Abstract
Background
The prognostic value of coronary artery calcium (CAC) or carotid intima-media thickness (CIMT) among asymptomatic adults with a family history (FH) of premature coronary heart disease (CHD) is unclear.
Methods and Results
MESA enrolled 6814 adults without known atherosclerotic cardiovascular disease (ASCVD). Hard ASCVD events were ascertained over a median follow-up of 10.2 years. We estimated adjusted-hazard ratios (HRs) across CAC and CIMT categories using Cox regression, both between and within FH status groups. Improvement in discrimination with CAC or CIMT added to variables from the ASCVD pooled-cohort equation was also evaluated using receiver operator characteristic curve and likelihood ratio analysis. Of 6125 individuals (62 ± 10 years, 47% males) who reported information on FH, 1262 (21%) had a FH of premature CHD. Among these, 104 hard ASCVD events occurred. Crude incidence-rates (per 1,000 person years) for hard ASCVD were 4.4 for CAC=0 (N=574, 46% of the sample), 8.8 for CAC 1–99 (N=368), 14.9 for CAC 100–399 (N=178), and 20.8 for CAC ≥400 (N=142). Relative to CAC=0, adjusted hard ASCVD HRs for each CAC category among persons with a FH were; 1.64 (95% CI, 0.94–2.87), 2.45 (1.31–4.58), and 2.80 (1.44–5.43), respectively. However, there was no increased hazard for hard ASCVD in high versus low CIMT categories. In participants with a FH of premature CHD, CAC improved discrimination of hard ASCVD events (p<0.001). However, CIMT did not discriminate ASCVD (p=0.70).
Conclusions
Nearly half of individuals reporting FH have zero CAC and may receive less net benefit from aspirin or statin therapy. Among persons with a FH, CAC is a robust marker of absolute and relative risk of ASCVD, whereas CIMT is not.
Keywords: coronary artery calcium, CIMT, primary prevention, family history, risk stratification
Journal Subject Codes: 121 (Primary Prevention), 29 (Coronary Imaging, angiography/ultrasound/Doppler/CC)
Hereditary factors play an important role in the development of atherosclerotic cardiovascular disease (ASCVD). Indeed, the presence of a family history (FH) of premature coronary heart disease (CHD) was one of the earliest recognized cardiovascular risk factors (1,2). For example, prior studies suggest that adjusted risk for ASCVD events is approximately two-fold higher in persons who report that both parents suffered premature CHD (relative to no parental history) (3). Elevated ASCVD risk has also been demonstrated in those who have a FH of premature CHD in any-first degree relative (4). Indeed, despite the lower sensitivity of self-reported FH (68–86% in some analyses) (5), the presence of FH has been shown to stratify disease risk when screening is applied to the general population (6).
However, the incorporation of FH into clinical practice guidelines and risk estimation algorithms has been limited as FH is both non-modifiable and often not independent of other established risk factors (7). In addition, the genetics of ASCVD is complex and there is marked heterogeneity in both penetrance and severity of the disease phenotype among relatives of probands with a history of premature CHD. Thus, information on FH of premature CHD is not routinely incorporated into traditional risk stratification methods, such as the Framingham risk score (FRS) and the recently released pooled cohort equation (PCE) for ASCVD (8). Nevertheless, the 2013 American Heart Association-American College of Cardiology (AHA-ACC) prevention guidelines recommend that providers assess FH status in order to further define, and potentially reclassify, ASCVD risk assessment for adults in whom the appropriate allocation of preventive therapies remains uncertain after the patient-provider risk discussion (8).
However, because of complex inheritance patterns and the fact that FH is self-reported and, thus, may be subject to reporting error (for example, there is low sensitivity with a tendency for under-reporting) (7), assessment of subclinical atherosclerosis could add clinically useful prognostic information in persons who report a FH of ASCVD. For example, prior studies have shown that patients with FH of premature CHD often have an increased prevalence of elevated coronary artery calcium (CAC) (9) and carotid intima-media thickness (CIMT) (10,11). Consequently, the yield from subclinical atherosclerosis testing is higher in persons with FH, an important consideration given that these noninvasive measures have consistently been shown to predict future ASCVD and CHD in cohorts of varied age and demographic make-up (12,13).
