Abstract
Background and Aims:
Perfusion index (PI) is a new parameter tried for predicting hypotension during spinal anaesthesia for the lower segment caesarean section (LSCS). This study aimed at investigating the correlation between baseline perfusion index and incidence of hypotension following SAB in LSCS.
Methods:
In this prospective observational study, 126 parturients were divided into two groups on the basis of baseline PI. Group I included parturients with PI of ≤3.5 and Group II, parturients with PI values >3.5. Spinal anaesthesia was performed with 10 mg of injection bupivacaine 0.5% (hyperbaric) at L3–L4 or L2–L3 interspace. Hypotension was defined as mean arterial pressure <65 mmHg. Statistical analysis was performed using Chi-square test, independent sample t-test and Mann–Whitney U-test. Regression analysis with Spearman's rank correlation coefficient was done to assess the correlation between baseline PI and hypotension. Receiver operating characteristic (ROC) curve was plotted for PI and occurrence of hypotension.
Results:
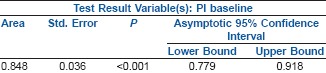
The incidence of hypotension in Group I was 10.5% compared to 71.42% in Group II (P < 0.001). There was significant correlation between baseline PI >3.5 and number of episodes of hypotension (rs0.416, P < 0.001) and total dose of ephedrine (rs0.567, P < 0.001). The sensitivity and specificity of baseline PI of 3.5 to predict hypotension was 69.84% and 89.29%, respectively. The area under the ROC curve for PI to predict hypotension was 0.848.
Conclusion:
Baseline perfusion index >3.5 is associated with a higher incidence of hypotension following spinal anesthesia in elective LSCS.
Keywords: Hypotension, perfusion index, pregnancy, spinal anaesthesia
INTRODUCTION
Hypotension following spinal anaesthesia results from the sympathetic blockade and decreased cardiac output.[1] Pregnant women are more sensitive to local anaesthetics, less responsive to vasopressors and have lower mean arterial pressure (MAP) at term.[2] Hence, parturients can develop profound hypotension following central neuraxial blockade for the lower segment caesarean section (LSCS).
Non-invasive blood pressure (NIBP) measurement is the standard method of monitoring intraoperative haemodynamics. However, beat to beat variation in perfusion dynamics cannot be measured by this method and limits its efficacy.
Perfusion index (PI) is defined as the ratio of pulsatile blood flow to non-pulsatile blood flow in the peripheral vascular tissue, measured using a pulse oximeter based on the amount of Infrared light absorbed.[3] Hence, PI can be used to assess perfusion dynamics and is being considered as a non-invasive method to detect the likelihood of development of hypotension following subarachnoid block (SAB).[4,5,6] Various studies carried out previously have employed perfusion index to assess haemodynamic parameters. However, there are limited data regarding its use for prediction of the incidence of hypotension occurring as a result of the central neuraxial blockade. We conducted this study to determine whether a baseline PI >3.5 predicts the development of hypotension after spinal anaesthesia in parturients.
METHODS
The prospective observational study was conducted from June 2014 to October 2014. Approval for the study was obtained from the Institutional Ethics Committee. Informed written consent was obtained from every participant in the study.
The study included parturients between 20 and 35 years of age posted for elective caesarean section. We hypothesised that parturients with higher baseline PI would have a higher incidence of hypotension. Anticipating equal distribution of baseline PI on either side of cut-off point of 3.5 suggested in a study by Toyama et al.,[7] we conducted a pilot study in 15 parturients and found a difference in the incidence of hypotension to be 20% when those 15 patients were divided into two groups based on cut-off point of 3.5 (Group I PI ≤3.5 [eight patients] and PI >3.5 [seven patients]). Keeping the confidence interval at 95%, a minimum of 120 parturients would be required, to achieve a power of 80%, if the same result had to be reproduced. We enrolled 126 parturients for the study. Parturients involved in the pilot study were not considered for final analysis. Parturients with placenta praevia, preeclampsia, cardiovascular or cerebrovascular disease, gestational diabetes, body mass index ≥40, gestational age <36 or >41 weeks, contraindications to spinal anaesthesia and those requiring emergency LSCS were excluded from the study. Standard monitoring with electrocardiography, automated NIBP, and pulse oximetry (SpO2) was performed for baseline values and intraoperative monitoring. The perfusion index was measured in the supine position using a specific pulse oximeter probe (Masimo Radical 7®; Masimo Corp., Irvine, CA, USA) which was attached to the left index finger of all parturients to ensure uniformity in measured PI values.
