Abstract
Background
Gut microbiota may play a role in non-alcoholic steatohepatitis (NASH). Previous studies showed that prebiotics and probiotics might halt the progression of steatohepatitis.
Aim
To clarify the potential effect of Synbiotic 2000®Forte (Synb) in preventing or ameliorating diet induced steatohepatitis, particularly in fibrosis progression and how this intervention correlates with gut microbiota composition and endotoxinemia.
Methods
Twenty-seven C57BL/6 mice were divided into three groups: chow diet (CD, n = 7); high-fat choline deficient diet (HFCD, n = 10) and HFCD diet supplemented with Synbiotic 2000®Forte (four probiotic strains and four prebiotics mixture) (HFCD + Synb, n = 10). At 6 and 18 weeks, blood samples (lipopolysaccharides assay – LPS), cecal feaces (gut microbiota) and liver tissue (histology) were collected for analysis.
Results
Both HCFD diet mice developed steatohepatitis with ballooning at 6 and 18 weeks, opposite to CD. Comparison of histological scores in HFCD and HFCD + Synb, at 6 and 18 weeks showed no significant difference regarding steatosis, inflammation, or ballooning. Evaluating fibrosis with Sirius Red, and degree of smooth-muscle cell activation, HFCD mice had significantly more fibrosis; addition of Synb significantly reduced fibrosis at 6 weeks and 18 weeks. Serum endotoxin levels were similarly increased in HFCD and HFCD + Synb at week 6; however at week 18 HFCD + Synb had significantly lower endotoxin levels than HFCD. Gut microbiota of HFCD vs CD, showed no significant differences regarding the phyla Firmicutes and Bacteroidetes, either at 6 or 18 weeks; Proteobacteria increased at 6 week (3.3) and 18 week (7.5), while the addition of Synb resulted in a decrease at week 18 (−3.90). Fusobacteria markedly increase at week 18 (10.0), but less so with the addition of Synb (5.2).
Conclusion
Synbiotic 2000®Forte is able to modulate the mouse gut microbiota reducing the degree of fibrosis while simultaneously decreasing endotoxemia.
Abbreviations: NASH, non-alcoholic steatohepatits; Synb, Synbiotic 2000®Forte; CD, control diet; HFCD, high fat choline deficient diet; LPS, lipopolysaccharide; NAFLD, non-alcoholic fatty liver disease; TLR4, toll-like receptor-4
Keywords: Microbiota, Mice, Non-alcoholic Fatty Liver Disease, Prebiotics, Probiotics
Resumo
Introdução
A microbiota intestinal pode ter um papel na esteatohepatite não alcoólica (EHNA). Estudos prévios mostraram que os prebióticos e próbioticos podem interromper a progressão da esteatohepatite.
Objetivo
Clarificar o efeito potencial do Synbiotic 2000®Forte (Synb) na prevenção ou melhoria da esteatohepatite induzida pela dieta, particularmente na progressão da fibrose e como essa intervenção se correlaciona com a microbiota intestinal e endotoxinemia.
Métodos
Vinte e sete ratinhos C57BL/6 foram divididos em 3 grupos: dieta de ração (CD, n = 7); dieta gorda deficiente em colina (HFCD, n = 10) e HFCD dieta suplementada com Synbiotic 2000®Forte (4 cepas de probioticos e mistura de 4 prebióticos), (HFCD + Synb, n = 10). Às 6 e 18 semanas, foi colhido sangue para dosear o lipopolisacárido (LPS); foram também analisadas fezes do cego (microbiota intestinal) e tecido hepático (histologia).
Resultados
Todos os ratinhos sujeitos a dieta HCFD, desenvolveram esteatohepatite com balonização às 6 e 18 semanas, em oposição aos CD. A comparação dos scores histológicos nos HFCD e HFCD + Synb, às 6 e 18 semanas não revelou diferenças significativas em relação à esteatose, inflamação, ou balonização. A avaliação da fibrose através do Sirius Red, e o grau de ativação das células do músculo liso, revelou que os ratinhos HFCD tinham significativamente mais fibrose; a adição do Synb reduziu significativamente a fibrose às 6 e 18 semanas. Os níveis de endotoxina sérica estavam aumentados de forma semelhante às 6 semanas nos HFCD e HFCD + Synb; contudo à semana 18, os HFCD + Synb tinham significativamente menores níveis de endotoxina em relação aos HFCD. A microbiota intestinal dos HFCD vs CD, não revelou diferenças significativas nos filos Firmicutes e Bacteroidetes, quer às 6 ou às 18 semanas; Proteobacteria aumentou às 6 semanas (3.3) e 18 semanas (7.5), enquanto a adição de Synb resultou numa redução à semana 18 (-3.9). Fusobacteria aumentou significativamente à semana 18 (10.2), mas menos com a adição de Synb (5.8).
Conclusão
O Synbiotic 2000®Forte foi capaz de modular a microbiota intestinal do ratinho reduzindo o grau de fibrose, enquanto simultaneamente reduziu a endotoxemia.
