-
3.
Doctor shopping: a routine of visiting several doctors complaining of similar symptoms in order to receive multiple prescriptions for the same medication.
-
4.
Scamming: coercion or manipulation of the doctor in order to obtain medication.
Longo et al21 stated that a patient who pressures the doctor into writing a prescription, after the physician has already denied their request, is “pathognomonic” of a prescription drug use disorder. Although asking for a specific narcotic by name may be concerning, the doctor must carefully decide whether this patient is drug-seeking or pain-relief seeking, as the patient's request may actually be based on a previous experience where the medication was effective in treating their pain.1
The Screener and Opioid Assessment for Patients with Pain is a questionnaire that can facilitate the identification of high-risk patients for opioid therapy.22 The components of the Screener and Opioid Assessment for Patients with Pain include 14 questions, scored from 0 to 4, concerning mood, personal prescription and illicit drug use, family drug use, tobacco and alcohol use, and legal problems. Patients are considered high-risk for developing addiction and abuse if they score greater than 7. A score greater than 7 has a sensitivity of 91% for predicting high-risk individuals.22
HOW TO ADDRESS A PATIENT WITH A SUSPECTED OPIOID ABUSE DISORDER
When a patient exhibits characteristics of an opioid use disorder, the practitioner should not hesitate, nor be afraid, to have a frank discussion with the patient about their habits. This conversation must be handled gently, nonjudgmentally, and using open-ended questions.
Bien23 proposed that the practitioner utilize brief interventional counseling involving three steps: Feedback, Advice, and Goal-Setting. Give feedback expressing concern about the patient's usage. For example, “I am concerned about your use of Vicodin. Continuing to use at this pace can result in harm to your body, relationships, and work.” Give advice about how they can safely use opioids, cut down their usage, or minimize their pain in other ways. Explain to the patient the health and overdose risks involved with their unhealthy habits. For example, “There are other options for managing your pain that may be just as effective and better for you in the long term.” Establish goals by soliciting the patient's reaction to your recommendations. “Do you think you are ready to make a change? What are some ways that you might go about making this change?” Ask for the patient's thoughts on the recommendations, whether they are ready to make a change, and how they plan to change.
The SMART Recovery Program24 uses a 5-step motivational interviewing process as a method of encouraging a change in behavior. The practitioner should start by developing a discrepancy between how the person functions with their current drug use as compared to how they envision themselves ideally. Through this, the patient may realize the consequence of their drug use. Express empathy by listening and viewing the world from the patient's perspective, a critical skill to minimizing the patient's resistance to change. Amplify ambivalence using interviewing to promote inner exploration of the 2 sides of the patient's addiction: the side that wants to keep using, and the side that wants to quit. Roll with resistance when the patient is not prepared to make a change. If the patient is not ready, it is a sign that the practitioner should modify his or her approach. Telling the patient what to do or arguing may only make the patient more resistant. Instead, ask whether he or she is willing to talk about their usage again at a future appointment. Support self-efficacy by encouraging the patient to explore their own ways to improve their situation and by helping them believe that change is possible.
Table 4 provides the 7 Es, proposed by Becker et al,25 as a method of confronting a patient in a nonjudgmental and effective manner.
Table 4.
The “7 Es” to Remember When Engaging a Patient in Conversation About Their Substance Use25

DRUGS FOR MANAGING POSTOPERATIVE PAIN
The following are common drugs that are used for the treatment of acute and chronic dental and maxillofacial pain.
