Abstract
Background and Aims:
Ambu® AuraGain™ (AG) (Ambu, Ballerup, Denmark) is a supraglottic device which has a design facilitating its use as a conduit for intubation. We designed this prospective observational study to assess the ease of AG placement in paralysed patients, determine its position and alignment to the glottis and assess its utility as a conduit for intubation.
Methods:
One hundred patients, aged 18–60 years, American Society of Anesthesiologists physical status I–II, undergoing elective surgery under general anaesthesia were included in the study. The ease and number of attempts for successful insertion, ease of gastric tube insertion, leak pressures, fibre-optic grade of view, number of attempts and time for tracheal intubation, time for AG removal and complications were recorded. The mean, standard deviation (SD), interquartile range (IQR) and range were calculated. The upper limit of confidence interval for overall failure rate was calculated using Wilson's score method.
Results:
AG was successfully inserted in all patients. The mean (SD) time taken for insertion was 17.32 (8.48) s. The median [IQR] leak pressures were 24 [20–28] cm of H2O. Optimal laryngeal view for intubation was obtained in 68 patients. Eighty-eight patients could be intubated in the first attempt. Five patients could not be intubated. The overall failure rate of device was 9%.
Conclusion:
AMBU® AuraGain™ serves as an effective ventilating aid, but caution is suggested before using it as a conduit for endotracheal intubation.
Keywords: Bronchoscopes fibreoptic, equipment, intratracheal, intubation, laryngeal masks
INTRODUCTION
Supraglottic airway devices (SGDs) are gaining popularity as preferred devices for elective and emergency airway management. The addition of a gastric port improves the safety profile of SGDs. The Fourth National Audit Project and the All India Difficult Airway Association (AIDAA) have encouraged the use of second-generation SGD equipped with the passage of a gastric tube in difficult airway scenarios.[1,2] AIDAA recommends SGD in step 2 of its difficult airway algorithm to maintain oxygenation and as a conduit for fibre-optic-guided intubation in the experienced hands.[2]
Ambu® AuraGain™ (Ambu, Ballerup, Denmark) is a novel cuffed supraglottic airway that has a preformed curve and also a built-in gastric port. It is marketed as having the capability of working as a conduit for intubation. Ambu® AuraGain™ (AG) is preformed to follow the anatomy of the human airway, and the soft rounded curve allows easy insertion. Furthermore, the low friction surface of the drain tube allows for easy gastric tube placement. The airway tube of AG is broader, and it accommodates a bigger endotracheal tube (ETT) as compared to similarly sized second-generation SGDs.[3,4] However, there are few studies with regards to the use of the AG in clinical practice. SGDs have been previously used as a conduit for intubation,[5,6] but there are no studies evaluating the utility of AG as a conduit for fibre-optic bronchoscope (FOB)-guided intubation.
The objectives of this prospective observational study were to evaluate the performance characteristics of AG by assessing: (1) Ease of placement of device, (2) the position of the ventilating orifice in relation to the larynx using a FOB, (3) its efficacy as a conduit for fibre-optic intubation with cuffed tracheal tubes in paralyzed adult patients, and (4) ease of removal of AG without dislodgement of the tracheal tube after successful tracheal intubation.