This was a double-blinded study. The baseline haemodynamic values including PI were recorded in the supine position by an anaesthesiologist who was not involved in the further intraoperative monitoring of the patient. Those with a baseline perfusion index of ≤3.5 fell into Group I and those with a perfusion index of >3.5 fell into Group II.[7]
Intravenous (IV) access was established in the left upper limb. Each parturient was prehydrated with 500 ml of Ringer lactate over 20 min. After prehydration, the baseline values were recorded. While administering neuraxial blockade, the Masimo® pulse oximeter was disconnected to prevent observer bias and SpO2 was recorded using a different pulse oximeter. Spinal anaesthesia was performed by an anaesthesiologist blinded to the baseline PI values, using Quincke's 25-gauge spinal needle in left lateral decubitus position with 10 mg of injection bupivacaine 0.5% (hyperbaric) at the L3–L4 or L2–L3 interspace. The parturient was returned to the supine position with a left lateral tilt of 15° to facilitate left uterine displacement. The Masimo® pulse oximeter was reconnected to monitor the patient till the end of surgery. Oxygen was given through face mask at 4 L/min.
Ringer's lactate was administered at a rate of 100 ml/10 min. The level of sensory block was checked 5 min after the spinal injection with a cold swab. If a T6 sensory block level was not achieved, these parturients were excluded from the study and managed according to institutional protocol. Maximum cephalad spread was checked 20 min after SAB. NIBP, heart rate (HR), respiratory rate (RR), SpO2 and PI were recorded at 2 min intervals after the SAB up to 20 min and then at 5 min intervals by the same anaesthesiologist who administered SAB till the end of surgery. Hypotension was defined as a decrease in MAP <65 mm of Hg and treated with IV bolus of 6 mg injection ephedrine and 100 ml of Ringer lactate. The first 60 min following spinal anaesthesia was considered for anaesthesia-induced hypotension. Bradycardia was defined as HR <55 beats/min and treated with injection atropine 0.6 mg IV bolus. Following extraction of the baby, Apgar score was recorded at 1st and 5th min. Injection oxytocin 10 units was given as uterotonic following baby extraction at a rate of 200 mU/min as a separate infusion. Patients requiring additional oxytocics and/or additional surgical interventions excluded from the study. The incidence of other side effects such as nausea, vomiting if observed were recorded.
Categorical and discrete data are presented as tables, and continuous data represented by graphs. Discrete and continuous data were analysed for normal distribution using Shapiro–Wilk test. Chi-square test was applied to assess statistical significance for discrete and categorical data. Independent sample t-test and Mann–Whitney U-test were applied for continuous data which showed normal and skewed distribution, respectively. Regression analysis with Spearman's rank correlation coefficient was done to assess the correlation between baseline PI with other parameters. A Receiver Operating Characteristic (ROC) curve was obtained for baseline PI compared with the hypotension episodes of 126 patients. Data were analysed using SPSS (Statistical Package for Social Sciences) version 20. (IBM SPSS Statistics for Windows, version 20.0, IBM Corp., Armonk, NY, USA) P < 0.05 was considered statistically significant.