Opioids26
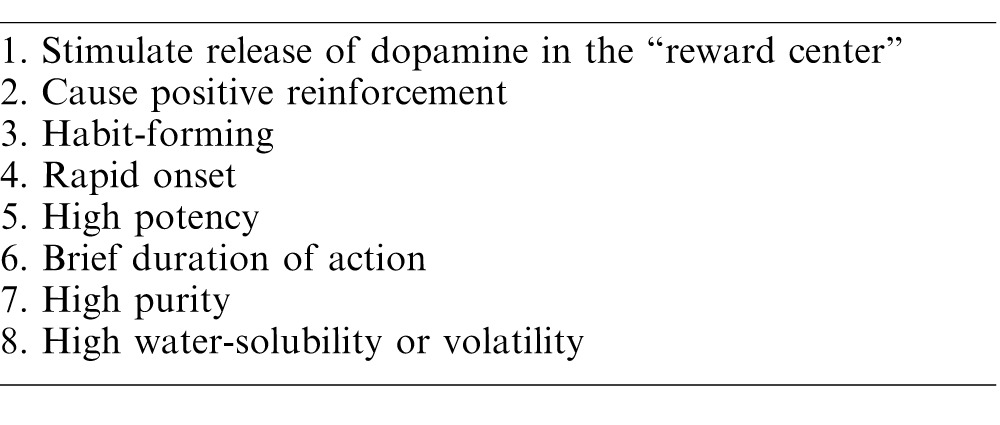
All of the frequently used opioids are simple modifications of morphine, or synthetic derivatives with actions similar to morphine. Generally, opioids are highly soluble weak bases, highly protein bound, and largely ionized at physiologic pH. The opioid receptor is a G-protein coupled receptor which, when activated, results in hyperpolarization of the cell and inhibition of neuronal excitability. In addition, opioids inhibit the release of central nervous system substance P, preventing pain transmission to the brain, and may also change the patient's affective response to pain. Most opioids are metabolized via the liver, although some are conjugated and excreted through the kidney. While opioids provide excellent analgesia, especially against C-fiber mediated pain, a major drawback is they activate the reward centers in the brain, causing sedation, euphoria, and the potential for addiction. Opioids also depress the brain's respiratory drive and central response to CO2, resulting in decreased minute ventilation, which can cause significant hypoxia. At small doses, tidal volume increases but respiratory rate deceases leading to this drop in minute ventilation. At higher doses, there is a drop in both tidal volume and respiratory rate. Fortunately, there are alternatives to opioids that are equally efficacious for relieving postoperative pain, but are nonaddictive and nonhabit forming. Table 5 shows the common properties of drugs of abuse.
Table 5.
Common Qualities of Drugs of Abuse21
NSAIDs and Acetaminophen
Nonsteroidal anti-inflammatory drugs, or NSAIDs, are effective analgesic, anti-inflammatory and antipyretic medications that inhibit the enzyme cyclooxygenase. In turn, prostaglandin formation, which is responsible for inflammation and pain, is prevented or reduced. NSAIDs can inhibit protective prostaglandin effects, such as increasing gastric mucous and bicarbonate secretion while decreasing acid secretion (thus protecting the gastric mucosa), as well as platelet aggregation via inhibition of thromboxane. Therefore, NSAIDs should be used cautiously in patients with active peptic ulcer disease or bleeding or in those with bleeding disorders. NSAIDs include medications such as aspirin, ibuprofen, diclofenac, naproxen, and ketorolac.
Acetaminophen, also known as paracetamol or APAP, is proposed to work through a similar central nervous system mechanism to NSAIDs, although unproven. It exhibits good analgesic and antipyretic action, but does not have anti-inflammatory properties. Acetaminophen is metabolized by the liver and its dose should be adjusted in patients with liver diseases and those taking warfarin. It is often combined with opioids to provide additional pain relief, such as with hydrocodone (Vicodin), oxycodone (Percocet), and codeine (Tylenol #2, #3, and #4).