METHODS
This prospective observational study was conducted from October 2016 to January 2017 after Institutional Ethics Committee approval in a tertiary care hospital. One hundred patients of American Society of Anesthesiologists (ASA) physical status I and II and age ranging from 18 to 60 years of either sex, undergoing elective surgeries were included after written informed consent. Patients with ASA physical status III and above, those with an anticipated difficult airway, gastroesophageal reflux or body mass index >30 kg/m2 were excluded from the study. In the operation theatre, monitoring included electrocardiogram, oxygen saturation, non-invasive blood pressure and capnography (PM-9000 Express, Penlon, Abingdon, UK). Intravenous glycopyrrolate 4 μg/kg, midazolam 0.02 mg/kg and fentanyl 2 μg/kg were given. After induction of anaesthesia with propofol 2–2.5 mg/kg and vecuronium 0.08 mg/kg, an appropriate sized, completely deflated and lubricated AG, was inserted with the head in neutral position after application of jaw thrust, using the midline approach, by the consulting anaesthesiologist. The size of AG was selected as per the weight of the patient (size 3 for 30–50 kg and size 4 for 50–70 kg). The cuff was inflated using the manufacturer's recommendation. Correct placement was confirmed by observing visible chest rise and square waveform on capnography. AG was inserted by an anaesthesiologist with an experience of inserting at least 30 AGs and 200 other SGDs. The ease of insertion was assessed using a scale of 1–4 (1 = no resistance, 2 = moderate resistance, 3 = high resistance, 4 = inability to place the device).[7] The oropharyngeal leak pressure was measured while observing the pressure gauge on the anaesthesia machine with the expiratory valve closed, with a fresh gas flow of 5 L/min until an audible noise was heard at the patient's larynx or noting the airway pressure at which the pressure gauge indicator moved no further. A 16 Fr lubricated gastric tube was inserted through the gastric port. Ease of insertion of gastric tube through the gastric channel was noted on a 3-point scale of 1–3 (1 = passed easily, 2 = passed with difficulty, 3 = impossible to pass).[7] A fibre-optic scope was loaded with appropriate sized ETT (6.5 mm internal diameter through size 3 and 7.5 mm internal diameter through size 4 AG) and inserted through the AG. The anatomical alignment of the device to the larynx was viewed through FOB just above the ventilating orifice. A grading scale was used to assess the grade of view as follows: 4 = only vocal cords seen; 3 = vocal cords plus posterior epiglottis seen; 2 = vocal cords plus anterior epiglottis seen; 1 = vocal cords not seen, but function adequate; 0 = failure to function where vocal cords not seen fibre-optically.[8] According to the discretion of the anaesthesiologist manoeuvres were allowed if the view was grade 2 or 1, in an attempt to optimise the laryngeal view and facilitate fibre-optic navigation into the glottis. Manipulations allowed were: Neck flexion or extension, jaw thrust or gentle advancement or withdrawal of device. Improvement in laryngeal view, if any, secondary to the manipulation was noted. On visualisation of the carina with the bronchoscope, the tracheal tube was passed into the trachea. The cuff of the ETT was inflated up to a pressure of 25 cm of H2O. The cuff of AG was deflated, and it was removed after confirmation of tracheal intubation by capnography. An ETT with 5.5 and 6.5 mm internal diameter for AG size 3 and 4, respectively, was used as a tube stabiliser while removing the device. Ease of removal was graded on a 4-point scale of 1–4 (1 = easy removal, 2 = requires deflation of pilot balloon of ETT, 3 = desaturation <90% and 4 = extubation or damage to pilot balloon or inflation line of ETT). To minimise the risk of desaturation, the patients were ventilated with 100% oxygen throughout the process. The following parameters were noted by an independent observer: (1) Time of insertion (from disconnection of mask ventilation till reconnection of breathing circuit and 1st square waveform on capnography, (2) oropharyngeal leak pressure, (3) time for intubation (from the time the fibre-optic scope entered the device until reconnection of anaesthesia circuit to the tracheal tube), (4) time for removal of device (from disconnection of circuit to time of reconnection), and (5) heart rate and mean arterial pressure measured pre-induction, post-insertion of AG and at 1 and 3 min after intubation. Criteria for failure of device as a conduit for intubation were: Inability to place AG in two attempts or failure to intubate the trachea using FOB after two attempts, or dislodgement of the tracheal tube during removal of AG, or clinically significant desaturation (SpO2 ≤90%), or any damage to pilot balloon and cuff of ETT requiring conversion to conventional laryngoscopy and intubation. The tracheal tube was removed at the end of the surgery after reversal of neuromuscular blockade. Complications such as traumatic placement evidenced by the presence of blood on the AG, aspiration, laryngospasm or bronchospasm and desaturation (SpO2 ≤90%) were noted. In patients where intubation with AG as a conduit was unsuccessful, the patients were intubated with direct laryngoscopy using Macintosh laryngoscope.
