Abstract
Interventional endoscopic ultrasonography (EUS) is currently becoming the less invasive therapeutic approach for the drainage of pancreatic fluid collections, of acute cholecystitis in patients unfit for surgery and for biliary drainage after failed endoscopic retrograde cholangiopancreatography. In addition, EUS-guided gastroenterostomy (EUS-GE) has recently emerged as a feasible procedure to treat patients with gastric outlet obstruction, as an alternative to surgery or to standard endoscopy when endoscopic stent placement is not possible. Prior animal studies have shown that the procedure is safe and can create a stable anastomosis. However, the major challenge in translating the results of the animal studies into clinical practice is represented by the proper identification of the distal duodenal or proximal jejunal loop to be accessed in order to create the anastomosis. Currently, there are three EUS-GE techniques available: the direct EUS-GE technique, assisted EUS-GE technique, and its variant called the EUS-guided double-balloon-occluded gastrojejunostomy bypass. The present review describes the current EUS-GE techniques, depicts the different procedural aspects of the procedure, and presents the clinical evidences available so far, with a focus on the future perspectives of this EUS-guided technique.
Keywords: Endoscopic ultrasonography, endoscopic ultrasonography-guided gastroenterostomy, endoscopic ultrasonography-guided gastroduodenostomy, endoscopic ultrasonography-guided gastrojejunostomy, gastric outlet obstruction
INTRODUCTION
Endoscopic ultrasonography (EUS) is naturally evolving from a pure diagnostic procedure into a more therapeutic one. Drainage of pancreatic fluid collections, malignant biliary obstruction after failure of endoscopic retrograde cholangiopancreatography, and the gallbladder in high surgical risk patients with acute cholecystitis have become accepted indications.[1] More recently, EUS-guided gastroenterostomy (EUS-GE) has emerged as a feasible procedure, alternative to surgery in patients with gastric outlet obstruction (GOO) in whom endoscopic stent placement is not feasible.[2] The technique was first presented in an animal model by Fritscher-Ravens et al. in 2002,[3] but required for completion endoscope exchange and the use of special devices; thus, it was judged too cumbersome to be translated into clinical practice.
Recently, the development of a lumen-apposing self-expandable fully covered metal stent (LA-SEMS) (AXIOS™, Boston Scientific Corp., Marlborough, MA, USA) able to safely tie together two juxtaposed luminal structures has brought new insights into the development of the EUS-GE procedure.[4] The stent is made up of braided nitinol that is fully covered with silicone, with wide flanges on both ends that provide anchoring between the two adjacent structures [Figure 1]. The 15 mm diameter, 10 mm length stent that is the largest available is delivered through a 10.8 French catheter, which is Luer-locked to the inlet port of the echoendoscope's working channel to provide fully controlled deployment of the stent by the endoscopist. When fully expanded, the anchor flanges have a diameter of 24 mm that is almost double than that of the saddle section and have a design that allows an even distribution of the pressure on the luminal wall, thus securely anchoring the stent and preventing its migration. Since the beginning of 2013, the AXIOS™ stent has been incorporated into a novel device, the AXIOS-EC™, in which an electrocautery wire has been added to the distal tip of the delivery catheter, which by applying cutting current allows penetration of the device through the gastrointestinal wall and the target structure without the need for prior dilation.
Figure 1.
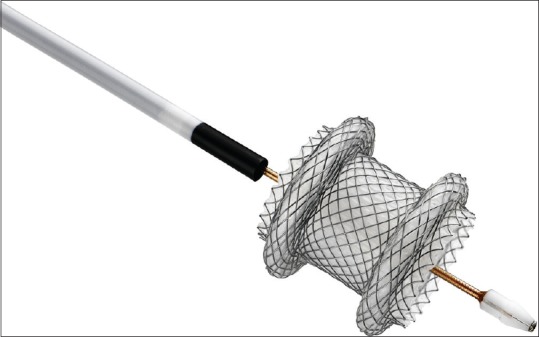
The novel developed lumen-apposing self-expanding metal stent incorporated into an electrocautery-enhanced device. Fully opened, it has wide flanges that provide tissue apposition, preventing stent migration
In 2012, Binmoeller and Shah[5] presented the results of the use of this newly developed LA-SEMS in performing gastrojejunostomy in five pigs. The procedure was technically successful in all animals with no bleeding or perforation. In one acute experiment, necropsy showed good placement of the stent with no tissue injury. In the four survival cases, all the stents remained patent, and the animals displayed normal eating behavior without signs of sepsis. All stents were removed without tissue trauma at 4.5 weeks (n = 3) or 5.5 weeks (n = 1), leaving a mature anastomotic tract, which was easily passed with a regular endoscope. Complete adhesion between the stomach and small bowel at the gastroenterostomy site was demonstrated at necropsy in all survival cases.
