Abstract
Perioperative fluid management is a key component in the care of the surgical patient. It is an area that has seen significant changes and developments, however there remains a wide disparity in practice between clinicians. Historically, patients received large volumes of intravenous fluids perioperatively. The concept of goal directed therapy was then introduced, with the early studies showing significant improvements in morbidity and mortality. The current focus is on fluid therapy guided by an individual patient's physiology. A fluid challenge is commonly performed as part of an assessment of a patient's fluid responsiveness. There remains wide variation in how clinicians perform a fluid challenge and this review explores the evidence for how to administer an effective challenge that is both reliable and reproducible. The methods for monitoring cardiac output have evolved from the pulmonary artery catheter to a range of less invasive techniques. The different options that are available for perioperative use are considered. Fluid status can also be assessed by examining the microcirculation and the importance of recognising the possibility of a lack of coherence between the macro and microcirculation is discussed. Fluid therapy needs to be targeted to specific end points and individualised. Not all patients who respond to a fluid challenge will necessarily require additional fluid administration and care should be aimed at identifying those who do. This review aims to explain the underlying physiology and describe the evidence base and the changes that have been seen in the approach to perioperative fluid therapy.
Keywords: Cardiac output, fluid, perioperative, physiology
INTRODUCTION
Worldwide, there are over 312 million surgical procedures performed annually.[1] While many of these patients fall into a low-risk group, it has been previously estimated that up to 10% of patients can be classified as a high surgical risk. Those patients in the high-risk group account for 80% of post-operative mortality.[2] The European Surgical Outcome Study demonstrated a 4% post-operative, in hospital mortality. Patients who develop post-operative complications have both increased morbidity and mortality.[3] Khuri et al. demonstrated that patients who developed any post-operative complication, in the first 30 days after surgery had a 30 day mortality of 13.3%, compared to 0.8% in those without post-operative complications. This effect persisted when comparing 1 and 5 year mortality between the two groups.[4] The association between perioperative morbidity and volume of fluid therapy administrated has been studied and a U-shaped curve described, with increased mortality associated with very high or very low volumes of fluid administration.[5]
Perioperative fluid management has been a focus for both anaesthetic and critical care research for a number of years. With an evolving and changing evidence base, there have been significant developments in clinical practice. Changes have been seen in all domains, from fluid choice, to volume of administration, to which end points to target. Historically, there was a trend for high volume or liberal fluid therapy, this shifted towards a focus on restrictive fluid therapy. A different way to look at this is by looking at goal directed fluid therapy rather than focusing on liberal or restrictive approaches. As an ever increasing evidence base demonstrates that perioperative fluid management alters patient outcomes, there is an increasing awareness of the need to individualise and target fluid administration to the specific patient. However, fluid therapy remains a particularly contentious area of perioperative medicine.[6] The consequences of hypovolaemia, with haemodynamic compromise, impaired oxygen delivery and subsequent organ dysfunction are well recognised. Conversely, fluid overload has resultant multisystemic effects, including interstitial oedema with implications on gas exchange, renal function and the gastrointestinal system.[7] So how do we optimally maintain patient's fluid balance?
This review aims to analyse the existing evidence and physiological principles guiding fluid management strategies. Literature searches were performed using PubMed, including the terms perioperative, surgery, fluid therapy, goal directed therapy (GDT), cardiac output and microcirculation to ensure relevant articles were included. Snowballing of references was used, as well as the authors’ existing knowledge of the publications in the field.
