Abstract
Pancreatic adenocarcinoma may account for more than 80% of all pancreatic neoplasms. Occasionally, other rare tumors such as lymphoma, metastatic tumor, and solid pseudopapillary neoplasm can be considered in the differential diagnosis. We report the case of an 82-year-old man with a pancreatic solid mass. This case suggests that endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA) with biopsy, that is, EUS-FNA is recommended in the differential diagnosis of the pancreatic solid mass apart from pancreatic adenocarcinoma. In particular, the histologic core obtained by EUS-guided biopsy is helpful for the immunostaining of molecular markers to confirm the final diagnosis.
Keywords: Endoscopic ultrasound (EUS), fine-needle aspiration (FNA), pancreatic neoplasm, plasmacytoma
INTRODUCTION
Pancreatic cancer usually refers to pancreatic adenocarcinoma, which may account for more than 80% of all pancreatic neoplasms. Occasionally, other rare diseases presenting as solid pancreatic masses can be considered as a differential diagnosis. Histological confirmation is required in this situation. Since the development of the linear-array echoendoscope, endoscopic ultrasound (EUS)-guided fine-needle aspiration (FNA), that is, EUS-FNA has emerged as the primary modality for histological diagnosis, with high accuracy and tolerability.[1] We report a case of pancreatic mass that was mimicking pancreatic cancer initially and was later diagnosed as extramedullary plasmacytoma by EUS-FNA and EUS-guided fine-needle aspiration and biopsy (EUS-FNAB).
CASE REPORT
An 82-year-old man presented with fever, which had persisted for more than 1 week. Laboratory investigations revealed anemia with a hemoglobin level of 11.8 g/dL accompanied by leukocytosis with a white blood cell (WBC) count of 12,470/μL. The source of fever could not be localized clinically. Contrast-enhanced abdominal computerized tomography (CT) showed a heterogeneous enhancing mass in the pancreas body and upstream dilatation of the main pancreatic duct [Figure 1a]. Magnetic resonance imaging (MRI) of the pancreas showed a mass with T2-weighted higher signal intensity than the liver parenchyma, accompanied by diffusion restriction, suggestive of pancreatic ductal adenocarcinoma [Figure 1b].
Figure 1.
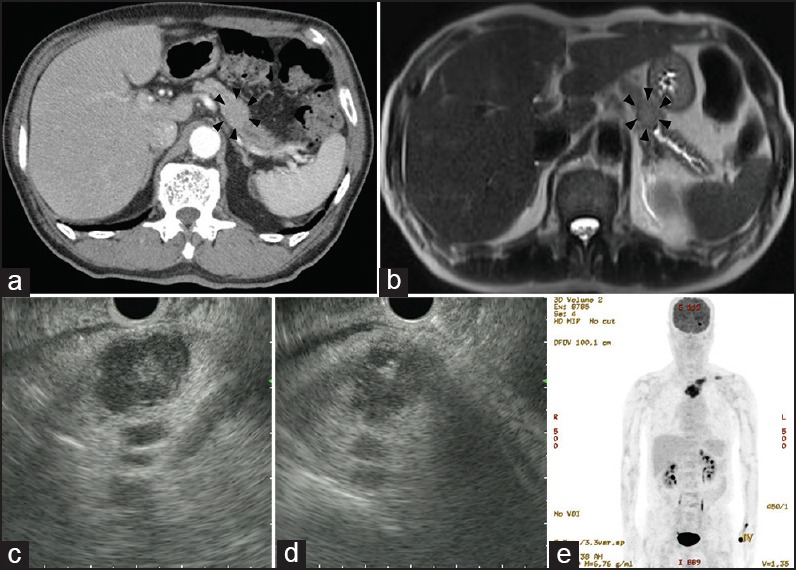
(a) Heterogeneously enhanced low-attenuation lesion accompanied by focal pancreatic ductal dilatation is observed at the pancreatic body in contrast-enhanced abdomen-pelvis CT (b) MRI of the pancreas showed a mass with T2-weighted higher signal intensity than the liver parenchyma accompanied by diffusion restriction (c) EUS showed an approximately 24-mm-sized, well-defined hypoechoic, and heterogeneous mass in the pancreatic body. The margins were clear, and a more hyperechoic rim than the echo level of the center was noted around the mass (d) EUS-FNA and EUS-FNAB was performed targeting the lesion (e) PET-CT showed strong FDG uptake at the pancreatic body, and multiple osteolytic lesions in the left clavicle, the left sternoclavicular junction, sternum, and the left posterior orbital wall
For further evaluation of the pancreatic mass, EUS was performed. During scanning of the pancreas from the stomach using a linear-array echoendoscope (GF-UCT240, Olympus Optical Co., Tokyo, Japan), an approximately 24-mm-sized, well-defined hypoechoic and heterogeneous mass was seen in the pancreatic body [Figure 1c]. This finding was different from the usual pancreatic cancers as the margins were clear and a more hyperechoic rim than the echo level of the center was noted around the mass. EUS-FNA with biopsy was performed targeting the mass using a 22-gauge EchoTip needle (Cook Endoscopy, Winston-Salem, NC, USA) [Figure 1d].