Successful intubation through AG on the first attempt was the primary end point on the basis of which the sample size was calculated. A study evaluating air-Q ILA™ had a first attempt intubation success rate of 97%.[5] The sample size was estimated to be 98 for a margin of error of 4% and confidence level of 98%. Statistical analysis was performed using Microsoft® Excel® 2016 (Redmond, WA, U.S.A) and SPSS version 16.0 (SPSS Inc., Chicago, Illinois, USA). A one-way repeated measure analysis of variance was conducted separately each to evaluate the change in heart rate and mean arterial pressure from baseline, over time, post-intubation in the study participants.
RESULTS
Demographic information and summary of results are presented in Tables 1 and 2, respectively. No patients were excluded after enrolment. Insertion of AG was successful in 98 patients in the first attempt, and two patients required a second attempt. Ventilation was successful in all patients. Sixty-eight patients had laryngeal view grade 3 or 4. All these patients were intubated at first attempt. Of the remaining 32 patients who had a fibre-optic visualisation grade 2 or 1, 26 required extension, one patient required jaw thrust, the AG was manipulated in one patient, and in two patients, a combination of extension and jaw thrust improved the grade to 3 or 4. Out of these 32 patients, 20 could be intubated in the first attempt, 7 in the second attempt and 5 could not be intubated. In one patient with grade 2 view, manoeuvres to improve the laryngoscopic view resulted in displacement of the device. In one patient with grade 1 view of larynx, none of the manoeuvres resulted in improvement of view, and in 3 patients, FOB could not be negotiated through the vocal cords [Figure 1].
Table 1.
Demographic data
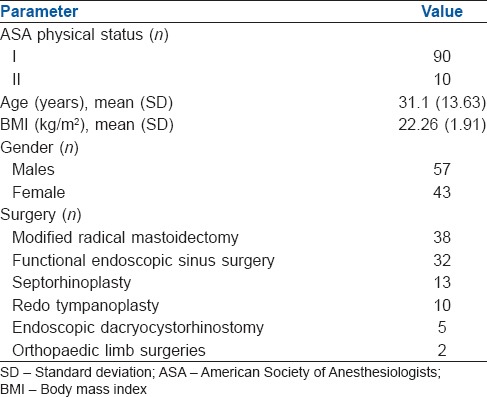
Table 2.
Descriptive statistics regarding performance characteristics of Ambu® AuraGain™
Figure 1.
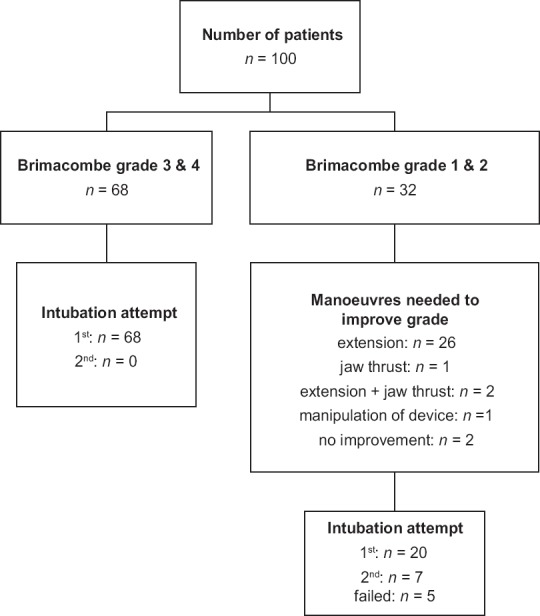
Summary of laryngeal view on fiberoptic bronchoscopy and success of intubation. Brimacombe grading scale to assess the grade of view is as follows: 4 = only vocal cords seen; 3 = vocal cords plus posterior epiglottis seen; 2 = vocal cords plus anterior epiglottis seen; 1 = vocal cords not seen, but function adequate; 0 = failure to function where vocal cords not seen fibre-optically
Four patients had grade 4 difficulty in removal, of which three had accidental extubation, and in one case, the pilot balloon got stuck in the lumen of AG and the whole assembly had to be removed. As per criteria mentioned earlier, the failure rate was 9%. The reasons for failure are mentioned in Table 3. The upper limit of 95% confidence interval using Wilson's score method was 16.23% for overall failure rate. Blood was present on AG in twenty patients. No patient experienced desaturation, laryngospasm or airway obstruction during the study [Table 4]. The haemodynamic response to AG insertion and intubation is depicted in Table 5.