RESULTS
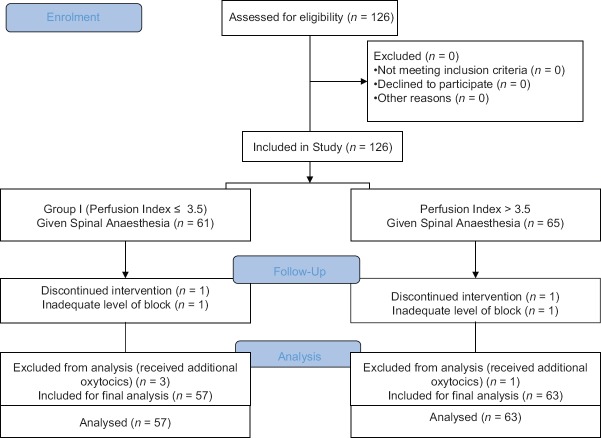
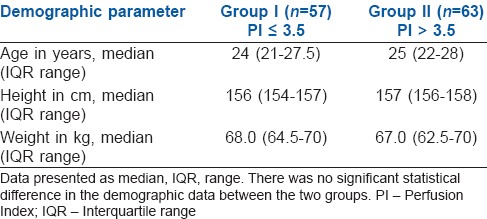
A total of 126 patients were included in the study. Two parturients were excluded from the study due to an inadequate level of the spinal blockade, and four parturients had to be excluded due to the requirement of additional oxytocics, as the drugs administered could influence the HR and blood pressure of the patients. Fifty-seven patients were in Group I and 63 patients were in Group II for final analysis [Figure 1]. The demographic parameters such as age, weight and height were comparable between the two groups [Table 1]. The average duration of surgery in both groups was comparable (Group I - 45.87 ± 11.14 min and Group II - 47.93 ± 9.78 [P = 0.2]).
Figure 1.
CONSORT Flow Diagram
Table 1.
Comparison of demographic characteristics between two groups
The median level of cephalad spread of sensory block achieved in both groups was T6. (interquartile range [IQR] - T4–T6).
The PI values in both groups on assessment showed skewed distribution and the median PI in Group I was 2.45 (IQR [1.8–2.8]), and in Group II was 5.4 (IQR [4.25–7.1]). The skewed distribution to the right around the PI value of 3.5, was observed when baseline PI values of both groups were combined and assessed for normal distribution.
Intraoperatively, the HR was comparable between the two groups.
The difference between the two groups with respect to systolic blood pressure (SBP), diastolic blood pressure (DBP) and MAP was statistically significant for the first 25 min [Figure 2]. The difference in SBP was most significant during the 2nd, 4th, 6th, 10th and 15th min with values being lower in Group II than Group I, whereas difference in DBP was most significant during the 4th, 10th, 15th, 20th and 25th min and the difference in MAP was most significant during the 2nd, 4th, 6th, 10th, 15th, 20th and 25th min. The DBP and MAP were also lower in Group II than Group I.
Figure 2.
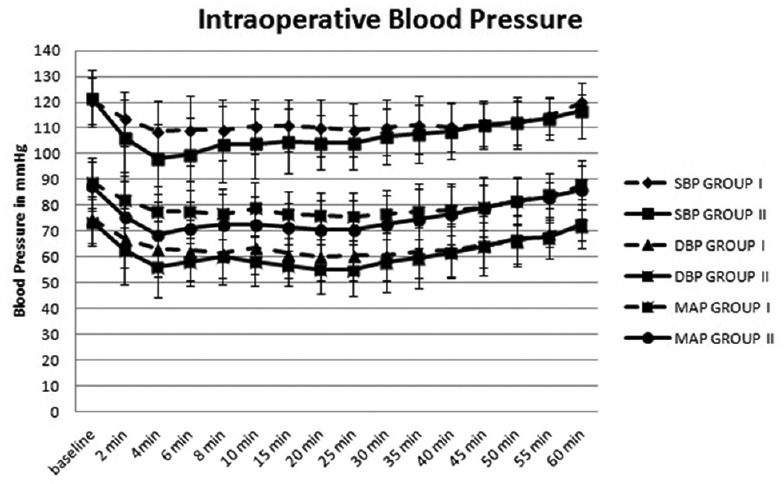
Comparison of systolic blood pressure, diastolic blood pressure and mean arterial pressure between the two groups intraoperatively. Systolic, diastolic and mean arterial pressure values presented as mean ± standard deviation. Statistical analysis done using independent t-test P > 0.05
The ROC curve yielded 3.85 as a more appropriate cut-off with a well balanced 76% sensitivity and specificity. The area under the ROC curve (AUC) was 0.848 [Figure 3].
Figure 3.