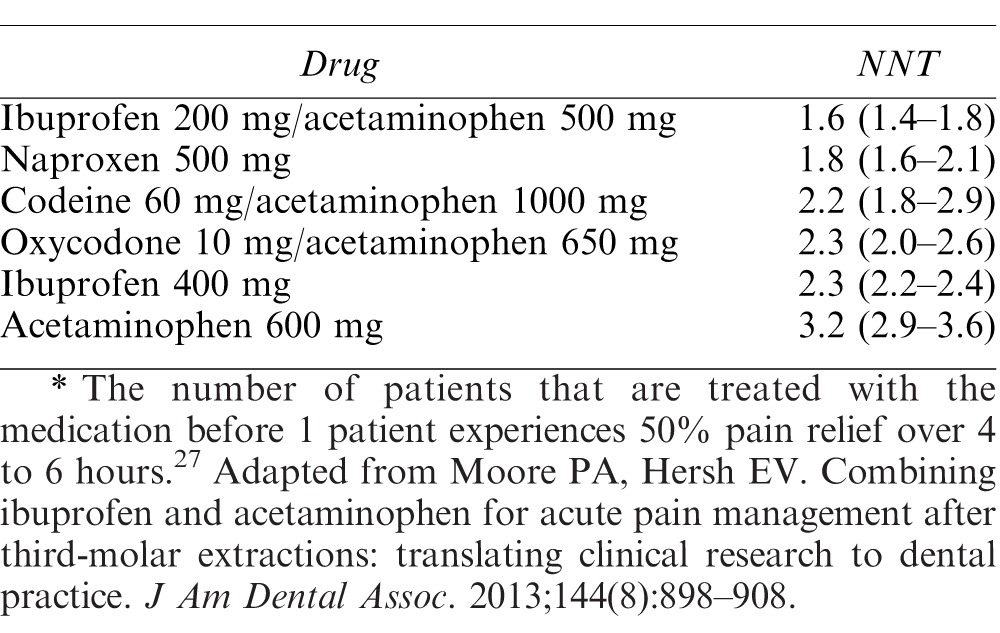
Moore et al27 conducted a Cochrane review on the use of medications for acute pain following third molar surgery in adults in order to assess their efficacy. Many of the included studies evaluated pain from dental procedures. The study used number needed to treat, defined as the number of patients treated before one experienced at least 50% maximum pain relief over 4 to 6 hours compared with placebo, in order to evaluate the effectiveness of each pharmaceutical intervention. Their results, found in Table 6, indicate that a combination of ibuprofen/acetaminophen is equally effective to preparations involving opioids, and that ibuprofen and acetaminophen alone are very comparable. The authors proposed guidelines for acute pain management in dentistry. For mild pain relief, ibuprofen 200 to 400 mg every 4 to 6 hours is recommended. For moderate-to-severe pain, they suggest ibuprofen 400 to 600 mg taken in conjunction with acetaminophen 500 mg every 6 hours. Opioids are only advised in cases of severe pain, using ibuprofen 400 to 600 mg, acetaminophen 650 mg, and hydrocodone 10 mg every 6 hours for 1 to 2 days, followed by discontinuation of hydrocodone, with ibuprofen and acetaminophen as needed. Patients can be assessed for their postoperative pain level using a scale such as the Visual Analog Scale or a numeric scale from 0 to 10.
Table 6.
Number Needed to Treat (NNT)*
Mehlisch et al28 showed that a combination pill of ibuprofen 400 mg/APAP 1000 mg had significantly better pain control than ibuprofen and APAP alone. Wong et al10 found that oxycodone (97.99%) and hydrocodone (92.12%) showed no significant difference in pain relief for invasive dental procedures when compared to acetaminophen (91.73%) and NSAIDs (100%), but that opioids resulted in side effects in 27.5% as compared to only 7.5% of people taking NSAIDs. They also noted that over-the-counter recommendations for analgesics resulted in misuse 10 times more often than for analgesics that were prescribed. Practitioners who choose to recommend over-the-counter NSAIDs and acetaminophen to their patients should do so with clear communication and written instructions to maximize patient benefit from the regimen.
REFERRAL TO AN ADDICTION SPECIALIST AND BUPRENORPHINE MANAGEMENT
When a patient has a suspected opioid use disorder, managing his or her acute pain versus feeding an underlying addiction can be confusing. Dental professionals should build a referral base that includes, among others, pain management doctors, addiction specialists, and psychiatrists. Becker et al25 stated the most important reasons to refer a patient to an addiction specialist include:
-
1.
Abusive medication use, such as early and consistent refill requests or positive drugs screens
-
2.
Excessive alcohol use
-
3.
Unwillingness to try other pain treatments or medication options
-
4.
Concurrent prescription for opioids and sedatives
-
5.
Mental health symptoms
-
6.
Opioid use disorder being treated with methadone or buprenorphine/naloxone experiencing persistent, impairing pain
Addiction specialists may test their patients for opioid use and these results can be requested when making decisions regarding pain management.29 Opioids have a short half-life in saliva and blood of only 6 to 12 hours and may not be the most useful tests. Instead, urine drug screens are used because the opioids stay in the system for 2 to 5 days, providing insight into the patient's recent use. Hair samples can be used for assessing long-term usage, as opioids can be detected for up to 90 days, but this is not a reliable indicator of acute or recent use.