Therefore, we sought to determine whether the extent of subclinical atherosclerosis burden (by either CAC or CIMT) could better stratify risk for ASCVD and CHD events beyond traditional risk factors among individuals with a self-reported FH of premature CHD. Such information could help determine the clinical value of pursuing further imaging prior to allocating lifelong preventive pharmacotherapy in persons who report a FH of premature CHD. Please note that, unless otherwise stated, the abbreviation “FH” throughout this text specifically denotes a family history of premature CHD.
Methods
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) study recruited 6,814 participants between 2000 and 2002 across six field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St Paul, Minnesota) (14). Participants were between 45 and 84 years of age, identified themselves as Caucasian, African American, Hispanic, or Chinese American, and were free of clinical ASCVD at baseline. Due to the fact that detailed FH data were only available from MESA visit 2 (including data on the age of CHD onset in relatives of MESA participants that is required to determine whether FH is premature or not), we only included persons who attended both visit 1 (when CAC and CIMT imaging were obtained) and visit 2. Of the 6201 persons who attended both visits, a total of 71 subjects were excluded for missing CIMT data (all subjects had CAC data), 5 were omitted due to missing outcomes data, 97 were missing low-density lipoprotein cholesterol information, and 113 were missing other variables of interest in the models. Notably, 150 study participants (2% of the MESA sample overall) died between visits 1 and 2. After exclusions, 6125 participants were available for the main analysis. Institutional review boards at each site approved the study, and all participants gave written informed consent.
Baseline Measurements
At the baseline MESA visit (July 2000–September 2002), study participants completed self-administered questionnaires, standardized interviews, and in-person examinations of lifestyle characteristics, medical history, anthropometric measurements, and laboratory data. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. High-density lipoprotein cholesterol (HDL-C) was measured using the cholesterol oxidase method. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation (15). Diabetes was defined using the 2003 American Diabetes Association criteria (16) of a fasting glucose ≥126 mg/dl or use of insulin or oral hypoglycemic medications. Hypertension was defined using the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure criteria (17) as either a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, a history of physician-diagnosed hypertension, or taking a medication for hypertension. Study participants also self-reported personal habits such as alcohol and current tobacco use (defined as having smoked a cigarette in the last 30 day and more than 100 cigarettes in a lifetime).
CAC and CIMT Assessment
The details of the MESA cardiac CT protocol have been published elsewhere (18). Briefly, baseline CAC at MESA visit 1 was quantified using the Agatston scoring method (19). CAC was measured either by electron-beam computed tomography or by multi-detector row helical computed tomography, depending on the field center. Inter-observer agreement (κ = 0.93) and intra-observer agreement (κ = 0.90) were high (20).
The intima-media layer of the carotid arterial wall was measured at visit 1 using high-resolution B-mode ultrasonography of the near and far walls of the left and right common carotid arteries; the mean maximum value, in millimeters, of the common CIMT was used in the analysis (21). The intra-class correlation coefficients for intra-reader and inter-reader reproducibility of common carotid measurements were 0.98 and 0.86, respectively (21). A standardized protocol with quality control procedures was used and interpretation was performed at a centralized reading station in Tufts Medical Center, Boston, Massachusetts (21).
Family History Assessment
Detailed information on FH of premature CHD was ascertained in the majority of participants (n=6,201) in an ancillary study at MESA visit 2 (September 2002- February 2004). Participants were asked if any member of their immediate family (first-degree relative: mother, father, siblings, or children) experienced a fatal or non-fatal myocardial infarction and/or cardiac procedure (coronary bypass surgery, balloon angioplasty, and intracoronary stenting). Response options were “yes,” “no,” and “do not know.” For the purposes of this analysis “Do not know” responses were counted as “no” responses. If a participant reported “yes” for a disease in a first degree relative, the age at diagnosis was also ascertained. We defined FH of premature CHD as having at least one first-degree relative with CHD occurring before the age of 55 years in male relatives and before the age of 65 years in female relatives.
Definition and Ascertainment of Events
A detailed description of the event adjudication process has been previously published (14). For the purpose of this study, we analyzed two separate clinical end points: 1) hard CHD: defined to include myocardial infarction, resuscitated cardiac arrest, or coronary heart disease death; and 2) hard ASCVD: defined as the events comprising of hard CHD, plus stroke or stroke death.