Palavras-chave: Microbiota, Ratos, Esteatohepatite Não Alcoólica, Prebióticos, Próbioticos
1. Introduction
Non-alcoholic steatohepatitis (NASH) is a metabolic liver disease of epidemic importance in many countries, and strongly related with the increased prevalence of obesity and features of the metabolic syndrome in western countries.1 It is characterized by the association of hepatic steatosis with liver cell injury, ballooning, lobular inflammation and variable degrees of fibrosis, that can progress to end-stage cirrhosis. Clinical and experimental research suggests that the pathogenesis of NASH involves a multifactorial mechanism by which, in the setting of a fatty liver, altered lipid metabolism, dysregulated cytokine production and oxidative stress cause injury and provoke hepatic inflammation and fibrosis.2
In the last years, there has been a great interest in the role that microbiota may play in liver diseases, particularly in non-alcoholic fatty liver disease.3, 4, 5, 6, 7, 8 In fact, several studies have highlighted the association between small intestinal bacterial overgrowth, increased intestinal permeability and nonalcoholic steatohepatitis (NASH), probably due to increased endotoxinaemia and activation of the Toll-like receptor-4 (TLR4) signalling cascade; studies have focused on the possibility that activation of liver fibrogenesis may depend on the stimulation of stellate cells and Kupffer cells by TLR4.9, 10, 11, 12, 13 An additional possible role for microbiota in NAFLD, is related with the pathogenesis of obesity, since there is evidence that some Gram-anaerobes, such as Bacteroides thetaiotaomicron are able to degrade plant polysaccharides,14 thus increasing the caloric extraction from diet; also, colonization of the gut in experimental models of germ-free mice, induces obesity,15 and in the opposite, microbiota depletion promotes browning of white adipose tissue and reduces obesity.16 Another potential contribution of microbiota to NAFLD relates to suppression of the lipoprotein lipase (LPL) inhibitor, angiopoietin-like 4, hence allowing continued expression of LPL that regulates fatty acid release from triglycerides in the liver.17
Our aim was to clarify the potential effects of the four probiotic strains and four prebiotics mixture (Synbiotic 2000®Forte) in preventing or ameliorating diet induced steatohepatitis, and particularly fibrosis progression. In order to get a model more similar to human NASH we have chosen a moderate high-fat diet (35%) simultaneously choline deficient (HFCD), since it has been shown that adding choline deficiency to a high-fat diet, amplifies liver fat accumulation.18 We also aimed to evaluate how this intervention correlates with microbiota composition and endotoxinemia.
2. Methods
2.1. Animal housing and diet
A total of 27 pathogen-free male C57BL/6 six week-old mice (Harlan Laboratories, Castellar, Spain) weighing 15–20 g were housed in individual cages and kept at a temperature of 22 °C, on a 12:12 h light/dark cycle, in the Portuguese National Institute of Health.
Upon arrival, the mice were acclimated for 14 days prior to being used in experiments, and had ad libitum access to tap water and feeding. This study was reviewed and approved by the National Animal Care Committee from Direcção Geral de Veterinária. All procedures with animals were in compliance with the Care and Use of Laboratory Animals.
Steatohepatitis was induced by a high-fat diet (35% total fat, 54% trans-fatty acid enriched) choline-deficient – HFCD diet, –Harlan Laboratories, Castellar, Spain and Standard chow diet was obtained from (Mmucedola srl – 4RF21 certificate batch: 250202).
2.2. Study design
After acclimatization period, mice were divided into three different groups according the type of diet and product administration: (1) regular chow diet (CD) n = 7; (2) high fat choline deficient diet (HFCD) n = 10; (3) HFCD plus Synbiotic 2000®Forte (HFCD + Synb) n = 10.
Using a 20-gauge ballpoint metal feeding tube (Harvard Apparatus, Inc., Holliston, MA), mice were inoculated intra-gastric, three times/week with either phosphate buffered saline (PBS), CD and HFCD groups or Synbiotic 2000®Forte: 50 mg/mice (4 × 108 bacteria/mouse), HFCD + Synb group.
At week 6, five mice of each group were euthanized. The remaining mice of each group were euthanized at week 18.
2.3. Bacterial viability of Synbiotic 2000®Forte
Each sachet consisted of a combination of 1011 CFU of each of four probiotics: Lactobacillus paracasei: 25%, Lactobacillus plantarum: 25%, Leuconostoc mesenteroides: 25%, Pediococcus pentosaceus: 25% with bioactive fibres, total weight: 10 g, oat bran: 2.5 g, pectin: 2.5 g, resistant starch: 2.5 g, inulin: 2.5 g.
To access the viability of the Synbiotic 2000®Forte mixture, assays in duplicate were performed. After recovery of the lyophilized, the mixture was cultured on Schaedler agar and incubated at 35 °C for 48 h under anaerobic conditions. Colonies were tested for biochemical activities and monitored microscopically by Gram stain. It was observed that the viability of Lactobacillus casei and L. plantarum was 83%, the Pediococus 100% and the Leuconostoc 72%.
2.4. Sampling
At the time of sacrifice by cervical dislocation, after 14 h fasting, total body and liver of each mice was weighed. Blood was collected to perform LPS assay. Liver tissue was processed for histology and cecal faeces were taken to evaluate gut microbiota. Serum and faeces were stored at −80 °C until used.
2.5. Blood analysis
Blood was collected and centrifuged for 10 min at 3000 rpm. Serum samples were used to perform LPS assay using the Cusabio Biotech CO., LTD, Wuhan, China according to the manufacturer's instruction.
2.6. Histology
In each time point, liver mice was obtained and divided into two fragments: one was flash-frozen in liquid nitrogen and kept at −80 °C until analysis. The tissue was homogenized and total proteins were extracted and isolated. The other fragment was placed in a 10% buffered formalin solution. The tissue was routinely processed, paraffin embedded and sectioned at a thickness of 4 μm. Haematoxylin–eosin and Sirius Red staining were performed. For the detection of activated hepatic stellate cells, α-SMA positive cells were immunohistochemically assessed by α-SMA antibody. Immunostaining was performed manually by the peroxidase-indirect-polymer method (Envision Dako – HRP, Code K4011) for 30 min. Sections 2 μm thick were cut, unto Superfrost plus slides, from paraffin-embedded routine tissue blocks. The sections were de-waxed, rehydrated and subjected to epitope antigen retrieval (20 min, 94 °C) with Target Retrieval Solution Low pH 50x Dako Envision™ Flex in a pre-treatment module PTlink (Dako, Model PT 10130). Primary polyclonal rabbit antibody Pan-Actin (Cell Signalling ref.: 4968) was incubated overnight at dilution 1:200. Appendix was used as positive control. For negative control, primary antibody was omitted during the staining. Endogenous peroxidise was blocked with 2% H2O2 in absolute methanol for 10 min. Sections were counterstained with Mayer's haematoxylin.