The main difficulty and major challenge in translating the results of animal studies into clinical practice is represented by the identification of the proper distal duodenal or proximal jejunal loop to be accessed from the gastric body in order to create the anastomosis. Because of this difficulty, the authors of the first published experience using the AXIOS™ stent to perform a gastroenterostomy utilized a natural orifice transluminal endoscopic surgery (NOTES) approach, with promising results.[6] Initial access to the peritoneal space was performed under EUS guidance in the gastric region close to the ligament of Treitz. After proper dilation of the fistulous tract, the peritoneal space was entered from the stomach over the guidewire by a double-channel endoscope. Then, the operator needed to recognize the different anatomical structures and between them to choose a proximal jejunal loop to create the anastomosis. This task can be very difficult even for expert endoscopists who are not trained for it, except if they are surgeons. Consequently, this has led to a barrier in the dissemination of this proposed approach. Interestingly, after proper recognition of the bowel structure, the procedure was then continued similarly to the EUS-guided approach: a 19-gauge needle was used to puncture the loop, followed by contrast injection and guidewire placement, then exchanged with an AXIOS-EC™ device that was utilized to cautery dilate the tract, followed by distal flange deployment into the bowel lumen, withdrawal of the endoscope and the bowel loop toward the gastric cavity where the proximal flange was finally deployed.
The EUS-guided approach to perform gastroenterostomy overcomes the need for a NOTES approach and is based on the capability of recognizing the distal duodenum or the proximal jejunum, which usually lay very close to the gastric wall at the level of the ligament of Treitz.[7]
TERMINOLOGY AND INDICATIONS
EUS-GE can be performed by puncturing the third or fourth portion of the duodenum (gastroduodenostomy). It represents an attractive alternative to surgical bypass in symptomatic GOO after failure of endoscopic transstenotic stenting, regardless of the etiology and the size of the stenosis.[2,8] The etiology of GOO and indications and contraindications to perform EUS-GE are shown in Tables 1 and 2, respectively.[2] An absolute contraindication to perform EUS-GE is the presence of a large quantity of ascites, which interfere with the bowel loops adherence and fixation.[2]
Table 1.
Etiology of gastric outlet obstruction
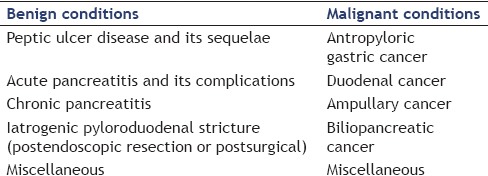
Table 2.
Endoscopic ultrasonography-guided gastroenterostomy procedural indications and contraindications according to the site of obstruction

No food intake or low-residue diet should be established days before the scheduled EUS-GE. On the other hand, in case of large amounts of gastric residue, its endoscopic removal using different devices should be accomplished before performing the procedure.
ENDOSCOPIC ULTRASONOGRAPHY-GUIDED GASTROENTEROSTOMY TECHNIQUES
As a preoperative roadmap, cross-sectional imaging confirmation of the close apposition between the stomach and the distal duodenum/proximal small bowel loops is recommended before attempting the EUS-GE procedure. To perform EUS-GE, it is possible to use two different techniques, the direct approach or the balloon-assisted EUS-GE [Figure 2].
Figure 2.