Chronic users are treated using opioid antagonists, such as naltrexone, or the long-acting opioids, methadone or buprenorphine.30 The most effective treatment approach, administered by licensed addiction-treatment facilities, uses these long-acting opioids to relieve withdrawal symptoms and gradually lower the dose until the patient no longer withdraws in its absence.30 Methadone is a long-acting opioid that can be dosed 1 time per day but requires the patient go to a “methadone clinic” every day. Because of this, buprenorphine has become the more common method for treating opioid use disorder in the United States. Buprenorphine is an opioid partial agonist that has very high affinity for the opioid receptor. As a partial agonist, the opioid receptor is stimulated but to a lesser degree than the full agonist opioids. When opioids of abuse are taken while on buprenorphine, they have minimal effects as they cannot easily displace buprenorphine from the opioid receptors. The most commonly prescribed form of buprenorphine is as Suboxone® which is a 4:1 combination of buprenorphine and naloxone in a sublingual formula. The amount of naloxone in the combination drug is not considered bioavailable if the medication is taken as prescribed, sublingually. The naloxone only becomes active if the medication is snorted or injected intravascularly. Long-term users who stop buprenorphine abruptly will begin experiencing withdrawal symptoms within several days, and these effects may last up to 10 days. Withdrawal can include poor appetite, mydriasis, cramping, insomnia, and fatigue, among other signs and symptoms.30 It is important to consult with the addiction specialist before prescribing drugs for pain management for those on buprenorphine therapy. Common postoperative opioids will generally not be effective.31 For most dental and oral surgical procedures, NSAIDs, with or without concomitant acetaminophen, are efficacious and should be prescribed in lieu of opioid containing medications while the patient continues buprenorphine. Macintyre et al32 in 2013 found that patients on buprenorphine therapy that was held on the day after surgery required more patient-controlled analgesia than those whose dose was continued. It is critically important to optimize local anesthesia during and after surgery to minimize perioperative complications. If more severe postoperative pain is anticipated, many addiction specialists will elect to discontinue buprenorphine therapy prior to surgery or a dental procedure with short-acting or long-acting opioids substituted.30 It generally takes 3 to 5 days for enough buprenorphine to dissociate from the opioid receptors for full agonist opioids to be effective.31 Consultation with the primary opioid prescriber should help direct the dentist as to how to manage more severe postoperative pain until nonopioid analgesics are effective and buprenorphine can be restarted. If the patient's physician recommends stopping buprenorphine prior to surgery, the protocol states that buprenorphine therapy should be restarted no less than 12 to 18 hours following termination of short-acting opioid administration, and should be re-administered in small test doses with observation for signs of withdrawal.31 The dose can be gradually increased over the next several days as per the prescribing physician. Furthermore, studies by Roux et al33 and Neumann et al34 in 2013 showed that buprenorphine with naloxone (Suboxone®) can provide good analgesia in patients at risk for opioid use not currently under therapy for opioid use disorder.
As with postoperative opioids, opioids used in intravenous sedation or general anesthesia for dental or oral surgery procedures are also likely to be ineffective. Benzodiazepines and propofol are still suitable but excellent local anesthesia is required. Ketamine can be used effectively for analgesia and dissociation. For dentist anesthesiologists, intubated inhalation anesthesia is also effective. As the focus of this article is on postoperative pain, the sedation practitioner should review additional literature regarding intraoperative management of patients undergoing opioid use disorder treatment.
LAWS, REGULATIONS, AND RESOURCES
Risk management is important when prescribing opioids.21 The American Dental Association has taken a strong stance against prescription drug abuse and offers continuing education and publications to quickly and efficiently inform its members.9,35 Continuing education is essential for keeping pace with the latest medications and trends in pain management. All patient encounters should be documented and informed consent obtained when prescribing opioids.21 Inform patients of the inherent risks, including drowsiness and respiratory depression, and remind them not to drive or use heavy machinery. Utilize prescription writing that is fraud-proof by using personal prescription pads and by writing out the amount of pills in letters. Prescribing only the exact number of doses needed until the next appointment can help to reduce overdose and diversion.21 Avoid prescribing opioids without concurrently employing other pain-relieving therapies, such as referral to an appropriate specialist, surgeon, or physical therapist.21 It is important to minimize diversion to prevent opioid medications from reaching the wrong hands. Practitioners should emphasize to their patients about safe storage, in a locked cabinet or safe, and on how to dispose of their unused medication.21 Proper disposal methods include local take-back programs, DEA-authorized collection sites, mixing with kitty litter or coffee grounds and disposing in household trash, and flushing down the toilet.1
The United States' Food and Drug Administration's Safe Use Initiative41 is developing an opioid patient-prescriber agreement that is intended to stimulate conversation between the prescriber and patient about the decision to use opioids to treat pain. The Food and Drug Administration hopes this will increase patient awareness of the risks and benefits of opioid use and to emphasize the responsibilities of the patient and prescriber in pain treatment.