The haematoxylin–eosin slides were evaluated blindly by an experienced pathologist and graded for steatosis and necro-inflammation as 0 (absent), 1 (sparse or mild, focal), 2 (noticeable) and 3 (severe) and hepatocyte ballooning was graded as 0 (absent), 1 (few ballooned cells), and 2 (prominent). Fibrosis was quantified by histomorphometric analysis of Sirius Red stained slides, using a digital camera (DFC 320, Leica, Germany) coupled to a bright field microscope (DM4000, Leica, Germany). Three randomly selected fields (10× magnification) of Sirius Red-stained slides (5-mm sections) were evaluated at a final magnification of 100×. Images were analyzed using the LAS Image Analysis from LAS – Leica Application Suite software. Using the ‘Image’ tool window, images were prepared for analysis using the ‘Adjust contrast’ tool to optimize contrast and the ‘Threshold’ function to select specific signal. Results were measured in terms of % area occupied by specific staining.19
The same method of histomorphometric analysis was used to quantify activated stellate cells. SMA-positive cells were evaluated in six fields per specimen, which were randomly selected at 200× magnification.
2.7. Gut microbiota
Constituents of cecal microbiota were quantified using Fluorescence in situ hybridization (FISH), targeting the phyla Firmicutes (Staphylococcus, Enterococcus, L. casei/paracasei, L. plantarum, Clostridium, Leuconostoc, Pediococcus, Bacilleacea and Streptococcus), Proteobacteria (Enterobacteriacea), Fusobacteria (Fusobacterium), Actinobacteria (Propionibacterium and Bifidobacterium) and Bacteroidetes (Bacteroides).
2.8. Choosing and preparing probes
Fourteen probes of 16S rRNA-targeted oligonucleotide probes were designed, validated and used to quantify predominant bacterial groups (SABiosciences Corporation, USA).
Sample preparation and fixation: Faeces samples were weighed and diluted in distillate water (1:10,000). One microlitre of each dilution was placed in slides previously washed with 70% ethanol. After that, all samples were fixed by adding 1 μl of 4% paraformaldehyde (Sigma–Aldrich, Sintra-Portugal) at room temperature for 20 min and washed in 3× PBS solution by 2 min, then repeated two more times. Permeabilization: Treatment with 0.05% (p/v) of SDS (sodium dodecyl sulphate) for 20 min and proteinase K (Sigma–Aldrich, Sintra-Portugal) (Cf = 10 μg/mL) for 10 min. Denaturation and hybridization: The probes (200 ng/μL) and samples were simultaneous denaturated in a bath at 80 °C for 3 min and the hybridization was at 42 °C for 4 h. Washes: After the hybridization, slides were immersed in 0.01× SSC (saline-sodium citrate) and BT/BSA (Sigma–Aldrich, Sintra-Portugal) solutions for 10 min each according the temperature (Table 1). DNA colouration and observation: DNA was labelled with DAPI (4,6-Diamino-2 Phenylindole; C16H15N5.2HCl-Vector Laboratories) (1 mg/ml). The slides were observed in a fluorescent microscopy Zeiss Axiovert 200M. This microscopy had simple filters that allowed to see separated the CY3, Texas Red, CY5, FITC and Pacific Blue and DAPI fluorochromes, through the Plan-Neofluar 100× immersion objective.
Table 1.
Probes sequence, flurochomes and hybridization temperature.
| Identification | Sequence | Fluorochome | Hybridization temp. (°C) |
|---|---|---|---|
| Pediococcus pentosaceus | 5′-GCC ATC TTT TAA AAG AAA ACC ATG C-3′ | Cy3 | 48 °C |
| Leuconostoc mesenteroides | 5′-TTT GTG TCA TGC GAC ACT AAG TTT T-3′ | Cy5 | |
| Lactobacillus casei/paracasei | 5′-CGT TCC ATG TTG AAT CTC GGT G-3′ | Pacific Blue | |
| Bacillaceae | 5′-CGT CCA TTR NKG AAG ATT CCC TA-3′ | FITC | |
| Fusobacterium | 5′-CGT CCA TTR NKG AAG ATT CCC TA-3′ | Taxas Red | 53 °C |
| Staphylococcus | 5′-CNC CGA AGR GGD ARN YTC TAT CTC TAG A-3′ | Cy3 | |
| Enterococcus | 5′-TTT CCA AGT GTT ATC CCY YNC TGA NG-3′ | Cy5 | |
| Bacteroides | 5′-CCT TCA CGC TAC TTG GCT GGT TCA G-3′ | Pacific Blue | |
| Enterobacteriacea | 5′-GAC NTY ATG CGG TAT TAG CYA CCG-3′ | FITC | |
| Propionibacterium | 5′-TTG ACC CCG GCG GTC TCC ACT GAG TCC-3′ | Texas Red | 58 °C |
| Clostridium | 5′-GTG GCT TCC TCC NHN GGT ACC GTC ATT ATC-3′ | Cy3 | |
| Lactobacillus plantarum | 5′-TGT TAT CCC CCG CTT CTG GGC AGG TT-3′ | Cy5 | |
| Bifidobacterium | 5′-CTG ATA GGA CGC GAC CCC ATC NCA-3′ | Pacific Blue | |
| Streptococcus | 5′-ACC AAC TAG CTA ATA CAN CGC AGG TNC ATC T-3′ | FITC |
After the bacteria's identification, pictures were taken for each sample. Using a MATLAB program, and entering into account the weight, dilution of each faeces sample used in the assay, as well the picture area, total of each bacteria was quantified.20, 21
2.9. Statistical analysis
General descriptive statistics used all available samples. Bacterial variations were considered only for values greater than 2.0 (2× SD/mean) in at least one of the groups.