FLUID CHOICE
In health, water constitutes approximately 60% of the total body weight. This is divided between intra and extracellular compartments. The extracellular volume can be further divided into interstitial, intravascular and transcellular compartments. Movement between compartments is limited by membranes. The vascular endothelium forms the barrier between the intravascular and interstitial spaces, with the endothelial glycocalyx present on the vascular side and the cleft between endothelial cells closed by tight junctions. In health water and electrolytes can diffuse across the endothelium and move freely down their respective pressure gradients but the movement of larger molecules such as protein is limited by the glycocalyx, and they require an active transport system. Perioperatively, fluid homeostasis can be altered by several mechanisms. Systemic vasodilatation decreases intracapillary pressure, altering the pressure gradient and therefore fluid shifts. Disruption to the glycocalyx is reported in a variety of disease states, leading to extravascular movement of larger molecules including proteins and an alteration in intra and extravascular oncotic pressure, with a subsequent movement of water extravascularly.[8]
The volume of fluid administered and fluid choice is thought to alter the movement of fluid between the body compartments. Historically, colloids were frequently used; it was believed that as colloids were more likely to remain intravascularly than crystalloids, and greater haemodynamic stability could be achieved with lower volumes. Several large studies have however refuted this. With consistent evidence of increased acute kidney injury, need for renal replacement therapy and 90 day mortality in patients receiving colloids, particularly starches, compared to patients receiving crystalloids, crystalloids now form the mainstay of treatment.[9] A number of the studies on the use of colloids have been performed in critically ill patients. However, a recent meta-analysis included surgical patients and reported similar results.[10] Given these findings, the safety profile of starches for perioperative use has been questioned, and it is the opinion of the authors that their use should be avoided, with crystalloids forming the mainstay of treatment.
Which crystalloid to use has also been investigated, generally either 0.9% saline or a balanced solution such as Hartmann's is used. 0.9% saline has a chloride concentration of 154 mmol which is significantly higher than serum chloride. It is well recognised that its use can lead to a hyperchloremic acidosis. Post-operative hyperchloremia has been linked with increased post-operative complications, such as acute kidney injury and increased 30-day mortality, although causation has not been proved.[11] Balanced solutions, which include Hartmann's solution or Plasmalyte®, are considered more physiological and generally preferred for perioperative fluid management.[12]
LIBERAL VERSUS RESTRICTIVE STRATEGIES
Estimating perioperative fluid losses can be challenging. The period of fasting preoperatively, intraoperative surgical time, surgical losses and insensible losses all need to be considered. Replacement based on these estimated values alone incorporates significant assumptions. In healthy volunteers, a period of pre-operative fasting was shown to have no effect on markers of preload. The value in replacing this volume has therefore been questioned.[13] Similarly, evidence for insensible losses, or third space losses, requiring liberal replacement, is inconsistent.[14]
The use of basic haemodynamic variables, for example changes from baseline heart rate and blood pressure, leads to reactive rather than proactive management, as it is well acknowledged that 20% of the circulating volume must be lost before any change in these parameters is seen. These are also variables with multifactorial influences, and a change may not indicate a change in fluid status. Both general anaesthesia and neuroaxial anaesthesia can cause perioperative hypotension, which is not necessarily indicative of intravascular fluid depletion, but decreased vascular tone. There can, however, be a tendency to manage this with aggressive fluid therapy.[14] Conversely, fluid overload may not manifest until adverse end organ effects are seen.[7]
The definition of what constitutes a liberal versus a restrictive fluid strategy varies but can be in the region of >5 L for a liberal strategy, and <3 L for a conservative strategy.[7] Studies comparing these two groups have shown differing results, but the trend is towards increased morbidity and mortality in the liberal fluid groups, particularly those undergoing high risk or major surgery.[14] A positive fluid balance has been shown to be associated with increased mortality.[15] However, as one strategy did not appear to fit all patient groups, some of these earlier studies commented on a need for individualised GDT.[16]
GOAL DIRECTED THERAPY
GDT aims to meet the patient's increased oxygen demand incurred in the perioperative period, by targeted intervention, guided by haemodynamic monitoring.[17] Increased perioperative oxygen delivery, with reduced oxygen debt in survivors compared to non-survivors was first observed by Shoemaker et al. in the 1980s. Shoemaker et al. thus hypothesised that targeting supranormal physiological parameters in the perioperative setting would therefore decrease morbidity and mortality. They described targets of a cardiac output >4.5 L/min/m2, and an oxygen delivery >600 ml/min/m2.[18] The management of perioperative fluid administration is a different entity to fluid management in the setting of a patient presenting acutely unwell, for example with sepsis. This difference is explained by the different processes occurring at the cellular level in the two conditions and the different reasons and goals of fluid therapy. Perioperative GDT describes fluid administration, with the aim of optimising a patient's cardiac function and ultimately oxygen delivery. It is used for a time limited period, both during and after a surgical intervention. Many of the patients given fluids postoperatively, as part of GDT, may have a heart rate and blood pressure within the normal range. The fluid is given with the aim of increasing preload and therefore stroke volume and cardiac output to potentially supranormal targets. GDT is conducted for a set time period postoperatively. This is in contrast to patients presenting with sepsis, where the time course may not initially be predictable and fluids and vasopressors may be required to ensure adequate end organ perfusion.