States have received money to develop prescription drug monitoring programs (PDMPs), which have been established or are currently under development, in order to reduce prescription drug misuse and to prevent diversion. In fact, many states now mandate use of the PDMP before prescribing controlled substances. For example, in Florida, this program is known as Electronic-Florida Online Reporting of Controlled Substance Evaluation Program.42 Electronic-Florida Online Reporting of Controlled Substance Evaluation Program collects information on the prescribing and dispensing of medications in Schedules II, III, and IV. This information is placed into a database that practitioners can access (known as the Patient Advisory Report) to help identify potential abusers and doctor-shoppers, and to guide their decision in prescribing.
Medicare Part D Overutilization Monitoring System43 is another new resource that is attempting to identify patients with opioid overutilization issues. Under this system, patients have quantity limits and undergo retrospective drug utilization reviews to identify those who may be at increased risk of an adverse event. Utilizing these reviews, pharmacies may be able to deny Part D coverage of opioid overutilization.
Narcotics Anonymous44 is a worldwide, community-based organization that supports addicts working with other addicts to overcome their opioid use disorders by sharing their successes and challenges with each other in a group-meeting setting. Attendance was positively correlated with improvement in family relationships, social connection, hobbies and interests, stable housing, employment, and education advancement. Patients with an opioid use disorder should be encouraged to attend NA meetings.
The Centers for Disease Control and Prevention (CDC) has taken a stance when it comes to the opioid prescription sales. Opioids are highly addictive and any claims otherwise have been proven false.45 Thomas Frieden,42 former Director of the CDC, stated that opioids are “just as addictive as heroin.” In response to the growing concern for opioid use and addiction, the CDC issued new recommendations for healthcare practitioners who prescribe such medication. On March 15, 2016, the CDC published a Guideline for Prescribing Opioids for Chronic Pain-United States 2016, a document that is “intended to improve communication between clinicians and patients about the risks and benefits of opioid therapy for chronic pain, improve the safety and effectiveness of pain treatment, and reduce the risks associated with long-term opioid therapy.”42 The recommendations are divided into 3 main areas, with 12 recommendations in total:
-
(1)
Determining When to Initiate or Continue Opioids for Chronic Pain
-
a.
Nonpharmacological and nonopioid therapy is the preferred course of treatment. Opioids should be considered only after doing a risk–benefit analysis, weighing the expected benefits versus the risks involved in their use. A combined approach of these methods to manage pain is preferred.
-
b.
If opioids are used, treatment goals are to be established, with thought given to how to stop therapy if the risks outweigh the benefits. If risks exceed any clinically meaningful improvement in pain and function, opioids should be discontinued.
-
c.
Practitioners should speak with their patients, at the beginning of treatment, and periodically thereafter, about the risks and realistic benefits of opioid therapy.
-
a.
-
(2)
Opioid Selection, Dosage, Duration, Follow-up, and Discontinuation
-
a.
Recognizing that long-term opioid use generally starts with the treatment of acute pain, practitioners should prescribe the lowest effective dose, using immediate-release opioids, rather than extended release or long-acting opioids.
-
b.
The quantity should not exceed the expected duration of the pain, ie, 3 days or less is often sufficient, with greater than 7 days rarely needed.
-
c.
Practitioners should continuously monitor the benefits versus the risks of treatment. In the beginning, evaluate after 1 to 4 weeks, and continue to monitor every 3 months or more frequently.
-
a.
-
(3)
Assessing Risk and Addressing Harms of Opioid Use
-
a.
At the onset, and during treatment, there should be a plan to mitigate the risks of opioid treatment.
-
b.
Practitioners should monitor their patient's history of controlled substance prescription use, utilizing state PDMPs. Using data from these programs, practitioners can see when their patients are either receiving doses or combinations that are high risk for overdose.
-
c.
Urine drug testing may be considered at the start of opioid therapy.
-
d.
Avoid giving opioids and benzodiazepines concurrently.
-
e.
Offer evidence-based treatment for patients with opioid use disorder.
-
a.