Bacteria in which the variations in all groups were lower than this value, were not considered. Results from different groups were compared using the Student's t test or Kruskal–Wallis non-parametric ANOVA. Values of p < 0.05 were considered statistically significant. All statistical analysis was performed using SPSS statistics (version 21).
3. Results
3.1. Body and liver weight
All mice had a body weight increase at each time-point during the study, but no significant differences were found between the study groups. However, both HFCD groups showed at 18 weeks an increased liver weight when compared with CD group, as shown in Table 2. The addition of Synb did not abrogate this increase in liver weight observed in mice undergoing HFCD diet.
Table 2.
Body weight and liver weight at different time points.
| Group | Week | Body weight (g) | Mean weight gain | Liver weight (g) | Liver/total body weight ratio (%) |
|---|---|---|---|---|---|
| CD | 0 | 18.6 | |||
| 6 | 23.4 ± 1.6 | 5.0 | 0.9 ± 0.1 | 3.8 | |
| 18 | 25.6 ± 1.7 | 7.2 | 1.2 ± 0.1 | 4.7 | |
| HFCD | 0 | 18.8 | |||
| 6 | 21.6 ± 0.7 | 2.8 | 1 ± 0.1 | 4.6 | |
| 18 | 27.9 ± 3.0 | 9.1 | 2 ± 0.2* | 7.1* | |
| HFCD + Synb | 0 | 18.9 | |||
| 6 | 23.4 ± 1.6 | 4.5 | 1.6 ± 1.3 | 6.8 | |
| 18 | 25.1 ± 0.4 | 6.2 | 1.7 ± 0.1* | 6.7* |
CD: chow diet; HFCD: high-fat choline deficient diet; Synb: synbiotic.
Statistically significant, p < 0.01.
At 18 weeks, liver weight as well as liver/body weight ratio was significant higher in either HFCD or HFCD + Synb diet than CD diet, p < 0.01.
3.2. Liver histology
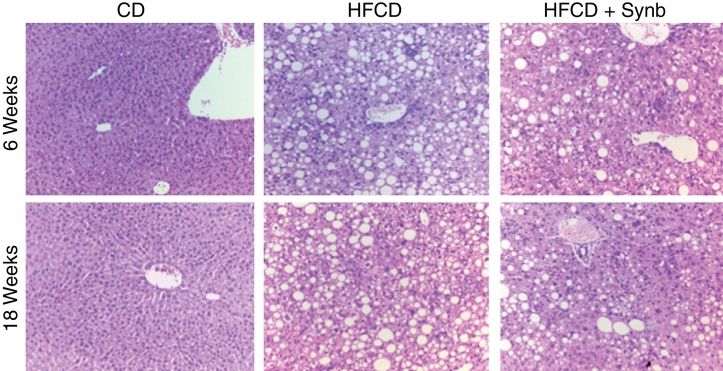
Control mice that were given chow diet, had normal livers throughout the entire study (Fig. 1). On the opposite, all mice undergoing the HFCD diet developed aspects of steatohepatitis, including steatosis, inflammation, ballooning and fibrosis. Consequently, the degree of steatosis, inflammation, ballooning and pericellular fibrosis was significantly higher (p < 0.05) in both HFCD groups than CD (where only mild lobular inflammation was seen); the use of Synb did not abrogate the severity of these histological parameters at any time point, as observed and scored using conventional H&E stained slides (Fig. 1) (Table 3).
Figure 1.
H&E stained liver sections 20× from representative mice fed with CD diet (CD) and mice fed high-fat choline-deficient diet (HFCD) diet or high-fat choline-deficient diet plus Synbiotic, as detailed in Methods. HFCD diet and HFCD diet + Synb showed aspects of steatohepatits at 6 and 18 weeks that were not present in mice undergoing CD diet. The addition of Synb did not abrogate these aspects.
Table 3.
Mean histological scores for each time point according to group (H&E).
| Group | Week | Steatosis | Intralobular inflammation | Ballooning | Pericelular fibrosis |
|---|---|---|---|---|---|
| CD | 6 | 0 | 0.75 | 0 | 0 |
| 18 | 0 | 0.6 | 0 | 0 | |
| HFCD | 6 | 3 | 3 | 1 | 1.6 |
| 18 | 2.4 | 2.6 | 2 | 3 | |
| HFCD + Synb | 6 | 3 | 2 | 2 | 1 |
| 18 | 3 | 2.2 | 2 | 1.6 |
Significant differences in mean scores (p < 0.05), at each time point for steatosis, intralobular inflammation, ballooning and pericellular fibrosis between CD vs HFCD and HFCD + Synb. No differences between HFCD and HFCD + Synb.
However, when evaluating fibrosis using Sirius Red, it was found that the percentage of fibrosis at 6 and 18 weeks was significantly decreased with the addition of Synb, as seen in Table 4, p = 0.001 at 6 weeks, and p = 0.04 at 18 weeks.
Table 4.