While the initial concepts developed by Shoemaker have been questioned and actually shown to be harmful in some settings,[19] GDT evolved mainly focusing on stroke volume optimisation with less invasive cardiac output monitors.[15] GDT carried on being studied in different settings, with multiple studies showing significant decreases in morbidity and mortality, particularly in higher risk patient groups.[17,20,21,22] Of interest, the improved outcomes persisted in long-term follow-up.[17,20,23] Arulkumaran et al. performed a meta-analysis which showed that high-risk surgical patients managed with GDT were not at increased risk of treatment related cardiovascular complications. It had been hypothesised that increased fluid and inotrope use, may predispose to increased complications but in fact, early GDT therapy was shown to be associated with decreased cardiovascular events.[24] More recent studies have failed to show the same results, the reasons for this are likely multifactorial.[25] The largest study on GDT to date, OPTIMISE, a multicentre, randomised study in patients undergoing major abdominal surgery, showed no difference in mortality or post-operative complications at 30 days.[26] Similarly, POEMAS, a multicentre, randomised study, conducted in patients undergoing major abdominal surgery showed no difference in outcomes between the two groups.[27] Clinical practice has changed significantly over the years, particularly with the development and increased practice of enhanced recovery after surgery programmes which advocate perioperative haemodynamic monitoring. As significant advances have been incorporated into standard care, it is likely that the overall quality of care routinely provided has improved. It can therefore become more challenging to conduct a study that identifies a single intervention that will statistically alter patient outcome.
ADVANCED ASSESSMENT OF FLUID STATUS
In addition to a thorough clinical examination, echocardiography is an increasingly useful bedside tool for the assessment of a patient's fluid status. It is becoming readily available, in intensive care, and is moving towards being as common place at the bedside as a stethoscope. Echocardiography can be used to assess preload, contractility and afterload and changes in these parameters in response to a fluid challenge observed. Although interpretability is dependent on the individual clinician's skill, it is an increasingly practiced skill. Continuous monitoring is required to provide ongoing assessment of a patient's fluid status and as such echocardiography is often coupled with cardiac output monitors to provide further information. Due to practical constraints it is more likely to be utilised in the post-operative phase rather than during surgery.
CARDIAC OUTPUT MONITORS
Significant developments have been made in methods of assessing cardiac output and oxygen delivery. Historically, this required a pulmonary artery catheter (PAC), but less invasive methods are now available and readily used, including Oesophageal Doppler and pulse contour analysis.
A PAC is still considered the gold standard method, however it is now used less in clinical practice. The catheter is inserted via a central vein, typically the internal jugular vein and a thermodilution technique used to directly measure central venous, right atrial, right ventricular, pulmonary arterial and pulmonary artery wedge pressures.[27] Some PACs have an automated semicontinuous cardiac output monitoring feature. However, as the cardiac output measurements are updated over a period of minutes, it is difficult to use as a method of assessing response to a fluid challenge where a faster data acquisition is necessary.[28]
The Oesophageal Doppler is placed at the mid thoracic level in the oesophagus and measures the velocity of flow in the descending aorta which is used to calculate cardiac output and change in stroke volume.