A positron emission tomography-computed tomography (PET-CT) scan was performed to evaluate operability. The PET-CT findings showed strong F-18 fluorodeoxyglucose (FDG) uptake at the pancreatic body and multiple osteolytic lesions in the left clavicle, the left sternoclavicular junction, sternum, and the left posterior orbital wall [Figure 1e].
EUS-FNA yielded a moderately cellular smear with loosely clustered or isolated tumor cells in addition to normal pancreatic acinar cells and ductal cells [Figure 2a]. The tumor cells showed plasmacytoid appearance with abundant finely granular cytoplasm, eccentric nuclei, and indistinct nucleoli [Figure 2b]. Binucleated forms and mitotic figures were also observed [Figure 2c]. Ancillary immunostaining was performed using the EUS-FNAB specimen. The tumor cells were positive for CD138 (cluster of differentiation 138 or syndecan-1) and negative for cytokeratin, leukocyte common antigen, synaptophysin, HMB45 (human melanoma black 45), and beta-catenin; these results were consistent with a diagnosis of plasmacytoma [Figure 2d]. In addition, marrow aspiration was performed at the sternal region, which showed increased uptake in the PET-CT. Multiple myeloma was diagnosed based on the finding of proliferation of the plasma cells.
Figure 2.
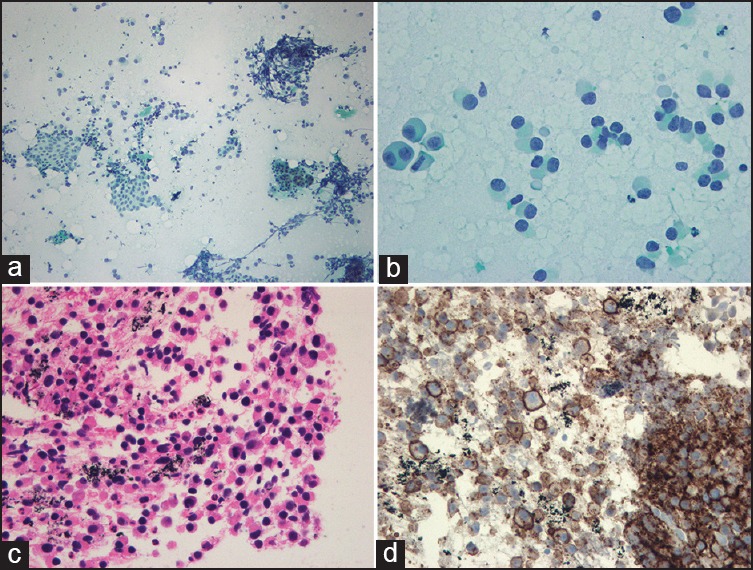
Pathological findings (a) FNA cytology smear showed a moderately cellular tumor cell population along with normal pancreatic acinar cells and ductal cells (Papanicolaou stain, 100×) (b) At higher magnification, tumor cells showed plasmacytoid appearance with eccentric nuclei and abundant deep basophilic cytoplasm (Papanicolaou stain, 400×) (c) The biopsy specimen demonstrates a sheet of plasmacytoid cells with mild anisonucleosis and mitotic activity (hematoxylin and eosin, 200×) (d) The tumor cells are diffusely positive for plasma cell marker (CD138) (200×)
Serum protein electrophoresis demonstrated a monoclonal spike in the gamma globulin region, which was identified as an immunoglobulin G (IgG) lambda light chain protein. The serum β2-microglobulin level was 1.73 mg/dL, which was within the normal range.