Not all medical organizations have been receptive to the guidelines as published. Organizations, such as the American Medical Association, the American Academy of Pain Medicine, and the American Academy of Pain Management,43 have “question(ed) the validity and quality of the guideline's featured recommendations.” The main emphasis of the criticism focuses on “low quality evidence.” For example, the CDC excluded all data from studies looking at the efficacy of opioid therapy recorded from 3 months to 1 year in duration. The American Academy of Pain Medicine supports the CDC's efforts but noted that “public health problems are typically complex; well-meaning but narrowly targeted, interventions often provoke unanticipated consequences.” By not including studies from 3 months to 1 year, when the guidelines are intended for treating pain that lasts longer than 3 months, they may encourage undertreatment of chronic pain sufferers. The American Medical Association was concerned that the guidelines may actually conflict with some state laws and product labeling. The American Academy of Pain Management was “saddened by the apparent lack of response by CDC to comments submitted by the Academy and numerous other pain management organizations and advocate.” They fear patients with chronic pain, who do not misuse or abuse their opioid medications, will be harmed by the new guidelines and they “stand ready to work with anyone, including CDC, to implement education and policy advocacy efforts designed to bring about (an) appropriately balanced result.”
In defense of the CDC, Friedman et al44 stated the “management of chronic pain is an art and a science. The science of opioids for chronic pain is clear: for vast majority of patients, the known, serious, and too often fatal risks far outweigh the unproven and transient benefits.”
CONCLUSIONS
Opioid use disorder is a growing issue in the United States and worldwide. Dental practitioners need to be prepared to manage pain and treat patients with opioid use disorder. The information in this article should be used to guide treatment decisions, make appropriate referrals, provide resources to patients, and abide by national and state laws regarding opioid prescribing.
CONTINUING EDUCATION QUESTIONS
This continuing education (CE) program is designed for dentists who desire to advance their understanding of pain and anxiety control in clinical practice. After reading the designated article, the participant should be able to evaluate and utilize the information appropriately in providing patient care.
The American Dental Society of Anesthesiology (ADSA) is accredited by the American Dental Association and Academy of General Dentistry to sponsor CE for dentists and will award CE credit for each article completed. You must answer 3 of the 4 questions correctly to receive credit.
Submit your answers online at www.adsahome.org. Click on “On Demand CE.”
CE questions must be completed within 3 months and prior to the next issue.
Opioid Use Disorder
-
1)
Which of the following opioid analgesics is the most abused?
-
a.
Fentanyl
-
b.
Heroin
-
c.
Methadone
-
d.
Oxycodone
-
a.
-
2)
Opioid analgesics bind to receptors that indirectly stimulate the reward center's
-
a.
Glutamate production.
-
b.
Dopamine production.
-
c.
Norepinephrine release.
-
d.
Follicle stimulating hormone release.
-
a.
-
3)
“Scamming” describes
-
a.
A continuous increase in frequency and dose needed to treat pain
-
b.
A relentless pursuit to obtain medication to feed cravings
-
c.
A routine visiting of several healthcare practitioners with a similar complaint to obtain multiple prescriptions for the same medication
-
d.
Coercion or manipulation of healthcare practitioner to obtain medication
-
a.
-
4)
What is the minimum number of days buprenorphine must be discontinued before oral analgesics will likely be effective?
-
a.
1 day
-
b.
3 days
-
c.
6 days
-
d.
9 days
-
a.
REFERENCES
- 1. National Survey on Drug Use and Health. The Substance Abuse and Mental Health Services Administration (SAMHSA). Nationwide trends. June 2015. Available at: https://www.drugabuse.gov/publications/drugfacts/nationwide-trends. Accessed November 5, 2016.