Currently, the most commonly used method of cardiac output measurement in intensive care uses pulse contour analysis to indirectly calculate cardiac output. This requires an arterial catheter, classically placed in either the radial or femoral artery and uses computer-based algorithms to calculate stroke volume from pulse pressure and compliance. There are five main commercially available devices; the PiCCO (Pulsion medical systems, Munich, Germany), LiDCOplus (LiDCO, Cambridge, UK), LiDCOrapid (LiDCO, Cambridge, UK), VolumeView/EV1000 (Edwards Lifesciences, Irvine CA, USA) and FloTrac (Edwards LifeSciences, Irvine CA, USA). All of these devices, except the FloTrac require calibration.[27,29]
Bioimpedance systems apply a high-frequency electrical current, of known amplitude and frequency, across the thorax and measure changes in voltage. The ratio between voltage and current amplitudes provides a measure of transthoracic direct current resistance or impedance. This varies in proportion to intrathoracic fluid volume. This is a non-invasive technique, however studies have shown poor correlation between bioimpedance measurements and cardiac output measurements made using invasive techniques and it has been deemed to be inaccurate in the intensive care population.[30] Systems using bioreactance to assess cardiac output and stroke volume variation have been developed. These are also non-invasive, however, in contrast to bioimpedance systems, they show promising early results in the assessment of patient fluid responsiveness. Waldron et al. showed the NICOM, non-invasive cardiac output monitor (Cheetah Medical, Berkshire, UK) performed similarly to the Oesophageal Doppler in guiding perioperative fluid therapy in elective colorectal surgery patients.[31]
Use of these techniques assists assessment of a patient's cardiac output and intravascular volume status. This often involves assessment of whether a patient's preload can be augmented to increase stroke volume with fluid administration. Perioperative fluid therapy can be guided by whether a patient is fluid responsive or not.
FLUID EXPANSION, FLUID RESPONSIVENESS AND ITS PREDICTION
Venous return is equal to cardiac output. The Frank-Starlin mechanism describes the process by which the heart is able to accommodate and then eject all blood returned to it, despite variations in venous return. An increased venous return, or increased preload increases ventricular filling and the end-diastolic volume. This increases stretch of the cardiac myocyte, which increases sarcomere length with resultant increased force of contraction leading to an increase in volume of blood ejected from the heart.[32] In order for a fluid challenge to be effective it therefore needs to be of sufficient volume to cause stretch of the cardiac myocytes and test the Frank-Starling principle. If the heart is able to accommodate the increased volume then stroke volume will increase, otherwise the volume will remain within the venous system.[29]
The total blood volume can theoretically be divided into the unstressed and stressed volumes. The unstressed volume is the volume that fills the blood vessels, without causing a rise in pressure. The stressed volume is any additional volume, which will cause both a pressure rise and elastic distension of the vessel wall. When a fluid challenge is given, the aim is to expand the stressed volume. The elastic properties, or compliance of the vessel, determine the resultant degree of distension in response to the fluid challenge. In the cardiovascular system, the pressure related to the stressed volume and vascular compliance is the mean systemic filling pressure (Pmsf).[33] Pmsf will increase in response to an increase in intravascular stressed volume, as demonstrated in Figure 1. The greater the vascular compliance, the smaller the rise in Pmsf in response to a fluid challenge. Pmsf was first described in 1894 by Bayliss and Starling using a dog model. It is defined as the pressure in the vascular system when the heart is stopped and there is no blood flow, conditions which only occur in cardiac arrest.[34]
Figure 1.