DISCUSSION
In resectable pancreatic cancers, pathological confirmation is not mandatory if preoperative chemoradiotherapy is not indicated. In cases where the clinical manifestation or imaging findings are atypical, a pathologic evaluation is required to differentiate it from other solid pancreatic tumors such as neuroendocrine tumor, mass-forming chronic pancreatitis, autoimmune pancreatitis, lymphoma, solid pseudopapillary neoplasm, and other metastatic tumors. EUS-FNA is a safe and well-established technique for the histological confirmation of pancreatic cancers. The adverse events after EUS-FNA include pancreatitis, bleeding, perforation, and needle-tract seeding. However, the rate of major adverse events is low, at approximately 1.1-3.0%.[2,3,4,5] Although the possibility of tract seeding after EUS-FNA is very low, it is a major concern in resectable pancreatic cancer, especially when the tumor is located in the body or tail of the pancreas. Two cases of pancreatic adenocarcinoma seeding through EUS-FNA have been reported, both of which involved the pancreatic tail region.[4] When EUS-FNA is performed targeting the pancreas body or tail, the needle should pass through the stomach wall where the aspiration route will not be included in the resection area.[5] Although cancer seeding after EUS-FNA in pancreatic head masses is also possible, no case has been reported yet as the puncture site of the duodenum and potential sites of tract seeding are resected during operation. In this case, the mass was located in the body of the pancreas and the pancreatic mass had been initially thought to be resectable. Therefore, EUS-FNA carried some risk of tract seeding. In cases where atypical findings are seen in the EUS, performing EUS-FNA regardless of the position of the mass in the pancreas will be of benefit in the identification of other malignancies, such as lymphoma or other metastatic cancers, thus avoiding any unnecessary operations.
It is hard to define the EUS characteristics of extramedullary plasmacytoma of the pancreas because there are only a few case reports about pancreatic plasmacytomas diagnosed by using EUS.[2,6,7,8] From our case report and literature review, extramedullary plasmacytoma of the pancreas usually shows a well-demarcated hypoechoic, heterogeneous mass, which is similar to that of metastatic pancreatic tumors.[2,6,7,8,9] The key EUS feature that can differentiate plasmacytomas from pancreatic adenocarcinoma is the well-defined margin of the tumor.[10,11] A pancreatic neuroendocrine tumor presents as a round, homogeneous, hypo- or isoechoic mass on EUS.[12] Plasmacytomas of the pancreas usually show a more uneven margin and more heterogeneous echogenicity than neuroendocrine tumors. However, it is still challenging to differentiate pancreatic tumors only by EUS findings. In the last decade, in an attempt to overcome some of the limitations of EUS-FNA, alternative sampling techniques to obtain tissue biopsy specimens for histologic examination under EUS guidance have been developed. Obtaining core biopsy specimens is important when architectural features are essential for the pathologic diagnosis or when a large number of cells are needed to confirm the malignancy potential of the tumors.[13] Therefore, EUS-guided tissue acquisition can be a useful diagnostic method when a core biopsy tissue is necessary for a pathological confirmation.[14,15] In the present case, based on the EUS-FNA cytology findings, other tumors demonstrating dispersed plasmacytoid cell patterns, such as pancreatic neuroendocrine tumor, melanoma, and lymphoma with plasmacytic differentiation, could be considered in the differential diagnosis. Additional immunostaining using the FNAB specimen was helpful in confirming the diagnosis.