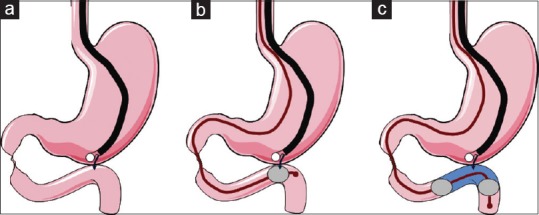
Schematic presentation of the different techniques that are currently used to perform endoscopic ultrasonography-guided gastroenterostomy. (a) Direct access with a standard 19-gauge needle of the bowel loop close to the angle of Treitz from the stomach. (b) The same loop is identified by placing a balloon device at the level of the desired anastomosis. Perforation of the balloon with the needle tip confirms proper positioning. (c) The same bowel loop is identified with the use of a novel occluding device, instillation of fluid between the two occluding balloons making it easily visible by endoscopic ultrasonography
In the direct EUS-GE technique, careful identification of a duodenal or a jejunal loop adjacent to the gastric body is performed first [Figure 2a]. In case where the target bowel loop is poorly distended or has air content that renders its visualization very poor, puncture with a 22-gauge needle is performed and distention of the bowel with injection of saline is done to allow its visualization by ultrasound. Identification of the water-filled bowel segment is then easily accomplished with the therapeutic linear echoendoscope, which is followed by puncture with a standard 19-gauge EUS-fine-needle aspiration needle. An enterogram is obtained to confirm the correct position and a 0.035” guidewire is then passed through the needle. Dilation of the newly created fistula using dilating balloons or electrocautery devices is then carefully performed over the guidewire followed by placement of the LA-SEMS. Efforts should be made to obtain just the proper dilation to allow penetration of the catheter sheet (10.8-Fr), while avoiding overdilation that carries on the risk of leakage and peritonitis. This step has been overcome by the advent of the novel cautery-enhanced delivery system and stent (AXIOS-EC™), which makes fistula creation and stent placement a one-step procedure, even without the need for guidewire placement after the correct identification of the distended duodenal or jejunal loop.[2]
In the assisted EUS-GE technique, a guidewire is initially placed under endoscopic or radiologic guidance across the luminal stenosis into the distal duodenum or proximal jejunum, followed by over-the-guidewire advancement of a water-instilling device (retrieval/dilating balloon, nasobiliary drain), which is placed in the region where the anastomosis is desired to be created [Figure 2b]. The balloon or the loop is then filled with water or saline, and the procedure is continued as described above for the direct EUS-GE technique.[2]
A different version of the balloon-assisted technique is the one named EUS-guided balloon-occluded gastrojejunostomy bypass (EPASS) for which a special double-balloon enteric tube (Tokyo Medical University type; Create Medic Co., Ltd, Yokohama, Japan) has been created to allow instillation of saline between the two inflated balloons [Figure 2c]. The value of this technique has been firstly documented in an animal study.[9] To place the double-balloon enteric tube, a gastroscope is passed into the third duodenal portion across the stenosis causing GOO. While the guidewire is advanced in the jejunum, the gastroscope is removed. The double-balloon tube is then inserted over the guidewire in combination with a 0.89” dedicated guidewire and placed with one balloon in the duodenum and one in the jejunum. Both balloons are filled with contrast until they become stable in their position. Saline with contrast is then introduced in the space between the two balloons that becomes the site where the EUS-GE is performed [Figure 2c].
Variations of the presented technique can be applied to treat other luminal stenoses of the upper GI tract, such as postoperative complete jejunal obstruction.[10]
AVAILABLE CLINICAL EVIDENCE
Up to now, there are three available studies conducted on ten or more patients.[7,11,12] The first is a multicenter USA experience[11] in ten patients (three with malignant GOO) using in almost all cases the balloon-assisted technique. The authors reported a technical success rate of 90% with one failure and clinical success in all nine patients in whom the procedure was accomplished. No procedure-related adverse events and no recurrence of symptoms during a mean follow-up of 150 days occurred.
The second is a prospective study in twenty patients with malignant GOO.[7] They used the EPASS technique in all patients, which resulted in a 90% technical and clinical success rate. Stent occlusion or migration was not observed in the study cohort of 18 successful stent placement cases during a median follow-up period of 100 days. In the two failed cases, maldeployment of the distal flange of the stent occurred and was recognized immediately after stent deployment by the combination of pneumoperitoneum on fluoroscopy and peritoneal endoscopic visualization through the LA-SEMS. Both of these cases were successfully managed conservatively. The authors thought that stent maldeployment was caused by the guidewire that pushed the jejunum away from the stomach while the catheter sheet was entering the fistula just formed. After they changed the technique to the one-step technique with electrocautery-enhanced stent advancement and deployment using the AXIOS-EC™, no more technical failures were observed.