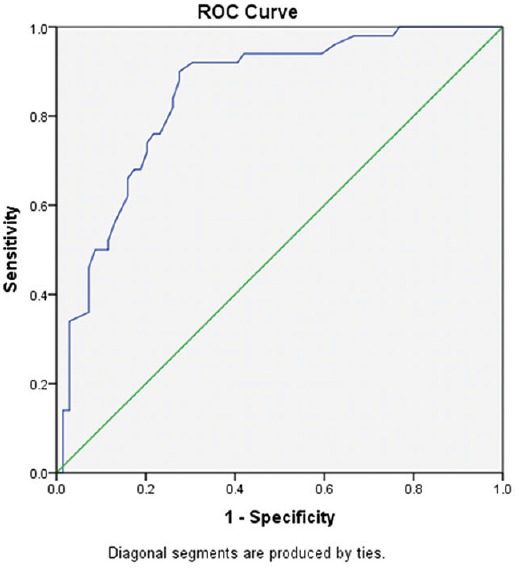
ROC curve depicting baseline PI against incidence of hypotension
Area Under the Curve
The incidence of hypotension in Group I was 10.5% (6/57) compared to 71.42% (45/63). This was clinically and statistically highly significant (P < 0.001, odds ratio –0.07). In Group I, four patients had one episode of hypotension, one patient had two episodes, and one patient had three. In Group II, twenty-four patients had one episode of hypotension, 16 patients had two episodes, four patients had three episodes, and one patient had four episodes [Table 2]. Eighty-nine percent of patients in Group I had no hypotension. Thirty-two percent of patients in Group II had multiple episodes of hypotension (P < 0.001).
Table 2.
Requirement of ephedrine and intravenous fluids and number of episodes of hypotension
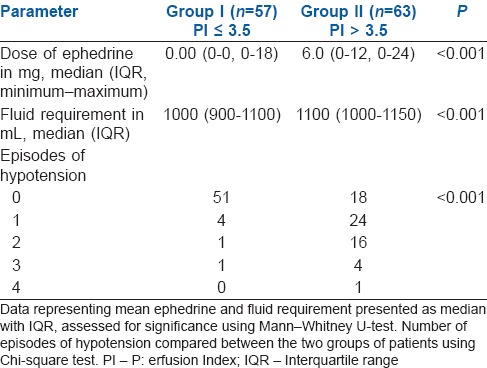
Median ephedrine usage in Group I was 0 mg (IQR 0–0 mg) and 6 mg (IQR 6–12) in Group II (P < 0.001) The amount of IV fluids required in Group I was also lower than Group II (P < 0.001) [Table 2]. One patient belonging to Group II developed bradycardia which was treated with injection atropine 0.6 mg IV.
On Spearman's rank correlation we found highly significant correlation between baseline PI >3.5 and number of episodes of hypotension (rs0.416, P < 0.001), total dose of ephedrine used (rs0.567, P < 0.001) and total IV fluids used (rs0.249, P = −0.019).
Post hoc power analysis comparing the incidence of hypotension and vasopressor use between the two groups showed a power of more than 90%, at confidence intervals of 95%. The sensitivity and specificity of baseline PI with a cut-off of 3.5 was 69.84% and 89.29% respectively.
The RR and SpO2 were comparable between the two groups throughout the study period. There was no significant difference in Apgar scores between the groups at 1st and 5th min. The incidence of nausea and vomiting was similar in both groups (Group I – 4/57 (7.01%), Group II 9/63 (14.28%), P = 0.20).
DISCUSSION
In the present study, the incidence and severity of hypotension, vasopressor requirement was higher in parturients whose baseline PI values were greater than 3.5. The ROC curve revealed that PI discriminated well between patients who developed hypotension versus those who did not; it yielded a new baseline PI value of 3.85 as the cut off point for predicting hypotension in parturients undergoing caesarean section under sub arachnoid block.