Percentage fibrosis (Sirius RED).
| Group | 6 weeks | 18 weeks |
|---|---|---|
| CD | 1.1 ± 0.2 | 0.6 ± 0.06 |
| HFCD | 7.4 ± 2.9 | 10.7 ± 2.6 |
| HFCD + Synb | 3.2 ± 1.0 | 6.3 ± 2.3 |
6 weeks: CD vs HFCD, p = 0.001; HFCD vs HFCD + Synb, p = 0.001.
18 weeks: CD vs HFCD, p = 0.000; HFCD vs HFCD + Synb, p = 0.04.
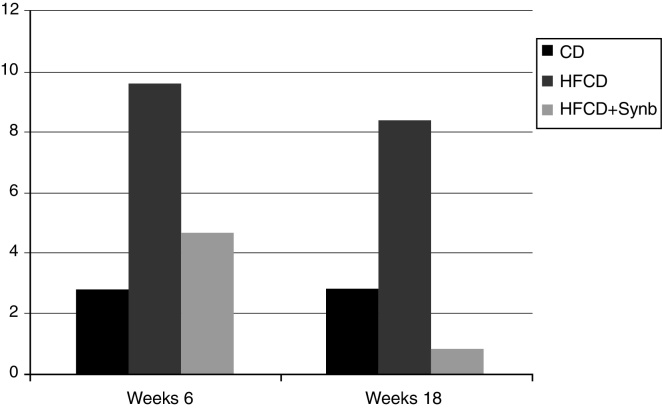
Activation of stellate cells was evaluated using α-SMA antibody. As seen in Fig. 2, significant increases were found at week 18 in mice undergoing HFCD diet when comparing with CD, p = 0.007. This increase was markedly abrogated by the addition of Synbiotic 2000®Forte to HFCD diet, p = 0.003.
Figure 2.
Percentage of α-SMA positive cells (activated stellate cells). Significant increases were found at week 18 in mice undergoing HFCD diet when comparing with CD, p = 0.007. This increase was markedly abrogated by the addition of Synb to HFCD diet, p = 0.003.
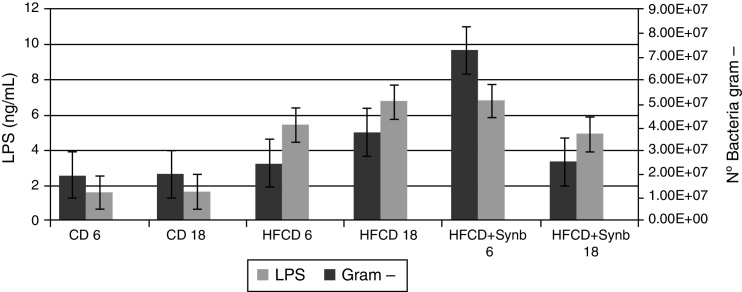
3.3. LPS and Gram-negative bacteria
At week 6, there was a significant increase in Gram-negative bacteria and LPS levels in the two HFCD diet groups, when comparing with CD diet, not abrogated by the addition of Synbiotic 2000®Forte. However, at week 18, although levels of LPS and Gram-negative bacteria continued to increase in HFCD group, the addition of Synbiotic 2000®Forte abrogated this effect, as shown in Fig. 3.
Figure 3.
LPS levels (ng/ml), and Gram-negative bacteria. At week 6, there was a significant increase in LPS levels and number of Gram-negative bacteria, in the two HFCD diet groups, when comparing with CD diet, not abrogated by the addition of Synb. At week 18, although levels of LPS and number of Gram-negative bacteria continued to increase in HFCD group, the addition of Synb partially abrogated this effect.
3.4. Gut microbiota
Data from microbiota will be presented at the phylum and species levels.
3.5. Phylum level (Table 5)
Table 5.
Phylum values.
| Firmicutes | Proteobacteria | Actinobacteria | Bacteroides | Fusobacteria | |
|---|---|---|---|---|---|
| HFCD vs CD (6 wk) | −1.7 | 3.3 | 1.1 | 1.1 | 2.8 |
| HFCD vs CD (18 wk) | 1.6 | 7.5 | −6.7 | 1 | 10.2 |
| HFCD + Synb vs CD (6 wk) | 2.4 | 5.3 | 1.1 | −4.6 | 41.7 |
| HFCD + Synb vs CD (18 wk) | −1.4 | −3.9 | −7.6 | −1.2 | 5.8 |
| HFCD + Synb vs HFCD (6 wk) | 4.2 | 2.1 | 1 | −5 | 15.1 |
| HFCD + Synb vs HFCD (18 wk) | −2.3 | −1.9 | −1.1 | −1.2 | −1.8 |
Bacterial variations were considered only for values greater than 2.0 (2× SD/mean).
In HFCD versus CD, there was an increase of Proteobacteria at week 6 (3.3) and at week 18 (7.5) and Fusobacteria (2.8) and (10.2) respectively; concerning Actinobacteria there was a decrease at week 18 (−6.7). Regarding Firmicutes and Bacteroidetes no major changes were observed at the same time course.
In HFCD + Synb vs CD, there was an increase of Proteobacteria at week 6 (5.3) and a decrease at week 18 (−3.9). Fusobacteria had a huge increase at week 6 (41.7), and at week 18 (5.8). Regarding Bacteroidetes there was a decrease at week 6 (−4.6). Actinobacteria had a decrease at week 18 (−7.6). No major changes at Firmicutes were found both times. In HFCD + Synb vs HFCD, no changes found at Proteobacteria and Actinobacteria, an increase of Fusobacteria at week 6 (15.1), a decrease of Bacteroidetes at week 6 (−5.0), and an increase of Firmicutes at week 6 (4.2) and decrease at week 18 (−2.3).
