Abstract
Background
There are only two single case reports describing double-balloon enteroscopy (DBE)-assisted endoscopic mucosal resection (EMR) of the jejunum. The aim of this case series was to evaluate the feasibility and utility of DBE-assisted EMR in patients with familial and non-familial jejunal polyps.
Patients and methods
Observational, open-label, retrospective, single-arm case series in two hospitals.
Results
Eight patients underwent DBE assisted jejunal EMR. Median age of patients was 42 years (range 24–62 years), male: female ratio 1.5:1. DBE was done through the antegrade (i.e. oral) route in all patients. Four patients had FAP; two had Peutz-Jeghers syndrome, one had a sporadic adenoma and one had a bleeding jejunal polyp, which on histological examination turned out to be lipoma. 3/8 underwent piece-meal EMR. No immediate adverse events occurred.
Conclusions
This is the first case series presenting the technical details, feasibility and outcomes of EMR of the small bowel. EMR of the jejunum is feasible and safe during DBE.
Keywords: Double-Balloon Enteroscopy, Intestinal Mucosa, Intestinal Polyps, Jejunum
Resumo
Introdução
Existem apenas duas séries clínicas na literatura a descrever os resultados da mucosectomia no jejuno por enteroscopia de duplo balão (DBE). O objetivo desta série de casos foi avaliar a exequibilidade e utilidade da mucosectomia por DBE em doentes com pólipos jejunais familiares e não familiares.
Métodos
Estudo observacional, retrospectivo, open-label, descrevendo uma série de casos em dois hospitais.
Resultados
Oito doentes realizaram mucosectomia por DBE. A idade mediana foi 42 anos (âmbito 24–62 anos), razão homem:mulher 1,5:1. Foi realizada DBE por via anterógrada (oral) em todos os doentes. Quatro doentes tinham polipose adenomatosa familiar (PAF); dois tinham síndroma de Peutz-Jeghers, um tinha um adenoma esporádico e um tinha um pólipo jejunal sangrante, cuja avaliação anatomopatológica revelou tratar-se de um lipoma. A mucosectomia foi fragmentada em 3 dos 8 doentes. Não se verificou nenhum efeito adverso imediato.
Conclusões
Este é o primeiro estudo que descreve os detalhes técnicos, exequibilidade e resultados da mucosectomia no intestino delgado. A mucosectomia no jejuno por DBE é exequível e segura.
Palavras-chave: Enteroscopia de Duplo Balão, Jejuno, Mucosa Intestinal, Pólipos Intestinais
1. Introduction
Endoscopic mucosal resection (EMR) has become a well-accepted and practiced method for treating neoplastic and non-neoplastic lesions of the esophagus, stomach, duodenum and colon.1 Until recently, primary surgical or intraoperative endoscopic resection was the only available means of treating polyps of the mid-small bowel.2 Since the advent of balloon-assisted enteroscopy (including double-balloon, single balloon) endoscopic polypectomy has become a viable option for treatment of small-bowel disorders. Indeed, at present balloon-assisted enteroscopy has become the primary mode for the removal of small bowel polyps. However, all yet published studies have only focused on standard polypectomy techniques.3, 4 Because the small bowel has a thinner wall as compared to other luminal parts of the gastrointestinal (GI) tract, the experience using EMR is very limited. Indeed, there are currently only two case reports describing EMR of the jejunum.5, 6 Therefore, the aim of this case series study was to report on the feasibility and utility of double balloon enteroscopy assisted mucosal resection (EMR) in patients with familial and non-familial jejunal polyps.