Hypotension following administration of spinal anaesthesia for caesarean delivery is common.[8] There is no definite monitoring system which may predict the likelihood of developing hypotension so that additional precautions may be taken. Studies have tried to evaluate the usefulness of perfusion index in predicting hypotension following spinal anaesthesia in casearean section.[7]
The principle of SpO2 is based on two light sources with different wavelengths 660 nm and 940 nm, emitted through cutaneous vascular bed of finger or earlobe.[6] The absorbance of both wavelengths has a pulsatile component, which represents fluctuations in the volume of arterial blood between the source and the detector. The non-pulsatile component is from connective tissue, bone and venous compartment. The perfusion index (PI) is the ratio of the pulsatile component (arterial) and non-pulsatile component of light reaching the detector.
Healthy pregnancy is characterised by a decrease in systemic vascular resistance, increased total blood volume and cardiac output.[9] The reduction of systemic vascular resistance may vary in parturients depending on various factors.[9,10,11,12,13] This decrease in tone will correspond to higher perfusion index values due to increase in pulsatile component due to vasodilatation. Induction of a sympathectomy by spinal anaesthesia will cause a further decrease peripheral vascular tone and increase pooling and hypotension. Parturients with high baseline perfusion index are expected to have lower peripheral vascular tone and hence are at higher risk of developing hypotension following spinal anaesthesia. PI has been used in the study by Mowafi et al. to detect intravascular injection of the epinephrine-containing epidural test dose, hence its reliability to detect vasoconstriction has been demonstrated successfully.[4] Ginosar et al. demonstrated that increase in PI following epidural anaesthesia was a clear and reliable indicator of sympathectomy.[5]
In contrast, a recent study performed by Yokose et al.[14] demonstrated that PI had no predictive value for hypotension in parturients undergoing LSCS following SAB. This discrepancy was attributed to various methodological differences, such as the definition of hypotension, co-loading with colloids and method of calculation of baseline PI.
The cut-off value of baseline perfusion index for prediction of hypotension following spinal anaesthesia was chosen as 3.5 based on a study conducted by Toyama et al.[7] who did regression analysis and ROC curve analysis and concluded that a baseline perfusion index cut-off point of 3.5 could be used to identify parturients at risk for such hypotension. An attempt was made to explore the predictive ability of this value in the Indian population, in this study. Further, only the baseline value was considered for analysis, since we did not try to explore the correlation between changes in serial PI values with the incidence of hypotension.
In this study, the baseline PI >3.5 and probability of hypotension were significantly correlating, a finding similar to study by Toyama et al.
On Spearman rank correlation, a highly significant correlation was found between baseline PI >3.5 and number of episodes of hypotension, the total dose of ephedrine used and total IV fluids used. A higher requirement of vasopressor was seen in parturients with baseline PI >3.5.
Toyama et al. found a sensitivity and specificity of 81% and 86%, respectively, for baseline PI with a cut-off of 3.5 to predict hypotension, whereas in this study, the specificity was comparable, 89.29%, but sensitivity was lower, 69.84%.
In this study, the consumption of IV fluid was higher than that in the study by Toyama et al. As we used injection ephedrine and fluid bolus to treat hypotension while they used only injection phenylephrine to treat hypotension.
Uterotonics such as prostaglandin F2 alpha, methylergometrine are powerful vasoconstrictors and would have influenced the observations and hence patients receiving these drugs were excluded from analysis, as they received these drugs between 20 and 25 min after spinal anaesthesia.
There are many limitations in this study. Patient movement and any stimulus increasing sympathetic activity like anxiety could easily change the PI values. In this study, we recorded baseline PI values with utmost care to avoid patient movement, especially while recording baseline values and all patients were counselled before taking them up for surgery to allay anxiety. The baseline value of PI could have been affected due to aortocaval compression in supine position while recording baseline values. Systemic vascular resistance was not measured, but it would be invasive and unnecessary for the uncomplicated caesarean section. Arterial blood gas analysis for both the mother and foetus was not done which could have ruled out hypoxia resulting from hypoperfusion.
Since PI is dependent on the vascular tone of digital vessels, its role in predicting hypotension in conditions where the tone of these vessels is affected is questionable and more studies regarding its use in other patients needs to be done before it can be accepted as a universal non-invasive tool to predict hypotension following spinal anaesthesia. In addition, further studies comparing PI with invasive and accepted tools of haemodynamic monitoring may throw more light regarding its utility.