3.6. Species level (Table 6)
Table 6.
Species values.
| L. casei | L. plantarum | Leuconostoc | Pediococcus | Streptococcus | Enterobacteriacea | Fusobacterium | Bifidobacterium | |
|---|---|---|---|---|---|---|---|---|
| HFCD vs CD6 | −8.7 | −1.1 | −14.9 | −10 | 1 | 2.5 | 2.8 | 1.7 |
| HFCD vs CD18 | −2.7 | −6.6 | −4.6 | 4.2 | −5 | 7.5 | 10.2 | −47.7 |
| HFCD + Synb vs CD6 | −8.6 | 1.3 | 1.6 | 1.5 | 2.5 | 5.3 | 41.7 | −8.8 |
| HFCD + Synb vs CD18 | −2.9 | −6.5 | −56.4 | 1.5 | −21.8 | 3.9 | 5.8 | −14.3 |
| HFCD + Synb vs HFCD6 | 1 | 1.4 | 23.6 | 14.7 | 2.4 | 2.1 | 15.1 | −14.6 |
| HFCD + Synb vs HFCD18 | −1.1 | 1 | −12.3 | −2.8 | −4.4 | −1.9 | −1.8 | 3.3 |
Bacterial variations were considered only for values greater than 2.0 (2× SD/mean).
In the species level, comparing HFCD versus CD, there is an increase of Enterobacteriaceae (2.5 and 7.5) and Fusobacterium (2.8 and 10.2) at 6 and 18 weeks, while there is a decrease of Bifidobacterium (−47.7) and Streptococcus (−5.0) at week 18. A decrease of Leuconostoc was found at both times (−14.9 and −4.6), a decrease of Pediococcus at week 6 (−10.0) and an increase at week 18 (4.2), a decrease of L. casei at both times (−8.7 and −2.7) and a decrease of L. plantarum only at week 18 (−6.6).
Regarding HFCD + Synb versus CD there is an increase of Enterobacteriaceae (5.3 and 3.9) and Fusobacterium (41.7 and 5.8) a decrease of Bifidobacterium (−8.8 and −14.3) at both times, Streptococcus and Leuconostoc a decrease at week 18 (−21.8) and (−56.4). Concerning L. casei there is a decrease at both times (−8.6 and −2.9) and a decrease of L. plantarum only at week 18 (−6.5).
When comparing HFCD + Synb versus HFCD, no changes in Enterobacteriaceae were found at both times, an increase of Fusobacterium at week 6 (15.1) a decrease of Bifidobacterium at week 6 (−14.6) and an increase at week 18 (3.3), a decrease of Streptococcus at both time (−2.2 and −4.4).
Regarding Leuconostoc and Pediococcus an increase at week 6 (23.6) and (14.7) and a decrease at week 18 (−2.3) and (−2.8) respectively. Concerning L. casei and L. plantarum, no changes found at both times.
There was no change in species not mentioned (Enterococcus, Clostridium and Propionibacterium).
4. Discussion
The main finding of the present study is that a symbiotic was able to reduce HFCD diet-induced fibrosis, when evaluated either by the measurement of fibrosis with Sirius Red or the percentage of activated stellate cells. Simultaneously, there was evidence that the addition of the Synbiotic 2000®Forte abrogated the HFCD-associated increase in the abundance of Gram-bacteria in the stools, this effect, simultaneously reducing serum levels of LPS. These results reinforce the concept that increased endotoxemia is one of the main mechanisms through which microbiota composition associates with the presence and severity of NAFLD, as previously shown.22, 23, 24 In fact, human studies were able to show elevated serum endotoxin levels in patients with NAFLD, when compared to controls,25 furthermore elevated in patients with NASH.26
The diet mice model used in the present study resulted in a full-blown picture of steatohepatitis. The high-fat choline deficient diet model was chosen in order to have more similarities with the human phenotype of NASH. The more classic methionine choline deficient diet model was avoided, since those mice in fact lose weight, in opposition to human NASH. Also, we did not use high-fat diet model, since although they get steatosis, NASH does not systematically develop, thus creating difficulties in the evaluation of an intervention. As intervention, the use of a Synbiotic was chosen instead of a probiotic alone, due to the potential advantage of improving the viability of the probiotic.27 In human studies, this particular Synbiotic (Forte 2000) has shown to induce lower intestinal permeability and fewer infections in trauma patients,28 but had no significant impact on the incidence of VAP in critically ill patients.29
The use of the Synbiotic 2000®Forte had no effect in abrogating steatosis or necro-inflammation induced by the HFCD diet, but was able to reduce the degree of fibrosis, with a good correlation with a decrease in Gram- bacteria and LPS. A previous study in rats had shown that a high-fat diet (45% fat) was associated with a decrease in total bacterial density and an increase in the relative proportion of Bacteroidales and Clostridiales, which may induce LPS elevation, gut inflammation and TLR4 activation.30, 31 In the present study, using a less fat diet (35%), but 54% trans-fatty acid enriched, we have not observed a huge increase in Firmicutes and a decrease of Bacteroidetes,32 but only a small effect (increase of Firmicutes only at week 18 (1.6) in population ratio HFCD versus CD group, and no changes in Bacteroidetes). Regarding Proteobacteria we observed an increase, that latter being reduced by the addition of the synbiotic. The reduction in Gram-bacteria may be due to increased production of short-chain fatty acids (SCFA), during the anaerobic metabolism of the prebiotic associated carbohydrates. In fact, SCFA are nonspecific antimicrobial substances that decrease pH, inhibiting growth of a wide range of Gram-bacteria.33
It is of note, that in one recent study where human microbiota was studied, it was found that the abundance of Proteobacteria increased from the healthy group to the obese group and then to the NASH group, being the only abundant phylum exhibiting a significant difference between the latter two groups.34 It was furthermore shown that the Proteobacteria were mostly Enterobacteria, and among those, mostly Escherichia coli that are ethanol producers; the authors also found NASH patients to have more elevated blood ethanol concentrations,34 similar to previous studies.35, 36 The increase of Bifidobacterium at week 18, may be responsible for the beneficial effect of the symbiotic; in fact it has been previously shown that an increase in Bifidobacterium associates with an anti-inflammatory effect.37