Table 3.
Reasons for failure of Ambu® AuraGain™ as a conduit for intubation

Table 4.
Complications

Table 5.
Haemodynamic parameters

DISCUSSION
The main finding of this study was that the AG can be an adequate ventilating device in terms of insertion and ventilating features, with an overall 100% insertion rate with timeframes comparable to other SGDs.[4,9] However, when assessed as conduit for intubation, it underperformed with a failure rate of 9%. To the best of our knowledge, there are no studies evaluating the utility of AG as a conduit for FOB-guided intubation.
This study was planned as AG has a wider airway tube and accommodates an adult size FOB with a bigger ETT through its lumen.[3,4] It allows intubation in a single step as compared to other SGD-guided intubations which may require intubation aids.[10] This avoids multiple, cumbersome and time-consuming steps, especially in an emergency.
The AG was successfully placed in all patients with two patients requiring a second attempt. In one patient, a size 4 AG selected as per weight had to be replaced with a size 3 due to a small oral cavity. In the second patient, there was resistance while inserting the device in the first attempt; hence, a second attempt was needed. This is higher than results obtained with AG by other authors[7,11] although 100% success rate at first attempt has also been reported.[12] The size of the AuraGain has to be selected on the basis of the weight of the patient as recommended by the manufacturers. However, this factor alone may lead to an error in size selection, and the size of the oral cavity may also be taken into account while selecting the appropriate size of AG.
The average time (standard deviation [SD]) taken for device insertion was 17.32 (8.48) s. This was comparable to other SGDs[7,9,13] though faster insertion times have been also reported.[12] AG was inserted with moderate (n = 11) or high resistance (n = 1) in 12 patients which is half of what has previously reported in paediatric patients[7] or with AMBU® Aura-i™.[9] While other authors have not commented on the ease of insertion of AG, they have reported a 100% success rate at first attempt, probably indicating that the device is easy to insert.[12] The ease of gastric tube insertion may indicate proper alignment of the device against the oesophageal inlet. While the gastric tube insertion has been reported easy in all patients previously,[12] we encountered some difficulty in 6% of patients. This difference is probably due to difference in the sample size.