CONCLUSION
Perfusion Index (PI) can be used as a tool for predicting hypotension in healthy parturients undergoing elective caesarean section under SAB. Parturients with baseline PI >3.5 are at higher risk of developing hypotension following SAB compared to those with baseline PI ≤3.5.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hanss R, Bein B, Ledowski T, Lehmkuhl M, Ohnesorge H, Scherkl W, et al. Heart rate variability predicts severe hypotension after spinal anesthesia for elective cesarean delivery. Anesthesiology. 2005;102:1086–93. doi: 10.1097/00000542-200506000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Gaiser R. Physiological changes of pregnancy. In: Chestnut DH, editor. Chestnut's Obstetric Anaesthesia: Principles and Practice. 5th ed. Philadelphia: Mosby Elsevier Publishing; 2014. pp. 15–38. [Google Scholar]
- 3.Hales JR, Stephens FR, Fawcett AA, Daniel K, Sheahan J, Westerman RA, et al. Observations on a new non-invasive monitor of skin blood flow. Clin Exp Pharmacol Physiol. 1989;16:403–15. doi: 10.1111/j.1440-1681.1989.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 4.Mowafi HA, Ismail SA, Shafi MA, Al-Ghamdi AA. The efficacy of perfusion index as an indicator for intravascular injection of epinephrine-containing epidural test dose in propofol-anesthetized adults. Anesth Analg. 2009;108:549–53. doi: 10.1213/ane.0b013e31818fc35b. [DOI] [PubMed] [Google Scholar]
- 5.Ginosar Y, Weiniger CF, Meroz Y, Kurz V, Bdolah-Abram T, Babchenko A, et al. Pulse oximeter perfusion index as an early indicator of sympathectomy after epidural anesthesia. Acta Anaesthesiol Scand. 2009;53:1018–26. doi: 10.1111/j.1399-6576.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 6.Lima AP, Beelen P, Bakker J. Use of a peripheral perfusion index derived from the pulse oximetry signal as a noninvasive indicator of perfusion. Crit Care Med. 2002;30:1210–3. doi: 10.1097/00003246-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Toyama S, Kakumoto M, Morioka M, Matsuoka K, Omatsu H, Tagaito Y, et al. Perfusion index derived from a pulse oximeter can predict the incidence of hypotension during spinal anaesthesia for caesarean delivery. Br J Anaesth. 2013;111:235–41. doi: 10.1093/bja/aet058. [DOI] [PubMed] [Google Scholar]
- 8.Park GE, Hauch MA, Curlin F, Datta S, Bader AM. The effects of varying volumes of crystalloid administration before cesarean delivery on maternal hemodynamics and colloid osmotic pressure. Anesth Analg. 1996;83:299–303. doi: 10.1097/00000539-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Ajne G, Ahlborg G, Wolff K, Nisell H. Contribution of endogenous endothelin-1 to basal vascular tone during normal pregnancy and preeclampsia. Am J Obstet Gynecol. 2005;193:234–40. doi: 10.1016/j.ajog.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Barwin BN, Roddie IC. Venous distensibility during pregnancy determined by graded venous congestion. Am J Obstet Gynecol. 1976;125:921–3. doi: 10.1016/0002-9378(76)90489-0. [DOI] [PubMed] [Google Scholar]
- 11.Sakai K, Imaizumi T, Maeda H, Nagata H, Tsukimori K, Takeshita A, et al. Venous distensibility during pregnancy. Comparisons between normal pregnancy and preeclampsia. Hypertension. 1994;24:461–6. doi: 10.1161/01.hyp.24.4.461. [DOI] [PubMed] [Google Scholar]
- 12.Bowyer L, Brown MA, Jones M. Forearm blood flow in pre-eclampsia. BJOG. 2003;110:383–91. [PubMed] [Google Scholar]
- 13.Clapp JF, 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997;80:1469–73. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 14.Yokose M, Mihara T, Sugawara Y, Goto T. The predictive ability of non-invasive haemodynamic parameters for hypotension during caesarean section: A prospective observational study. Anaesthesia. 2015;70:555–62. doi: 10.1111/anae.12992. [DOI] [PubMed] [Google Scholar]