Previous studies showed that obesity associates with a low-grade chronic inflammation that contributes to the development of insulin resistance, type 2 diabetes and cardiovascular diseases.38, 39 In our HFCD diet model, mice did not in fact get obese, but had all the features of steatohepatitis and an increase in LPS suggesting an on-going low-grade inflammation. The mechanisms explaining the high-fat induced, increase in endotoxin levels, may relate to the increase in Gram-bacteria, but it is also possible that high-fat feeding might induce an increase in intestinal permeability. In fact, Stenman and col, found that high-fat feeding in mice significantly increased intestinal permeability of the jejunum and colon, associating with a decreased proportion of faecal UDCA and increased FXR expression.40 There is also evidence, that high-fat diet induces hyper reactivity against low-dose LPS, through an up-regulation of CD14 by leptin-mediated signalling.41
The observation of increased activation of hepatic stellate cells (HSC), and increased fibrosis, is probably due to LPS-mediated signalling through toll-like receptor 4 (TLR4),42 which has been identified as a fibrogenic signal in HSC.43 It is however intriguing that we were not able to find any reduction in the degree of necro-inflammation in mice supplemented with the synbiotic. It is possible that conventional histology was not sensitive enough to detect minor changes in necro-inflammation, since also regarding fibrosis degree, no significant changes were changes were found in conventional histology, although using either Sirius Red, or alpha smooth-muscle immunohistochemistry to detect fibrosis or stellate cell activation, a significant reduction was found. It is possible that a more sensitive approach, eventually using immunohistochemistry to quantify inflammatory cells (macrophages, polymorphonuclear leukocytes, and lymphocyte subpopulations) could clarify the severity of cell-death and associated inflammation.
The present findings reinforce the rational for modulating microbiota in the setting of obesity and NAFLD. In fact, there are a large number of studies evaluating the effect of probiotics in murine models and in humans with NAFLD, as reviewed.44, 45 However, we are still missing well-designed trials, to evaluate the effect of mixtures of pre and probiotics in NAFLD that are very difficult to accomplish. The difficulty results from the slow and rather unpredictable course of NAFLD, but also to the difficulty of assuring the stability and the amount of the pre and probiotics preparations.
5. Conclusions
So, in summary we found that mice undergoing a high-fat choline deficient diet develop steatohepatitis, while simultaneously leading to microbial dysbiosis, with increased endotoxemia and liver fibrosis, this effect being at least partially corrected with the use a of a mixture of pre and probiotics. The prospect for using these substances in clinical practice, although attractive, is not yet demonstrated in good clinical trials in humans. Also, we need to be aware that in this study, the Synbiotic 2000®Forte intervention was done simultaneously with the diet. In practice, we usually intervene when the situation is already established, what can make a large difference.
Ethical disclosures
Protection of human and animal subjects
The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Funding
This work is supported by a grant from Fundação para a Ciência e a Tecnologia (PTDC-SAU-OSM/66323/2006).
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Lonardo A., Bellentani S., Argo C.K., Ballestri S., Byrne C.D., Caldwell S.H. Epidemiological modifiers of non-alcoholic fatty liver disease: focus on high-risk groups. Dig Liver Dis. 2015;47:997–1006. doi: 10.1016/j.dld.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Machado M.V., Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician needs to know. World J Gastroenterol. 2014;20:12956–12980. doi: 10.3748/wjg.v20.i36.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley E.M., Monsour H.P. The gut microbiota and nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:262–269. doi: 10.1055/s-0035-1562946. [DOI] [PubMed] [Google Scholar]
- 4.Machado M.V., Cortez-Pinto H. Gut microbiota and nonalcoholic fatty liver disease. Ann Hepatol. 2012;11:440–449. [PubMed] [Google Scholar]
- 5.Le Roy T., Llopis M., Lepage P., Bruneau A., Rabot S., Bevilacqua C. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 6.Mouzaki M., Comelli E.M., Arendt B.M., Bonengel J., Fung S.K., Fischer S.E. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120–127. doi: 10.1002/hep.26319. [DOI] [PubMed] [Google Scholar]
- 7.Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W.Z., Strowig T. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boursier J., Mueller O., Barret M., Machado M., Fizanne L., Araujo-Perez F. The severity of NAFLD is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2015 doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csak T., Velayudham A., Hritz I., Petrasek J., Levin I., Lippai D. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G433–G441. doi: 10.1152/ajpgi.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura K., Seki E., Ohnishi H., Brenner D.A. Role of toll-like receptors and their downstream molecules in the development of nonalcoholic fatty liver disease. Gastroenterol Res Pract. 2010;2010:362847. doi: 10.1155/2010/362847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soares J.B., Pimentel-Nunes P., Roncon-Albuquerque R., Leite-Moreira A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol Int. 2010;4:659–672. doi: 10.1007/s12072-010-9219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasinariu O.E., Ceccarelli S., Alisi A., Moraru E., Nobili V. Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig Liver Dis. 2013;45:543–551. doi: 10.1016/j.dld.