The third experience is a multicenter international retrospective study in 26 patients (malignant obstruction in 17 cases), in which multiple EUS-GE techniques were utilized.[12] Technical success was achieved in 24 (92%) and clinical success in 22 (85%) of the cases. In two patients, despite a patent anastomosis, symptoms did not improve. One patient died before initiation of the oral nutrition, and one underwent surgery for a suspected perforation. Overall, the rate of adverse events was 11.5%.
More recently, two studies comparing EUS-GE with the standard endoscopic stenting (ES) and surgical gastrojejunostomy (SGJ) became available.[13,14] The first study showed that technical and clinical success were not significantly different: 86.7% EUS-GE versus 94.2% ES (P = 0.2) and 83.3% EUS-GE versus 67.3% ES (P = 0.12), respectively.[13] On the other hand, GOO recurrence and need for reintervention were significantly lower in the EUS-GE group (4.0 vs. 28.6%, [P = 0.015]), even if the size of the endoscopically placed stent was larger (22 or 20 mm in diameter in the ES vs. 15 mm in the EUS arm). Rates of adverse events (16.7 vs. 11.5%, P = 0.5) and severe adverse events (10% vs. 9.6%) were also similar. Of note, in three of the EUS-GE cases, the stents were initially misdeployed in the peritoneum; of these, in two cases, it was possible to remove these stents during the initial procedure and to manage the cases conservatively, while in the other case, surgery was deemed necessary.
In the second study,[14] with a total of 93 patients with malignant GOO (thirty in the EUS-GE arm, in which multiple EUS-guided techniques were used), EUS-GE was compared with SGJ. A major difference in the patients enrolled was that peritoneal carcinomatosis was present in a significantly higher percentage in the EUS-GE group than in the SGJ group (43% vs. 11%). Despite this, and the fact that the technical success rate was lower in the EUS-GE group (87% vs. 100% in the SGJ group), the clinical success rate was not different between the two groups (87% vs. 90%), and there was a lower rate of adverse events in the EUS-GE group versus the SGJ group (16% vs. 25%). In addition, the rate of recurrent GOO (3% vs. 14%) and the mean time to reintervention (88 d vs. 121 d) were not statistically different between the two groups. The conclusion of the authors was that EUS-GE is a noninferior but less invasive approach for patients with malignant GOO compared with surgery.
CONCLUSIONS AND FUTURE PERSPECTIVES
Despite still limited, the available evidence indicates that EUS-GE is a safe and effective minimally invasive treatment modality alternative to surgery in patients with malignant GOO after failure of standard endoscopic transstenotic stenting. It offers long-lasting luminal patency and avoids the occurrence of stent obstruction, with a reasonable procedural risk, without the morbidity associated with a surgical procedure. Moreover, it appears to significantly decrease recurrence of GOO and need for reintervention when compared to standard ES. These indications are based on retrospective studies[13,14] and need to be verified in prospective randomized trials before they can become the standard of care. Similarly, the criteria to perform EUS-GE in patients with benign GOO need to be standardized and should not be performed unless surgery is strictly contraindicated.
The procedure is technically demanding, thus at present, it should be performed only by highly trained endosonographers and experts in interventional EUS. The actual procedure requires filling of the target loops with saline or water to allow for sufficient distention of the small bowel in order to correctly identify it and perform a secure puncture. This task is obtained using a prior needle puncture, a single balloon (balloon-assisted technique), or a double lumen balloon device (EPASS), the latter two not being feasible in complete GOO. Moreover, targeting the highly movable jejunum can be difficult because it easily tents away during needle puncturing, especially when the puncture is performed with a 19-gauge needle that is required to pass a 0.035” guidewire. Similarly, dilation of the fistulous tract can also be difficult for the same reason and may determine leakage of bowel contents into the peritoneum. These challenges seem to be overcome by the use of the AXIOS-EC™ device, the recently developed lumen-apposing SEMS mounted on an electrocautery-enhanced delivery system, which allows penetration and stent placement in a single-step procedure. The advantages of the one-step procedure over the two steps have been reported by Itoi et al., which showed a technical success rate of 100% versus 82%, respectively.[7]
Currently, available LA-SEMSs have a maximum diameter that does not seem to be completely appropriate for EUS-GE, where a bigger anastomosis is usually required. A 20 mm diameter stent will be soon available to overcome this limitation of the actual LA-SEMS. Their design with a short central portion and wide biflanges, which minimizes the risk of stent obstruction and migration, seems appropriate to develop a tight anastomosis. However, the duration of stent placement that determines the formation of a permanent patent gastroenteric fistula after removal of the stent is still unknown.