- 2. Florence CS, Zhou C, Luo F, Xu L. . The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Medical Care. 2016; 54: 901– 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manchikanti L. . National drug control policy and prescription drug abuse: facts and fallacies. Pain Physician. 2007; 10: 399– 424. [PubMed] [Google Scholar]
- 4. Meyer R, Patel A, Rattana S, Quock T, Mody S. . Prescription opioid abuse: a literature review of the clinical and economic burden in the United States. Population Health Management. 2014; 17: 372– 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rudd RA, Seth P, David F, Scholl L. . Increases in drug and opioid-involved overdose deaths–United States, 2010-2015. Centers for Disease Control and Prevention. Weekly. 2016; 65: 1445– 1452. [DOI] [PubMed] [Google Scholar]
- 6. Williams A, Bisaga A. . From AIDS to opioids–how to combat an epidemic. N Engl J Med. 2016; 375: 813– 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birnbaum HG, White AG, Reynolds JL, et al. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clin J Pain. 2006; 22: 667– 676. [DOI] [PubMed] [Google Scholar]
- 8. Volkow ND, McLellan TA, Cotto JH. . Research letter: characteristics of opioid prescriptions in 2009. JAMA. 2011; 305: 1299– 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denisco RC, Kenna GA, O'Neil MG, et al. Prevention of prescription opioid abuse: the role of the dentist. J Am Dental Assoc. 2011; 142: 800– 810. [DOI] [PubMed] [Google Scholar]
- 10. Wong YJ, Keenan J, Hudson K, et al. Opioid, NSAIDs, and OTC analgesic medications for dental procedures: PEARL Network findings. Compendium. 2016; 37: 710– 718. [PubMed] [Google Scholar]
- 11. International Association for the Study of Pain. IASP Taxonomy. 2012. Available at: http://www.iasp-pain.org/Taxonomy. Accessed November 5, 2016.
- 12. Carr DB, Goudas LC. . Acute pain. Lancet. 1999; 353: 2051– 2058. [DOI] [PubMed] [Google Scholar]
- 13. American Society of Addiction Medicine. Pain and addiction. http://www.asam.org/public-resources/pain-and-addiction. Accessed November 5, 2017.
- 14. Kircher S, Zacny J, Apfelbaum SM, et al. Understanding and treating opioid addiction in a patient with cancer pain. J Pain. 2011; 12: 1025– 1031. [DOI] [PubMed] [Google Scholar]
- 15. American Psychiatric Association. Substance-Related and Addictive Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric publishing; 2013. [Google Scholar]
- 16. Uhl GR. . Molecular genetics of substance abuse vulnerability: remarkable recent convergence of genome scan results. Ann N Y Acad Sci. 2004; 1025: 1– 13. [DOI] [PubMed] [Google Scholar]
- 17. Uhl GR, Drgon T, Jonson C, Liu QR. . Addiction genetics and pleiotropic effects of common haplotypes that make polygenic contributions to vulnerability to substance dependence. J Neurogenet. 2009; 23: 272– 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Detar DT. . Understanding the disease of addiction. Prim Care Clin Office Pract. 2011; 38: 1– 7. [DOI] [PubMed] [Google Scholar]
- 19. Hyman SE. . Addiction: a disease of learning and memory. Am J Psychiatry. 2005; 162: 1414– 1422. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz RH, Johnson NP, Hornung CA, Phelps GL, Berg EW. . Awareness and substance abuse in orthopedic patients: a survey of orthopedic surgeons. South Med J. 1991; 84: 1455– 1457. [DOI] [PubMed] [Google Scholar]
- 21. Longo L, Parran T Jr, Johnson B, Kinsey W. . Addiction: Part II. Identification and management of the drug-seeking patient. Am Fam Phys. 2000; 61: 2401– 2408. [PubMed] [Google Scholar]
- 22. Screener and Opioid Assessment for Patients with Pain (SOAPP). Version 1.0-14Q. Available at: https://www.nh.gov/medicine/documents/soappversion1.0.pdf. Accessed November 14, 2016. [DOI] [PMC free article] [PubMed]
- 23. Bien TH, Miller WR, Tonigan JS. . Brief interventions for alcohol problems: a review. Addiction. 1993; 88: 315– 335. [DOI] [PubMed] [Google Scholar]
- 24. Braastad J. . Using motivational interviewing techniques in SMART Recovery. Available at: http://www.smartrecovery.org/resources/UsingMIinSR.pdf. Accessed November 14, 2016.
- 25. Becker WC, Merlin JS, Manhapra A, Edens E. . Management of patients with issues related to opioid safety and/or misuse: a case series from an integrated, interdisciplinary clinic. Addiction Sci Clin Pract. 2016; 11. [DOI] [PMC free article] [PubMed]
- 26. Egan TD. . Opioids. : Stoelting RK Miller RD. . Basics of Anesthesia. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011: 115– 129. [Google Scholar]
- 27. Moore PA, Hersh EV. . Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice. J Am Dental Assoc. 2013; 144 8: 898– 908. [DOI] [PubMed] [Google Scholar]
- 28. Mehlisch DR, Aspley S, Daniels S, Bandy D. . Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: a randomized, double-blind, placebo-controlled, parallel group, single-dose, two-center, modified factorial study. Clin Therapeutics. 2010; 32: 882– 895. [DOI] [PubMed] [Google Scholar]
- 29. Quest Diagnostics. Employer drug testing solutions. Available at: questdiagnostics.com. Accessed January 10, 2017.