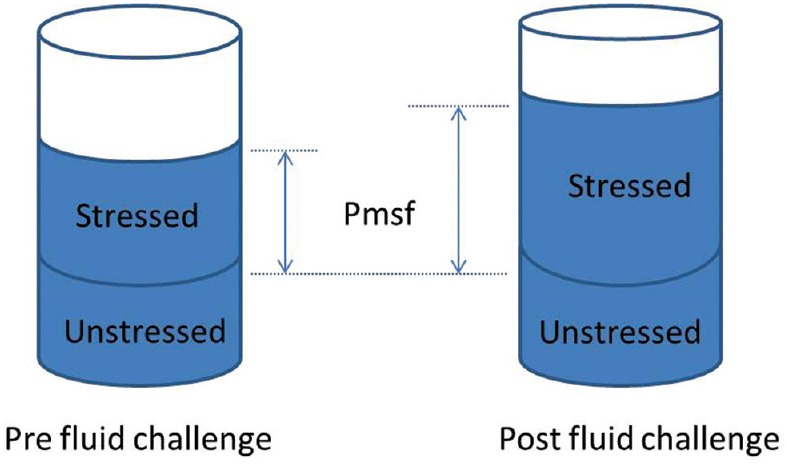
Blood volume can be divided into stressed and unstressed volumes. Following an effective fluid challenge the stressed volume increases, with a subsequent rise in mean systemic filling pressure
The determinants of venous return are Pmsf, right atrial pressure and resistance to venous return, as first defined by Guyton.[35] The driving pressure for venous return is the pressure gradient between Pmsf and central venous pressure (CVP), which then determines cardiac output.[33] Cecconi et al. measured a Pmsf analogue (Pmsa) and CVP in response to an effective fluid challenge. They showed that in both responders and non-responders Pmsa increased similarly, however in the non-responders CVP also increased. This means the pressure gradient, which as explained is the driving pressure for venous return, only increased in the responders.[36]
Assessing fluid responsiveness is important every time fluids are given. If possible the response to fluids should be predicted before their administration. The prediction of a patient's fluid responsiveness aims to identify those who may benefit from an increase in intravenous volume with an increase in stroke volume and cardiac output. Figure 2, uses the Frank Starling Curve to demonstrate whether an increase in preload will result in an increased stroke volume. Predictors of fluid responsiveness include high pulse pressure variation, stroke volume variation, vena cava collapsibility index, dynamic passive leg raising test and end occlusion expiratory test.[14] These assess beat to beat variations in either pulse pressure or stroke volume, however, are only validated in ventilated patients with tidal volumes of more than 8 ml/kg and no arrhythmias. Myatra et al. recently demonstrated that in patients ventilated with a low tidal volume strategy, fluid responsiveness can be predicted by transiently incrementing the tidal volume from 6 to 8 ml/kg and recording changes in either pulse pressure variation or stroke volume variation.[37]
Figure 2.
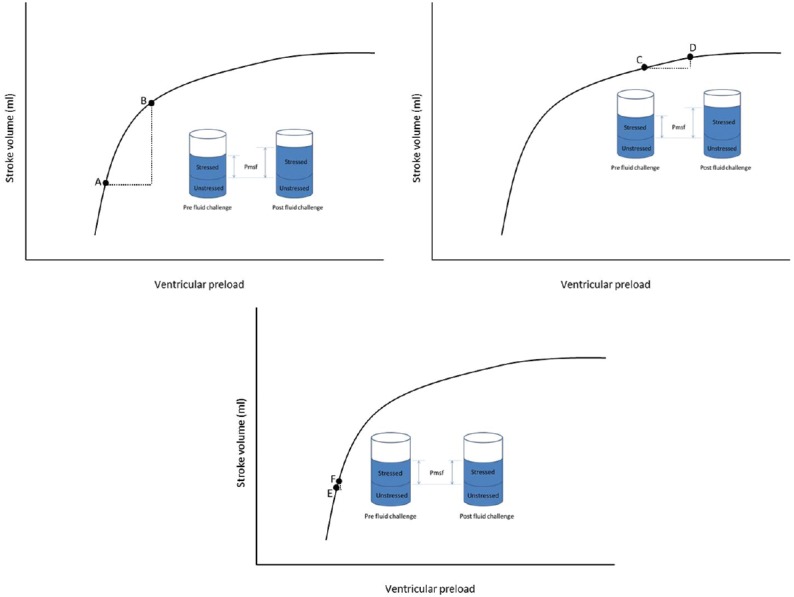
Frank–Starling curve. A fluid challenge at Point A would increase preload and mean systemic filling pressure, with a subsequent increase in stroke volume, as demonstrated by Point B. This patient would therefore be fluid responsive. At Point C, the same fluid challenge would again increase pre-load and mean systemic filling pressure, but there is no significant increase in stroke volume, as shown by Point D. At Point E, an inadequate fluid challenge is given, too small a volume is given to significantly increase preload and mean systemic filling pressure, therefore, no increase in stroke volume is seen and this patient would incorrectly be labelled as a non-responder
When the decision to administer fluids has been made, the best way to do it is with a fluid challenge. A fluid challenge can be used to assess for fluid responsiveness without the limitations associated with pulse pressure variation or stroke volume variation and it can be both diagnostic and therapeutic. The aim of using a small volume of fluid is to reduce the risk associated with fluid overload if additional resuscitation is not required.[30] However, if the volume of fluid given is not adequate to stress the system then the response of patients who could be fluid responsive may be misinterpreted. A patient is deemed fluid responsive if stroke volume or cardiac output increase by at least 10% following a fluid challenge. Previous work has shown that only 50% of patients admitted to intensive care respond to fluid loading.[38]
FLUID CHALLENGE
The assessment of fluid responsiveness relies on the administration of a fluid challenge, however the method of administration of a fluid challenge shows significant inter-user variability. At present, the type of fluid, the volume of fluid and the rate at which the fluid is administered can vary. One large, multicentre, observational study showed a wide disparity in practice. Crystalloids accounted for 74.3% of fluids used for the challenge, of these 45.9% were 0.9% saline and 53.5% balanced solutions including Hartmann's solution, a range of colloids accounted for the remaining 25.6%. The median amount of fluid given as a challenge was 500 ml, interquartile range 500–1000 ml, median time of administration 24 min and median rate of administration 1000 ml/h, interquartile range 500–1333 ml/h.[39]