The airway leak pressure in this study 24 (20–28) (10–40) cm of H2O (median, [interquartile range] [range]) is lower than those reported in other adult patients with the same device[12] but similar to those found in paediatric patients.[7,14] Similar leak pressures have been reported previously suggesting that AG is as effective as other second-generation SGDs.[13,15,16]
Visibility of epiglottis through the airway tube may be a marker of difficulty during blind intubation attempts through the AG. Our observation of a frequent incidence (71%) of visualisation of the epiglottis on fibre-optic examination is consistent with earlier reports with AG in adult (67%) and paediatric patients (56%).[7,12] Similar incidence of epiglottis visualisation has also been reported with intubating laryngeal mask airway (ILMA).[13] Although the success of blind intubation through AG was not assessed in the current study, use of fibre-optic bronchoscope to view the laryngeal inlet helped us in assessing the feasibility of blind intubation through the AG, especially in emergency, where it may be considered as a conduit. The Brimacombe grade was ≤2 in 32 patients which improved with manoeuvres. Such visually guided manoeuvres may not be possible when blind intubation is attempted. Previous reports of blind intubation through the AG have had a poor success rate (17.5%) as compared to ILMA Fastrach™(70%).[11] This, along with high rates of epiglottis visualisation noted in our study, leads us to opine against blind intubations with AG. This is in consonance with the recommendations of AIDAA which advises against blind intubation through any SGD, especially when it has been placed as a rescue device for ventilation.[2]
Five patients could not be intubated despite two attempts [Table 3]. In three of these patients, intubation was not possible in spite of the FOB being in the trachea; out of which oesophageal intubation occurred in one patient. Multiple reasons for inability in passing ETT even after tracheal placement of FOB have been described, ranging from abutment against epiglottis or arytenoid cartilage, accidental slippage into the oesophagus or advancement of the tube directly into oesophagus ignoring the course of the FOB.[17,18] The incidence of oesophageal intubation despite FOB being in trachea can be high[17] varying from 2 in 84[19] to 2 in sixty patients.[20] In two patients, the FOB could not be negotiated beyond the vocal cords as it kept abutting against the arytenoid cartilage despite two attempts and the manoeuvres to improve the fibre-optic view resulted in displacement of the AG prompting switch over to direct laryngoscopy and endotracheal intubation. These problems can be explained on the basis of the trajectory of the FOB and the ETT when it exits the airway tube of the AG. Similar problems have also been reported during intubation through AMBU Aura-i™ where five out of six failed intubation attempts were due to inability to railroad the tracheal tube over the fibrescope into the trachea. The authors have reasoned that Aura-i has a flat angle of exit, which may result in the fibrescope or tracheal tube exiting more posteriorly and heading towards the oesophagus.[21] Furthermore, all patients who required two attempts at intubation had an initial Brimacombe grade of ≤2.
The time taken for intubation represents the apnoeic period, and it was slightly more than the time taken for intubation through the air-Q ILA™ (33.50 [6.79] s, mean [SD]) but similar to that through ILMA (39.50 [6.56] s mean [SD]) using a similar technique.[13] However, the longest intubation times during our study were within clinically acceptable limits.[22] This also correlates with our findings that none of the patients had oxygen desaturation during the entire process. The time taken for intubation is dependent on the user, and we ensured that anaesthesiologists who intubated were well conversant with the use of FOB through SGDs.