2. Patients and methods
Forty-two patients with jejunal polyp(s) (familial adenomatous polyposis syndrome (FAP), n = 17, Peutz-Jeghers syndrome (PJS), n = 12, sporadic adenomas, n = 7, nodular lymphoid hyperplasia, n = 3, lipomas, n = 3) undergoing DBE-assisted resection of their lesions at the Marienhospital Bottrop, University of Magdeburg Medical Center between December 2007 and December 2012 were included and their data recorded in a computerized database. For this case study we only included patients undergoing EMR. EMR was defined as the resection of the entire mucosal layer and part of the submucosa using advanced endoscopic resection techniques (i.e. mucosectomy). Patients undergoing standard snare polypectomy were excluded. The patients provided written informed consent to undergo endoscopy with the double balloon enteroscopy system. Double balloon enteroscopy was performed using the Fujinon enteroscope (Fujinon EN-450T5, Fujifilm, Saitama, Japan). The study was approved by the ethics committee of the University of Magdeburg and conducted out in accordance with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
2.1. Mucosectomy technique
Before resection the mucosal Kudo pit pattern of the lesion was analyzed using high resolution white-light and chromoendoscopy methods (i.e. standard and virtual chromoendoscopy) to better define their surface, borders and assist during resection (Fig. 1).7 The basis for a successful mucosectomy is the creation of a “submucosal cushion”, thus lifting the lesion from the submucosa. The submucosal cushion was created using epinephrine-saline and indigo-carmine solution (1:20,000 epinephrine:saline; 0.1 ml of indigo-carmine 3% in 100 ml of saline). A maximum of 10 ml of this solution was used, as there are reports of bowel ischemia induced by epinephrine.8 If more injection was required to raise the polyp before or during the mucosectomy just normal saline was used. The submucosal cushion was initiated by injecting 1–3 ml of epinephrine-saline solution to the most distal part of the lesion. This maneuver places the polyp “en-face” to the endoscopist. Then one or both lateral sides were injected, allowing for a homogenous lifting (Fig. 2). If no lifting was observed mucosectomy was aborted. In addition, no mucosectomy was attempted in patients with inflammatory polyps as perforation is a likely occurrence in these types of lesions and also not indicated (13). Polyps were divided into those <10 mm and those >10 mm. Polyps <10 mm in size were removed en-bloc (Fig. 3), whereas large polyps (>10 mm) were removed using the piecemeal mucosectomy technique (Fig. 4). The resected specimens were captured with the snare itself, a Roth net or with a basket. When performing piecemeal mucosectomy all pieces of the resected lesion were removed through the overtube (Fig. 5). The overtube was left in place to allow for reinsertion and removal of the enteroscope during mucosectomy. One to three clips (Olympus, Hamburg, Germany) were used for closure of defects larger than 20 mm. The aim was to visibly close the defect.
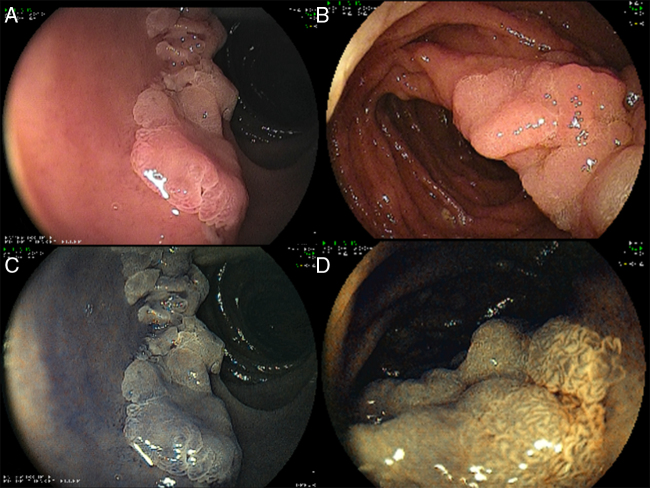
Figure 1.
The mucosal surface was carefully analyzed using high resolution white light endoscopy (A) and standard or virtual chromoendoscopy methods (B). Chromoendoscopy may be useful for determining the borders and extend of the lesion (B).
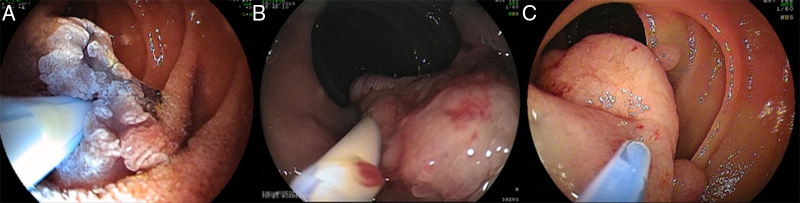
Figure 2.