No doubt that refinement of the stent and expansion of dedicated accessories to perform EUS-GE are needed to develop a complete armamentarium dedicated to the procedure. Nevertheless, EUS-GE is here to stay with possible future expansion of indications to patients with obesity and diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
Alberto Larghi and Guido Costamagna are consultants for Boston Scientific Corp. The other author has no relevant competing interests to declare.
REFERENCES
- 1.Fuccio L, Attili F, Vanella G, et al. Interventional endoscopic ultrasonography. Curr Treat Options Gastroenterol. 2014;12:183–210. doi: 10.1007/s11938-014-0015-x. [DOI] [PubMed] [Google Scholar]
- 2.Itoi T, Baron TH, Khashab MA, et al. Technical review of endoscopic ultrasonography-guided gastroenterostomy in 2017. Dig Endosc. 2017;29:495–502. doi: 10.1111/den.12794. [DOI] [PubMed] [Google Scholar]
- 3.Fritscher-Ravens A, Mosse CA, Mills TN, et al. Athrough-the-scope device for suturing and tissue approximation under EUS control. Gastrointest Endosc. 2002;56:737–42. doi: 10.1067/mge.2002.129084. [DOI] [PubMed] [Google Scholar]
- 4.Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–6. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: A porcine study. Endoscopy. 2012;44:499–503. doi: 10.1055/s-0032-1309382. [DOI] [PubMed] [Google Scholar]
- 6.Barthet M, Binmoeller KF, Vanbiervliet G, et al. Natural orifice transluminal endoscopic surgery gastroenterostomy with a biflanged lumen-apposing stent:First clinical experience (with videos) Gastrointest Endosc. 2015;81:215–8. doi: 10.1016/j.gie.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Itoi T, Ishii K, Ikeuchi N, et al. Prospective evaluation of endoscopic ultrasonography-guided double-balloon-occluded gastrojejunostomy bypass (EPASS) for malignant gastric outlet obstruction. Gut. 2016;65:193–5. doi: 10.1136/gutjnl-2015-310348. [DOI] [PubMed] [Google Scholar]
- 8.Itoi T, Tsuchiya T, Tonozuka R, et al. Novel EUS-guided double-balloon-occluded gastrojejunostomy bypass. Gastrointest Endosc. 2016;83:461–2. doi: 10.1016/j.gie.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Itoi T, Itokawa F, Uraoka T, et al. Novel EUS-guided gastrojejunostomy technique using a new double-balloon enteric tube and lumen-apposing metal stent (with videos) Gastrointest Endosc. 2013;78:934–9. doi: 10.1016/j.gie.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Majmudar K, Wagh MS. EUS-guided jejuno-jejunostomy with lumen-apposing metal stent for complete jejunal obstruction after gastric bypass. Gastrointest Endosc. 2016;84:853–4. doi: 10.1016/j.gie.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Khashab MA, Kumbhari V, Grimm IS, et al. EUS-guided gastroenterostomy: The first U.S. clinical experience (with video) Gastrointest Endosc. 2015;82:932–8. doi: 10.1016/j.gie.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Tyberg A, Perez-Miranda M, Sanchez-Ocaña R, et al. Endoscopic ultrasound-guided gastrojejunostomy with a lumen-apposing metal stent: A multicenter, international experience. Endosc Int Open. 2016;4:E276–81. doi: 10.1055/s-0042-101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YI, Itoi T, Baron TH, et al. EUS-guided gastroenterostomy is comparable to enteral stenting with fewer re-interventions in malignant gastric outlet obstruction. Surg Endosc. 2017;31:2946–52. doi: 10.1007/s00464-016-5311-1. [DOI] [PubMed] [Google Scholar]
- 14.Khashab M, Bukhari M, Baron T, et al. International multicenter comparative trial of endoscopic ultrasonography-guided gastroenterostomy versus surgical gastrojejunostomy for the treatment of malignant gastric outlet obstruction. Endosc Int Open. 2017;5:E275–81. doi: 10.1055/s-0043-101695. [DOI] [PMC free article] [PubMed] [Google Scholar]