- 30. Benumof JL. . Treatment of opioid-use disorders. N Engl J Med. 2016; 375: 1596. [DOI] [PubMed] [Google Scholar]
- 31. Fiellin D, Sullivan MA. . Treatment of acute pain in patients receiving buprenorphine/naloxone. Providers clinical support system for medication assisted treatment (PCSS-MAT). 2014. Available at: http://pcssmat.org/wp-content/uploads/2014/03/PCSS-MATGuidanceTreatmentOfAcutePainInPatients ReceivingBup.Fiellin.pdf. Accessed January 10, 2017.
- 32. Macintyre PE, Russell RA, Usher KA, Gaughwin M, Huxtable CA. . Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesthesia Intensive Care. 2013; 41: 222– 230. [DOI] [PubMed] [Google Scholar]
- 33. Roux P, Sullivan MA, Cohen J, et al. Buprenorphine/naloxone as a promising therapeutic option for opioid abusing patients with chronic pain: reduction of pain, opioid withdrawal symptoms, and abuse liability of oral oxycodone. Pain. 2013; 154: 1442– 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neumann AM, Blondell RD, Jaanimagi U, et al. A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexist opioid addiction. J Addictive Disorders. 2013; 32: 68– 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. ADA House of Delegates. Statement on the use of opioids in the treatment of dental pain. October 2016. Available at: http://www.ada.org/en/about-the-ada/ada-positions-policies-and-statements/statement-on-opioids- dental-pain. Accessed January 15, 2017.
- 36. US Food and Drug Administration. Opioid patient-prescriber agreement. Safe Use Initiative - Current Projects. Available at: http://www.fda.gov/Drugs/DrugSafety/SafeUseInitiative/ucm188762.htm#opioidppa. Accessed January 15, 2017.
- 37. E-FORSCE, the Florida Prescription Drug Monitoring Program. Available at: http://www.floridahealth.gov/statistics-and-data/e-forcse/. Accessed January 22, 2017.
- 38. Centers for Medicare & Medicaid Services. Improving drug utilization review controls in Part D. https://www.cms.gov/Medicare/Prescription-Drug-coveragePrescriptionDrug CovContra/RxUtilization.html. Accessed January 22, 2017.
- 39. Narcotics Anonymous. Available at: http://www.na.org/admin/include/spaw2/uploads/pdf/pr/Info_about_NA_2016.pdf. Accessed January 22, 2017.
- 40. Markus PA, Thomas AL. . Prudent prescribing: an overview of recent federal and state guidelines for opioid prescriptions. Available at: http://www.americanbar.org/publications/aba_health_esource/2016-2017/opioids/prescriptions.html. Accessed January 23, 2017.
- 41. Tavernise S. . CDC painkiller guidelines aim to reduce addiction risk. Available at: https://www.nytimes.com/2016/03/16/health/cdc-opioid-guidelines.html?_r=0. Accessed January 23, 2017.
- 42. Dowell D, Haegerich TM, Chou R. . CDC guideline for prescribing opioids for pain–United States, 2016. MMWR Recomm Rep. 2016; 65(No. RR-1):1–49. doi: http://dx.doi.org/10.15585/mmwr.rr6501e1 [DOI] [PubMed]
- 43. Ciccone TG, Kean N.. Responses and criticisms over new CDC opioid guidelines, March 17, 2016. Available at: https://www.practicalpainmanagement.com/resources/news-and-research/responses-criticisms-over-new-cdc-opioid- prescribing-guidelines. Accessed January 23, 2017.
- 44. Friedman TR, Houry D. . Reducing the risk of relief – the CDC opioid-prescribing guideline. N Engl J Med. 2016; 374: 1501 doi: 10.1056/NEJMp1515917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mehta P, Rathmell JP. . Chronic pain management. : Stoelting RK Miller RD. . Basics of Anesthesia. 6th ed. Philadelphia, PA: Churchill Livingstone; 2011: 698–714. [Google Scholar]