Different authors have reported differing changes in Pmsf in response to a fluid challenge. This may reflect the different techniques used to administer the fluid challenge. If the fluid challenge is to be effective in testing fluid responsiveness then it needs to increase Pmsf. Otherwise a patient may be incorrectly labelled as a non-responder, when in fact the volume given may have just filled the unstressed volume. Aya et al. have studied fluid challenge volumes of 1, 2, 3 and 4 ml/kg in post-surgical patients, and shown that a fluid challenge of 4 ml/kg is the option which most reliably stresses the cardiac system, to differentiate responders from non-responders.[40]
Whilst work aims to identify those who respond to intravenous fluids, it is important to realise that not all who respond will necessarily require the additional volume. In a study of healthy volunteers, they were shown to demonstrate a significant increase in stroke volume following a head down tilt. However, it is likely that these healthy volunteers do not necessarily require an increased intravascular volume.[29] It is important that the assessment of fluid responsiveness is used as part of a complete assessment of a patient's clinical condition and fluid status to determine whether they need or it is appropriate for them to receive further intravenous fluids.
MACRO AND MICROCIRCULATION COHERENCE
The focus in fluid management has historically been on the macrocirculation, with an assumption there will be haemodynamic coherence or that correction of the haemodynamics at the macrocirculatory level will translate to normalisation of perfusion and oxygen delivery in the microcirculation.[41] However, several studies have shown that coherence does not persist in states of illness. Jhanji et al. perioperatively studied the microcirculation and macrocirculation in patients undergoing gastrointestinal surgery. They observed that there were no significant differences in the systemic haemodynamic variables between patients who did and did not develop post-operative complications. However, at the microcirculation level, both the proportion and density of perfused small vessels and the microvascular flow index were reduced both pre and post-operatively in those patients who developed post-operative complications.[42]
It has been shown a low microcirculatory flow, independent of systemic haemodynamics, predicts the response to a fluid challenge. Surrogate markers of microcirculation such as lactate or skin temperature correlate poorly with direct visualisation of flow. The microcirculation can be examined at the bedside using a hand held microcirculation camera. At present, three generations of this technology have been developed. Patients with macrocirculatory markers of hypoperfusion may have either low or normal microcirculatory flow. Pranskunas et al. demonstrated that a fluid challenge only resulted in improvement in clinical markers of hypoperfusion in those with an initial low microcirculatory flow, which increased or normalised in response to a fluid challenge. Of those with an initial normal microcirculation no improvement in macrocirculatory markers of hypoperfusion was seen following a fluid challenge. The study participants’ change in stroke volume in response to fluid administration was independent of the microcirculatory changes seen. In studies guiding fluid therapy based on parameters within the microcirculation, significantly lower volumes of fluid were administered overall, with the same macrocirculatory outcomes.[42,43] This may be beneficial given the previously discussed increased mortality associated with a positive fluid balance.[15]
The choice of fluid has been shown to alter coherence. 0.9% saline has been shown to be the least effective of the commonly used fluids in improving microcirculatory flow and promoting haemodynamic coherence. It has been hypothesised that a focus on microcirculation guided fluids may improve outcomes, but this remains to be proved in large studies.[41]
SUMMARY
In summary, fluid therapy remains a contentious area of perioperative medicine, with differing approaches seen. It is vital that patients receive fluids targeted to their individual physiology. When a fluid challenge is given it should be an effective challenge of the patient's physiology. Fluid responsiveness should be assessed with an effective fluid challenge that increases Pmsf and the response or possible change in cardiac output monitored. Of those that do respond, not all of these patients will still require ongoing fluid resuscitation and there may be a role for bedside microcirculatory assessment, to guide this in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Cecconi is a Consultant and Speaker for Edwards Lifesciences, Cheetah, LidCO, Masimo, and is on the Medical Advisory Board of Directed Systems.