Multiple myelomas account for more than 10% of all hematological malignancies, the second highest prevalence among hematological malignancies. Extramedullary plasmacytoma is rare, accounting for only approximately 5% of plasma cell neoplasms. Pancreatic involvement in extramedullary plasmacytoma is very rare, being found in only approximately 2.3% of all autopsies.[2,7,16] Our experience suggests that differential diagnosis including extramedullary plasmacytoma could be considered when pancreatic masses show atypical imaging findings. If ambiguous findings are shown, pathological confirmation with EUS-FNAB will be helpful.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass. A meta-analysis and systematic review? Pancreas. 2013;42:20–6. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 2.Roh YH, Hwang SY, Lee SM, et al. Extramedullary plasmacytoma of the pancreas diagnosed using endoscopic ultrasonography-guided fine needle aspiration. Clin Endosc. 2014;47:115–8. doi: 10.5946/ce.2014.47.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hue SS, Azhar R. Plasmacytoma of the pancreas: An unusual manifestation of multiple myeloma. Singapore Med J. 2013;54:e105–7. doi: 10.11622/smedj.2013066. [DOI] [PubMed] [Google Scholar]
- 4.Chong A, Venugopal K, Segarajasingam D, et al. Tumor seeding after EUS-guided FNA of ancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933–5. doi: 10.1016/j.gie.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalo-Marin J, Vila JJ, Perez-Miranda M. Role of endoscopic ultrasound in the diagnosis of pancreatic cancer. World J Gastrointest Oncol. 2014;6:360–8. doi: 10.4251/wjgo.v6.i9.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artifon EL, Okawa L, Baba ER, et al. Diagnosis of pancreatic plasmacytoma by endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2011;43(Suppl 2 UCTN):E79–80. doi: 10.1055/s-0030-1255767. [DOI] [PubMed] [Google Scholar]
- 7.Akyuz F, Sahin D, Akyuz U, et al. Rare pancreas tumor mimicking adenocarcinoma: Extramedullary plasmacytoma. World J Gastrointest Endosc. 2014;6:99–100. doi: 10.4253/wjge.v6.i3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padda MS, Milless T, Adeniran AJ, et al. Pancreatic and gastric plasmacytoma presenting with obstructive jaundice, diagnosed with endoscopic ultrasound-guided fine needle aspiration. Case Rep Gastroenterol. 2010;4:410–5. doi: 10.1159/000321050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWitt J, Jowell P, Leblanc J, et al. EUS-guided FNA of pancreatic metastases: A multicenter experience. Gastrointest Endosc. 2005;61:689–96. doi: 10.1016/s0016-5107(05)00287-7. [DOI] [PubMed] [Google Scholar]
- 10.Johnson EA, Benson ME, Guda N, et al. Differentiating primary pancreatic lymphoma from adenocarcinoma using endoscopic ultrasound characteristics and flow cytometry: A case-control study. Endosc Ultrasound. 2014;3:221–5. doi: 10.4103/2303-9027.144530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J Gastroenterol. 2014;20:7864–77. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jani N, Khalid A, Kaushik N, et al. EUS-guided FNA diagnosis of pancreatic endocrine tumors: New trends identified. Gastrointest Endosc. 2008;67:44–50. doi: 10.1016/j.gie.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 13.Paik WH, Park Y, Park do H, et al. Prospective evaluation of new 22 gauge endoscopic ultrasound core needle using capillary sampling with stylet slow-pull technique for intra-abdominal solid masses. J Clin Gastroenterol. 2015;49:199–205. doi: 10.1097/MCG.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 14.Fuccio L, Larghi A. Endoscopic ultrasound-guided fine needle aspiration: How to obtain a core biopsy? Endosc Ultrasound. 2014;3:71–81. doi: 10.4103/2303-9027.123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EY. Fine-needle biopsy: Should this be the first choice in endoscopic ultrasound-guided tissue acquisition? Clin Endosc. 2014;47:425–8. doi: 10.5946/ce.2014.47.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiller N, Goitein O, Ashkenazi YJ. Plasmacytoma of the pancreas. Isr Med Assoc J. 2004;6:704–5. [PubMed] [Google Scholar]