For a device to be considered as a conduit of intubation, it is important that removal should be smooth and easy without accidental extubation. The manufacturers have not provided any stabilising rod or removal stylet to facilitate removal. We used an appropriate sized ETT as per manufacturer's recommendation to serve as a stabilising rod. Removal of AG was easy in majority of cases. The pilot balloon of the ETT had to be deflated in one case, while in another it had to be pushed back in with the help of Magill's forceps to facilitate easy removal. The pilot balloon got stuck in the lumen of AG in one patient leading to conversion to direct laryngoscopy and intubation. Accidental extubation occurred in three patients. The duration of apnoea during removal was also within clinically acceptable limits.[22] The high failure rate (9%), which includes failed intubation and extubation while removal, mandates caution while using this device as a backup conduit for intubation. The incidence of the blood on device, indicating trauma during the procedure, was similar to other studies.[11]
Our study has a few limitations. First, we have studied patients with normal airway anatomy instead of patients with difficult airways where a SGD is more likely to be used as a conduit. However, the paucity of evidence for AG as a conduit even in normal airways prevented its use in known difficult airways. Second, we have not compared AG with any established SGD, as our primary aim was to evaluate the feasibility of AG to work as a conduit for intubation. However, our study is in accordance with other single arm studies where newer SGDs were evaluated for their clinical utility.[4,5,14,23,24,25] We aimed to do a stage two study for evaluating AG as a conduit for intubation, as per recommendations for studying newer SGDs.[26] Further prospective randomised trials are needed to compare the AG with other established second-generation SGDs as per the ADEPT guidelines of Difficult Airway Society.[27]
CONCLUSION
We conclude that AG demonstrates a good level of utility as an alternative SGD with respect to ease of insertion, seal pressures and ventilation characteristics. It aligns well with the glottis in a majority of patients. It may serve as a ventilating aid but may not prove to be an effective conduit for tracheal intubation. If it is to be used as a conduit, then the availability of FOB is recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cook TM, Woodall N, Frerk C. Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth. 2011;106:617–31. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 2.Myatra SN, Shah A, Kundra P, Patwa A, Ramkumar V, Divatia JV, et al. All India Difficult Airway Association 2016 guidelines for the management of unanticipated difficult tracheal intubation in adults. Indian J Anaesth. 2016;60:885–98. doi: 10.4103/0019-5049.195481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez AM, Sala-blanch X, Valero R, Prats A. Cross-over assessment of the Ambu® AuraGain™, LMA supreme new cuff and intersurgical I-Gel in fresh cadavers. Open J Anesthesiol. 2014;4:332–9. [Google Scholar]
- 4.Attarde VB, Kotekar N, Shetty SM. Air-Q intubating laryngeal airway: A study of the second generation supraglottic airway device. Indian J Anaesth. 2016;60:343–8. doi: 10.4103/0019-5049.181596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagannathan N, Kozlowski RJ, Sohn LE, Langen KE, Roth AG, Mukherji II, et al. Aclinical evaluation of the intubating laryngeal airway as a conduit for tracheal intubation in children. Anesth Analg. 2011;112:176–82. doi: 10.1213/ANE.0b013e3181fe0408. [DOI] [PubMed] [Google Scholar]
- 6.Kundra P, Sujata N, Ravishankar M. Conventional tracheal tubes for intubation through the intubating laryngeal mask airway. Anesth Analg. 2005;100:284–8. doi: 10.1213/01.ANE.0000139348.00435.33. [DOI] [PubMed] [Google Scholar]
- 7.Jagannathan N, Hajduk J, Sohn L, Huang A, Sawardekar A, Gebhardt ER, et al. A randomised comparison of the Ambu® AuraGain™ and the LMA® supreme in infants and children. Anaesthesia. 2016;71:205–12. doi: 10.1111/anae.13330. [DOI] [PubMed] [Google Scholar]
- 8.Brimacombe J, Berry A. A proposed fiber-optic scoring system to standardize the assessment of laryngeal mask airway position. Anesth Analg. 1993;76:457. [PubMed] [Google Scholar]
- 9.Yahaya Z, Teoh WH, Dintan NA, Agrawal R. The AMBU® Aura-i™ laryngeal mask and LMA Supreme™: A randomized trial of clinical performance and fibreoptic positioning in unparalysed, anaesthetised patients by novices. Anesthesiol Res Pract 2016. 2016:4717061. doi: 10.1155/2016/4717061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong DT, Yang JJ, Mak HY, Jagannathan N. Use of intubation introducers through a supraglottic airway to facilitate tracheal intubation: A brief review. Can J Anaesth. 2012;59:704–15. doi: 10.1007/s12630-012-9714-8. [DOI] [PubMed] [Google Scholar]