REFERENCES
- 1.Weiser TG, Haynes AB, Molina G, Lipsitz SR, Esquivel MM, Uribe-Leitz T, et al. Estimate of the global volume of surgery in 2012: An assessment supporting improved health outcomes. Lancet. 2015;385(Suppl 2):S11. doi: 10.1016/S0140-6736(15)60806-6. [DOI] [PubMed] [Google Scholar]
- 2.Pearse RM, Harrison DA, James P, Watson D, Hinds C, Rhodes A, et al. Identification and characterisation of the high-risk surgical population in the United Kingdom. Crit Care. 2006;10:R81. doi: 10.1186/cc4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: A 7 day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy MC. Wet, dry or something else? Br J Anaesth. 2006;97:755–7. doi: 10.1093/bja/ael290. [DOI] [PubMed] [Google Scholar]
- 6.Cannesson M, Gan TJ. Pro: Peri-operative goal directed therapy is an essential part of an enhanced recovery protocol. Int Anaesth Res Soc. 2016;122:1258–60. doi: 10.1213/ANE.0000000000001144. [DOI] [PubMed] [Google Scholar]
- 7.Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting – A clinical review. J Intensive Care. 2016;4:27. doi: 10.1186/s40560-016-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards MR, Mythen MG. Fluid therapy in critical illness. Extrem Physiol Med. 2014;3:16. doi: 10.1186/2046-7648-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serpa Neto A, Veelo DP, Peireira VG, de Assunção MS, Manetta JA, Espósito DC, et al. Fluid resuscitation with hydroxyethyl starches in patients with sepsis is associated with an increased incidence of acute kidney injury and use of renal replacement therapy: A systematic review and meta-analysis of the literature. J Crit Care. 2014;29:185.e1–7. doi: 10.1016/j.jcrc.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD000567.pub6. CD000567. [DOI] [PubMed] [Google Scholar]
- 11.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: A propensity-matched cohort study. Anesth Analg. 2013;117:412–21. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney RM, McKendry RA, Bedi A. Perioperative intravenous fluid therapy for adults. Ulster Med J. 2013;82:171–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Alves DR, Ribeiras R. Does fasting influence preload responsiveness in ASA 1 and 2 volunteers? Braz J Anesthesiol. 2017;67:172–9. doi: 10.1016/j.bjane.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Aditianingsih D, George YW. Guiding principles of fluid and volume therapy. Best Pract Res Clin Anaesthesiol. 2014;28:249–60. doi: 10.1016/j.bpa.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: Data from the prospective FINNAKI study. Crit Care. 2012;16:R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holte K, Foss NB, Andersen J, Valentiner L, Lund C, Bie P, et al. Liberal or restrictive fluid administration in fast-track colonic surgery: A randomized, double-blind study. Br J Anaesth. 2007;99:500–8. doi: 10.1093/bja/aem211. [DOI] [PubMed] [Google Scholar]
- 17.Cecconi M, Corredor C, Arulkumaran N, Abuella G, Ball J, Grounds RM, et al. Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176–86. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 19.Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330:1717–22. doi: 10.1056/NEJM199406163302404. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 21.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9:R687–93. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lobo SM, Ronchi LS, Oliveira NE, Brandão PG, Froes A, Cunrath GS, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care. 2011;15:R226. doi: 10.1186/cc10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhodes A, Cecconi M, Hamilton M, Poloniecki J, Woods J, Boyd O, et al. Goal-directed therapy in high-risk surgical patients: A 15-year follow-up study. Intensive Care Med. 2010;36:1327–32. doi: 10.1007/s00134-010-1869-6. [DOI] [PubMed] [Google Scholar]
- 24.Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, et al. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: A meta-analysis. Br J Anaesth. 2014;112:648–59. doi: 10.1093/bja/aet466. [DOI] [PubMed] [Google Scholar]