The submucosal cushion serves many purposes: (a) it raises the lesion making it more visible (A), it lifts up the lesion from hiding folds (B), (c) it separates the lesion form the deeper mucosal layers potentially decreasing the risk of perforation during snare resection (C).
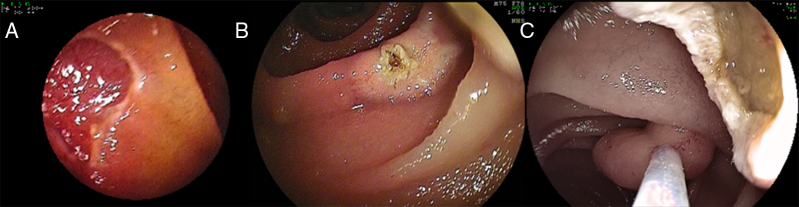
Figure 3.
Polyps <15 mm in size were removed en-bloc (A). EMR differs from standard polypectomy in the depth of resection. Whereas during standard polypectomy the polyp is resected near its base at the mucosal level (B), during EMR a deeper resection ensues (C). The injection of submucosal substance (“submucosal cushion”) allows for a deeper and likely safer resection (A and C).
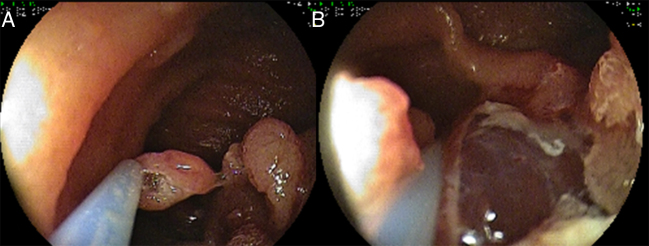
Figure 4.
Large sessile polyps (>10 mm) were removed using the piecemeal technique (A and B).
Figure 5.
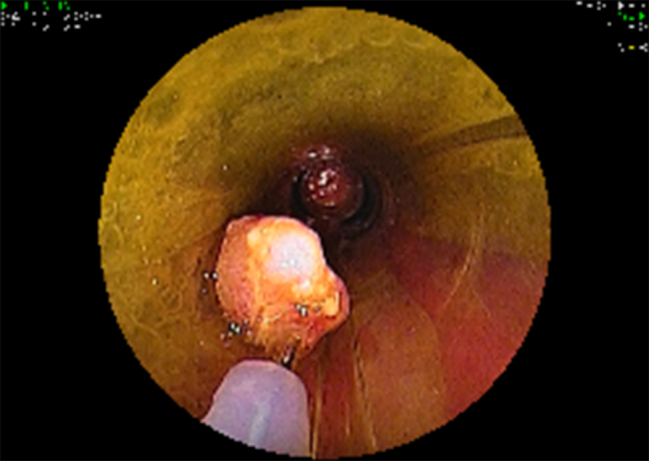
When performing piece meal mucosectomy all pieces of the resected lesion were removed through the overtube.
A total of eight patients underwent double-balloon-assisted jejunal EMR. The median age of patients was 42 years (range 24–62 years), male:female ratio 1.5:1 (Table 1). BAE was done through the antegrade (i.e. oral) route in all patients. Four patients had FAP; two had PJS, one had a sporadic adenoma and one had a bleeding jejunal polyp, which on histological examination turned out to be lipoma. The two patients with PJS had sessile lesions. The mean size of the lesions was 20 mm, range 10–30 mm. Thirty seven percent of patients (3/8) underwent piece-meal EMR. In FAP all lesions <15 mm could be resected in one piece, whereas lesions >20 mm were resected using piece-meal EMR technique. In contrast, in PJS lesions up to 25 mm could be resected in one piece using EMR technique. Post-EMR adverse events like perforation, bleeding or pancreatitis were not observed in any of these patients.
Table 1.