- 11.Correa TL, Sastre JA, Garzón JC. Blind tracheal intubation through 2 supraglottic devices: The Ambu® AuraGain™ vs. the LMA Fastrach. Emergencias. 2016;28:83–8. [PubMed] [Google Scholar]
- 12.Lopez AM, Agusti M, Gambus P, Pons M, Anglada T, Valero R. A randomized comparison of the Ambu AuraGain versus the LMA supreme in patients undergoing gynaecologic laparoscopic surgery. J Clin Monit Comput 2016. doi: 10.1007/s10877-016-9963-0. [Epub 2016 Nov 26] [DOI] [PubMed] [Google Scholar]
- 13.Abdel-halim TM, Abo MA, Enin E, Elgoushi MM, Afifi MG, Atwa HS. Comparative study between Air-Q and intubating laryngeal mask airway when used as conduit for fiber-optic. Egypt J Anaesth. 2014;30:107–13. [Google Scholar]
- 14.Whyte SD, Cooke E, Malherbe S. Usability and performance characteristics of the pediatric air-Q® intubating laryngeal airway. Can J Anaesth. 2013;60:557–63. doi: 10.1007/s12630-013-9918-6. [DOI] [PubMed] [Google Scholar]
- 15.Bakker EJ, Valkenburg M, Galvin EM. Pilot study of the air-Q intubating laryngeal airway in clinical use. Anaesth Intensive Care. 2010;38:346–8. doi: 10.1177/0310057X1003800217. [DOI] [PubMed] [Google Scholar]
- 16.Liew GH, Yu ED, Shah SS, Kothandan H. Comparison of the clinical performance of i-gel, LMA supreme and LMA ProSeal in elective surgery. Singapore Med J. 2016;57:432–7. doi: 10.11622/smedj.2016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asai T, Shingu K. Difficulty in advancing a tracheal tube over a fibreoptic bronchoscope: Incidence, causes and solutions. Br J Anaesth. 2004;92:870–81. doi: 10.1093/bja/aeh136. [DOI] [PubMed] [Google Scholar]
- 18.Jackson AH, Orr B, Yeo C, Parker C, Craven R, Greenberg SL. Multiple sites of impingement of a tracheal tube as it is advanced over a fibreoptic bronchoscope or tracheal tube introducer in anaesthetized, paralysed patients. Anaesth Intensive Care. 2006;34:444–9. doi: 10.1177/0310057X0603400409. [DOI] [PubMed] [Google Scholar]
- 19.Hakala P, Randell T. Comparison between two fibrescopes with different diameter insertion cords for fibreoptic intubation. Anaesthesia. 1995;50:735–7. doi: 10.1111/j.1365-2044.1995.tb06108.x. [DOI] [PubMed] [Google Scholar]
- 20.Koga K, Asai T, Latto IP, Vaughan RS. Effect of the size of a tracheal tube and the efficacy of the use of the laryngeal mask for fibrescope-aided tracheal intubation. Anaesthesia. 1997;52:131–5. doi: 10.1111/j.1365-2044.1997.31-az0058.x. [DOI] [PubMed] [Google Scholar]
- 21.de Lloyd LJ, Subash F, Wilkes AR, Hodzovic I. A comparison of fibreoptic-guided tracheal intubation through the Ambu® Aura-i™, the intubating laryngeal mask airway and the i-gel™: A manikin study. Anaesthesia. 2015;70:591–7. doi: 10.1111/anae.12988. [DOI] [PubMed] [Google Scholar]
- 22.Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87:979–82. doi: 10.1097/00000542-199710000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Hughes C, Place K, Berg S, Mason D. A clinical evaluation of the I-gel™ supraglottic airway device in children. Paediatr Anaesth. 2012;22:765–71. doi: 10.1111/j.1460-9592.2012.03893.x. [DOI] [PubMed] [Google Scholar]
- 24.Jagannathan N, Sohn LE, Chang E, Sawardekar A. A cohort evaluation of the laryngeal mask airway-Supreme™ in children. Paediatr Anaesth. 2012;22:759–64. doi: 10.1111/j.1460-9592.2012.03832.x. [DOI] [PubMed] [Google Scholar]
- 25.López AM, Muñoz-Rojas G, Fontanals M, de San José I, Hermoso A, Valero R. Clinical evaluation of the Baska Mask laryngeal mask in adult patients in ambulatory surgery. Rev Esp Anestesiol Reanim. 2015;62:551–6. doi: 10.1016/j.redar.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Cook TM. Novel airway devices: Spoilt for choice? Anaesthesia. 2003;58:107–10. doi: 10.1046/j.1365-2044.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 27.Pandit JJ, Popat MT, Cook TM, Wilkes AR, Groom P, Cooke H, et al. The Difficult Airway Society ‘ADEPT’ guidance on selecting airway devices: The basis of a strategy for equipment evaluation. Anaesthesia. 2011;66:726–37. doi: 10.1111/j.1365-2044.2011.06787.x. [DOI] [PubMed] [Google Scholar]