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Portela-Cidade J., Borges-Canha M., Leite-Moreira A., Pimentel-Nunes P. Systematic review of the relation between intestinal microbiota and toll-like receptors in the metabolic syndrome: what do we know so far? GE-Port J Gastroenterol. 2015;22:240–258. doi: 10.1016/j.jpge.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martens E.C., Lowe E.C., Chiang H., Pudlo N.A., Wu M., McNulty N.P. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 16.Suarez-Zamorano N., Fabbiano S., Chevalier C., Stojanovic O., Colin D.J., Stevanovic A. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raubenheimer P.J., Nyirenda M.J., Walker B.R. A choline-deficient diet exacerbates fatty liver but attenuates insulin resistance and glucose intolerance in mice fed a high-fat diet. Diabetes. 2006;55:2015–2020. doi: 10.2337/db06-0097. [DOI] [PubMed] [Google Scholar]
- 19.Malkusch W., Rehn B., Bruch J. Advantages of Sirius Red staining for quantitative morphometric collagen measurements in lungs. Exp Lung Res. 1995;21:67–77. doi: 10.3109/01902149509031745. [DOI] [PubMed] [Google Scholar]
- 20.Moter A., Gobel U.B. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J Microbiol Methods. 2000;41:85–112. doi: 10.1016/s0167-7012(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 21.Amann R., Fuchs B.M., Behrens S. The identification of microorganisms by fluorescence in situ hybridisation. Curr Opin Biotechnol. 2001;12:231–236. doi: 10.1016/s0958-1669(00)00204-4. [DOI] [PubMed] [Google Scholar]
- 22.Ferolla S.M., Armiliato G.N., Couto C.A., Ferrari T.C. The role of intestinal bacteria overgrowth in obesity-related nonalcoholic fatty liver disease. Nutrients. 2014;6:5583–5599. doi: 10.3390/nu6125583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 24.Farhadi A., Gundlapalli S., Shaikh M., Frantzides C., Harrell L., Kwasny M.M. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver Int. 2008;28:1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harte A.L., da Silva N.F., Creely S.J., McGee K.C., Billyard T., Youssef-Elabd E.M. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 2010;7:15. doi: 10.1186/1476-9255-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz A.G., Casafont F., Crespo J., Cayon A., Mayorga M., Estebanez A. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374–1380. doi: 10.1007/s11695-007-9243-7. [DOI] [PubMed] [Google Scholar]
- 27.Pandey K.R., Naik S.R., Vakil B.V. Probiotics, prebiotics and synbiotics – a review. J Food Sci Technol. 2015;52:7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spindler-Vesel A., Bengmark S., Vovk I., Cerovic O., Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parenter Enteral Nutr. 2007;31:119–126. doi: 10.1177/0148607107031002119. [DOI] [PubMed] [Google Scholar]
- 29.Knight D.J., Gardiner D., Banks A., Snape S.E., Weston V.C., Bengmark S. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35:854–861. doi: 10.1007/s00134-008-1368-1. [DOI] [PubMed] [Google Scholar]
- 30.de La Serre C.B., Ellis C.L., Lee J., Hartman A.L., Rutledge J.C., Raybould H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–G448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D.Y., Yang M., Edwards S., Ye S.Q., Nonalcoholic fatty liver disease: for better or worse, blame the gut microbiota? JPEN J Parenter Enteral Nutr. 2013 doi: 10.1177/0148607113481623. [DOI] [PubMed] [Google Scholar]
- 32.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carr F.J., Chill D., Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- 34.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 35.Nair S., Cope K., Risby T.H., Diehl A.M. Obesity and female gender increase breath ethanol concentration: potential implications for the pathogenesis of nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:1200–1204. doi: 10.1111/j.1572-0241.2001.03702.x. [DOI] [PubMed] [Google Scholar]
- 36.Cope K., Risby T., Diehl A.M. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 37.Turroni F., Marchesi J.R., Foroni E., Gueimonde M., Shanahan F., Margolles A. Microbiomic analysis of the bifidobacterial population in the human distal gut. ISME J. 2009;3:745–751. doi: 10.1038/ismej.2009.19. [DOI] [PubMed] [Google Scholar]
- 38.Hotamisligil G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes (Lond) 2008;32(Suppl. 7):S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoelson S.E., Goldfine A.B. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15:373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenman L.K., Holma R., Korpela R. High-fat-induced intestinal permeability dysfunction associated with altered fecal bile acids. World J Gastroenterol. 2012;18:923–929. doi: 10.3748/wjg.v18.i9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imajo K., Fujita K., Yoneda M., Nozaki Y., Ogawa Y., Shinohara Y. Hyperresponsivity to low-dose endotoxin during progression to nonalcoholic steatohepatitis is regulated by leptin-mediated signaling. Cell Metab. 2012;16:44–54. doi: 10.1016/j.cmet.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Feng C., Stamatos N.M., Dragan A.I., Medvedev A., Whitford M., Zhang L. Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS ONE. 2012;7:e32359. doi: 10.1371/journal.pone.0032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vespasiani-Gentilucci U., Carotti S., Perrone G., Mazzarelli C., Galati G., Onetti-Muda A. Hepatic toll-like receptor 4 expression is associated with portal inflammation and fibrosis in patients with NAFLD. Liver Int. 2015;35:569–581. doi: 10.1111/liv.12531. [DOI] [PubMed] [Google Scholar]
- 44.Sepideh A., Karim P., Hossein A., Leila R., Hamdollah M., Mohammad E.G. Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr. 2015:1–6. doi: 10.1080/07315724.2015.1031355. [DOI] [PubMed] [Google Scholar]
- 45.Iacono A., Raso G.M., Canani R.B., Calignano A., Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]