Clinical, demographic and endoscopic findings.
| N | Age | Sex | Route | Indication | Finding | Type of polyp(s) and size | Endoscopic/technical aspects |
|---|---|---|---|---|---|---|---|
| 1 | 24 | M | Oral | FAP | Diminutive polyps in the duodenum, one 10 mm jejunal polyp | Adenomatous, LGIN (10 mm) | EMR, one piece |
| 2 | 54 | F | Oral | FAP | 25 small polyps and one 30 mm proximal jejunal polyp | Adenoma, HGIN (30 mm) | Piece-meal mucosectomy |
| 3 | 45 | M | Oral | FAP | 20 polyps in duodenum, one 20 mm in jejunum | Adenoma (15 mm) | EMR, one piece |
| 4 | 28 | M | Oral | FAP | 15 polyps in duodenum, one 20 mm in jejunum | Adenoma (20 mm) | Piece-meal mucosectomy |
| 5 | 39 | F | Oral | Adenoma | One 25 mm in jejunum | Adenoma (25 mm) | Piece-meal mucosectomy |
| 6 | 62 | M | Oral | PJS | Multiple jejunal polyps, ranging from 5 mm to 25 mm | Sessile, broad based, hamartoma (25 mm) | EMR, one piece |
| 7 | 36 | M | Oral | PJS | Multiple jejunal polyps, ranging from 6 mm to 30 mm | Sessile, broad based, hamartoma (20 mm) | EMR, one piece |
| 8 | 58 | F | Oral | OGIB | Bleeding polyp | Lipoma (20 mm) | EMR, one piece |
DBE: double balloon enteroscopy; EMR: endoscopic mucosal resection; FAP: familial adenomatous polyposis syndrome; HGIN: high-grade intraepithelial neoplasia; LGIN: low-grade intraepithelial neoplasia; OGIB: obscure gastrointestinal bleeding; PJS: Peutz-Jeghers syndrome.
3. Discussion
Although resection of small bowel polyps using standard polypectomy techniques is now performed routinely in many centers we are not aware of previous studies describing advanced resection methods such as EMR for jejunal or ileal polyps. Indeed, there are only two case reports describing the role of EMR of the jejunum.5, 6 In a series describing endoscopic resection in patients with PJS the concept of injection-assisted endoscopic resection (i.e. EMR or mucosectomy) was also described.9 In our center EMR was performed in 19% of patients with small bowel polyps without any complications. Therefore, we believe that our study is important as it dwells into the practice of advanced endoscopic resection methods within the small bowel. Our report may also be of clinical impact as we provide a description of small bowel EMR, carefully describing the endoscopic technique, which may be useful to avoid complications. It is well known that resection of small bowel polyps is associated with higher risks of perforation or bleeding when compared with polyps form other parts of the luminal GI tract. The complications rate associated with small bowel polypectomy can be as high as 5%.10, 11 Thus, careful utilization of the submucosal cushion and piece-meal EMR technique should be used for flat or broad-based lesions. Furthermore, judicious use of epinephrine is mandatory as there are case reports of small bowel necrosis.11 Spiral enteroscopy is another deep enteroscopy technique that allows for therapeutic interventions. Whether its usefulness for endoluminal resections has advantages over DBE or SBE is unknown. We also believe that small bowel interventions should only be performed by a therapeutic endoscopist who also has undergone dedicated training in small bowel techniques. In our opinion, the endoscopist performing small bowel resections should be an expert colonoscopist. Unfortunately, there is not a minimum number to decide who can perform this or not. To us, it mainly depends on the skill and, more importantly on the concept and understanding that the small bowel is different and much more care should be applied to endoscopic resections here.
There are several potential explanations that might account for the higher incidence of complications during small bowel polypectomy. First, the thinner wall of the small intestine may be more prone to perforation. Second, the difficulty in maneuvering the enteroscope within the small bowel may limit the application of the snare around the lesion. And lastly, inexperience in therapeutic small bowel enteroscopy may also increase the chance of inducing complications. Thus, a careful and methodical approach to small bowel polyp resection is mandatory. We hypothesize that in our series the complication rate was non-existent due to a combination of several factors including advanced endoscopic training, cautious use of epinephrine, avoiding the resection of atypical appearing lesions and the use of submucosal cushion technique. We also want to emphasize that occasionally the endoscopist might be tempted to remove inconspicuous appearing lesions. A perforation of the small bowel is likely to happen when attempting the resection of inflammatory pseudopolyps, small bowel duplication cysts or intussusception. In addition, failure to use advanced resection methods may also lead to perforation.10, 11
We want to acknowledge potential limitations of this case series. First, the number of cases is relatively small. Nonetheless, our study represents the largest experience performing EMR of the small bowel. Second, the study is retrospective and has the inherent deficits of such a study design. However, our database is set up prospectively, thus diminishing the potential bias of retrospective studies. Finally, the study was performed by experienced therapeutic endoscopists. Therefore, the findings may not be applicable to other centers. However, we believe that advanced small bowel therapeutics should only be performed by expert centers were these skills are available, as perforation of the small bowel can be a devastating medical catastrophe. In most patients with small bowel polyps these lesions are usually diagnosed during a routine study such as capsule endoscopy. Thus, their therapeutic endoscopic procedure can be scheduled electively.