- 25.Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, et al. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth. 2012;108:53–62. doi: 10.1093/bja/aer273. [DOI] [PubMed] [Google Scholar]
- 26.Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland G, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. JAMA. 2014;311:2181–90. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 27.Pestaña D, Espinosa E, Eden A, Nájera D, Collar L, Aldecoa C, et al. Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: A prospective, randomized, multicenter, pragmatic trial: POEMAS Study (PeriOperative goal-directed thErapy in Major Abdominal Surgery) Anesth Analg. 2014;119:579–87. doi: 10.1213/ANE.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 28.Cove ME, Pinsky MR. Perioperative hemodynamic monitoring. Best Pract Res Clin Anaesthesiol. 2012;26:453–62. doi: 10.1016/j.bpa.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17:290–5. doi: 10.1097/MCC.0b013e32834699cd. [DOI] [PubMed] [Google Scholar]
- 30.Marik PE. Noninvasive cardiac output monitors: A state-of the-art review. J Cardiothorac Vasc Anesth. 2013;27:121–34. doi: 10.1053/j.jvca.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Waldron NH, Miller TE, Thacker JK, Manchester AK, White WD, Nardiello J, et al. A prospective comparison of a noninvasive cardiac output monitor versus esophageal Doppler monitor for goal-directed fluid therapy in colorectal surgery patients. Anesth Analg. 2014;118:966–75. doi: 10.1213/ANE.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 32.Patterson SW, Piper H, Starling EH. The regulation of the heart beat. J Physiol. 1914;48:465–513. doi: 10.1113/jphysiol.1914.sp001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maas JJ. Mean systemic filling pressure: Its measurement and meaning. Neth J Crit Care. 2015;19:6–11. [Google Scholar]
- 34.Bayliss WM, Starling EH. Observations on venous pressures and their relationship to capillary pressures. J Physiol. 1894;16:159–318. doi: 10.1113/jphysiol.1894.sp000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev. 1955;35:123–9. doi: 10.1152/physrev.1955.35.1.123. [DOI] [PubMed] [Google Scholar]
- 36.Cecconi M, Aya HD, Geisen M, Ebm C, Fletcher N, Grounds RM, et al. Changes in the mean systemic filling pressure during a fluid challenge in postsurgical intensive care patients. Intensive Care Med. 2013;39:1299–305. doi: 10.1007/s00134-013-2928-6. [DOI] [PubMed] [Google Scholar]
- 37.Myatra SN, Prabu NR, Divatia JV, Monnet X, Kulkarni AP, Teboul JL. The changes in pulse pressure variation or stroke volume variation after a “tidal volume challenge” reliably predict fluid responsiveness during low volume ventilation. Crit Care Med. 2017;45:415–21. doi: 10.1097/CCM.0000000000002183. [DOI] [PubMed] [Google Scholar]
- 38.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: A critical analysis of the evidence. Chest. 2002;121:2000–8. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 39.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, et al. Fluid challenges in intensive care: The FENICE study: A global inception cohort study. Intensive Care Med. 2015;41:1529–37. doi: 10.1007/s00134-015-3850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aya HD, Rhodes A, Chis Ster I, Fletcher N, Grounds RM, Cecconi M. Hemodynamic effect of different doses of fluids for a fluid challenge: A quasi-randomized controlled study. Crit Care Med. 2017;45:e161–8. doi: 10.1097/CCM.0000000000002067. [DOI] [PubMed] [Google Scholar]
- 41.Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8. doi: 10.1186/cc14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: A randomised controlled trial. Crit Care. 2010;14:R151. doi: 10.1186/cc9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. 2013;39:612–9. doi: 10.1007/s00134-012-2793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