In summary, in this case series we have shown that small bowel EMR is feasible and safe when as a strict endoscopic resection approach is followed.
Ethical disclosures
Protection of human and animal subjects
The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data
The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Soetikno R., Kaltenbach T., Yeh R., Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 2.Lin B.C., Lien J.M., Chen R.J., Fang J.F., Wong Y.C. Combined endoscopic and surgical treatment for the polyposis of Peutz-Jeghers syndrome. Surg Endosc. 2000;14:1185–1187. doi: 10.1007/s004640000029. [DOI] [PubMed] [Google Scholar]
- 3.Gao H., van Lier M.G., Poley J.W., Kuipers E.J., van Leerdam M.E., Mensink P.B. Endoscopic therapy of small-bowel polyps by double-balloon enteroscopy in patients with Peutz-Jeghers syndrome. Gastrointest Endosc. 2010;71:768–773. doi: 10.1016/j.gie.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Kopácová M., Bures J., Ferko A., Tachecí I., Rejchrt S. Comparison of intraoperative enteroscopy and double-balloon enteroscopy for the diagnosis and treatment of Peutz-Jeghers syndrome. Surg Endosc. 2010;24:1904–1910. doi: 10.1007/s00464-009-0868-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuno A., Yamamoto H., Kita H., Sunada K., Hayashi Y., Sato H. Double-balloon enteroscopy through a Roux-en-Y anastomosis for EMR of a jejunal adenoma in the afferent duodenal limb. Gastrointest Endosc. 2004;60:1032–1034. doi: 10.1016/s0016-5107(04)02191-1. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki H., Yamada A., Watabe H., Kobayashi Y., Hirata Y., Yamaji Y. Successful treatment of early-stage jejunum adenocarcinoma by endoscopic mucosal resection using double-balloon endoscopy: a case report. Diagn Ther Endosc. 2012:521960. doi: 10.1155/2012/521960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mönkemüller K., Fry L.C., Ebert M., Bellutti M., Venerito M., Knippig C. Feasibility of double-balloon enteroscopy-assisted chromoendoscopy of the small bowel in patients with familial adenomatous polyposis. Endoscopy. 2007;39:52–57. doi: 10.1055/s-2006-945116. [DOI] [PubMed] [Google Scholar]
- 8.Yen H.H., Chen Y.Y., Su W.W., Soon M.S. Intestinal necrosis as a complication of epinephrine injection therapy during double-balloon enteroscopy. Endoscopy. 2006;38:542. doi: 10.1055/s-2006-925184. [DOI] [PubMed] [Google Scholar]
- 9.Serrano M., Mão-de-Ferro S., Pinho R., Marcos-Pinto R., Figueiredo P., Ferreira S., Claro I., Mascarenhas-Saraiva M., Dias-Pereira A. Double-balloon enteroscopy in the management of patients with Peutz-Jeghers syndrome: a retrospective cohort multicenter study. Rev Esp Enferm Dig. 2013;105:594–599. doi: 10.4321/s1130-01082013001000004. [DOI] [PubMed] [Google Scholar]
- 10.Spahn T.W., Kampmann W., Eilers M. Small-bowel perforation after endoscopic resection of a Peutz-Jeghers polyp in an infant using double-balloon enteroscopy. Endoscopy. 2007;39(S1):E217. doi: 10.1055/s-2007-966411. [DOI] [PubMed] [Google Scholar]
- 11.Möschler O., May A., Müller M.K., Ell C., German DBE Study Group Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy. 2011;43:484–489. doi: 10.1055/s-0030-1256249. [DOI] [PubMed] [Google Scholar]