Statistical Analysis
Baseline characteristics in each of the two FH of premature CHD status groups (positive or negative) were compared using ANOVA for continuous variables and X2 or Wilcoxon rank sum testing for categorical variables. CAC score was classified into the following categories: 0, 1–99, 100–399, and ≥400 Agatston Units. Because no established clinical cut-points exist for CIMT and in order to create subgroups with comparable numbers of events to the CAC categories, CIMT was categorized according to the following percentile distributions: ≤50, 51–75, 76–90 and >90th percentiles.
For each event, we used Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) comparing persons with a FH of premature CHD to those without. The proportionality assumption was confirmed visually using log-log plots. Models were adjusted for age, sex, race, MESA site, cigarette smoking, hypertension status, diabetes status, BMI, LDL-C, HDL-C, and cholesterol lowering medications. We further adjusted for CAC as a continuous variable, log[CAC +1], in a mediation analysis. We also tested for multiplicative interaction between FH status and each of sex, race, and age categories (<55 or >=55 years) in the association with incident events.
In addition, we constructed adjusted Cox models, within each FH status group, according to CAC and CIMT categories using CAC=0 and CIMT ≤50th percentile as the reference groups, respectively. These models were adjusted for the same variables listed above. Sensitivity analyses for CIMT were also conducted by quartile. Furthermore, both transformed CAC (log[CAC +1]) and untransformed CIMT (mm) were also modeled in a continuous fashion. We also formally tested for multiplicative interaction between FH and either CAC or CIMT categories in the association with incident events.
For each event, we calculated the area under the receiver operating characteristic curves (AUC) using Harrell’s C-statistic for models with and without CAC or CIMT (both modeled continuously) in those with a FH of premature CHD. The base model for this AUC analysis consisted of the variables included in the PCE equation for ASCVD risk (age, sex, race, systolic blood pressure, treatment for hypertension, HDL-C, total cholesterol, diabetes, and smoking). Improvement in discrimination was determined using the likelihood ratio test (22). All statistical analyses were performed with the use of Stata version 13.0 (StataCorp). A two-sided p-value of <0.05 was considered statistically significant.
Results
The final study population consisted of 6125 individuals (mean baseline age 62 ± 10 years, 48% men). Overall, 21% (n = 1262) of MESA participants reported a FH of premature CHD. The FH group had a higher percentage of subjects who were women, white or black ethnicity, overweight, diabetic, hypertensive, or on lipid-lowering therapy (Table 1). Patients with a FH of premature CHD also had a higher proportion of persons with CAC ≥400 or CIMT values >90th percentile.
Table 1.
Baseline characteristics of a multi-ethnic, asymptomatic cohort according to family history of premature coronary disease status.
| Negative FH (n= 4863) | Positive FH (n= 1262) | p-value* | |
|---|---|---|---|
|
| |||
| Characteristics | |||
| Age, mean ± SD | 62 ± 10 | 62 ± 10 | 0.73 |
| Gender, n (%) | <0.001 | ||
| Male | 2411 (50) | 505 (40) | |
| Female | 2452 (50) | 757 (60) | |
| Race/Ethnicity, n (%) | <0.001 | ||
| White | 1884 (39) | 547 (43) | |
| Chinese American | 685 (14) | 39 (3) | |
| Black | 1239 (26) | 398 (32) | |
| Hispanic | 1055 (22) | 278 (22) | |
| BMI (kg/m2), mean ± SD | 28 ± 5 | 29 ± 6 | <0.001 |
| LDL-C (mg/dL), mean ± SD | 117 ± 31 | 118 ± 31 | 0.30 |
| HDL-C (mg/dL), mean ± SD | 51 ± 15 | 52 ± 15 | 0.21 |
| HTN, n (%) | 2065 (43) | 627 (50) | <0.001 |
| Diabetes Mellitus, n (%) | 478 (10) | 174 (14) | <0.001 |
| Lipid-lowering medication use, n (%) | 747 (15) | 245 (19) | <0.001 |
| Cigarette smoking, n (%) | 0.004 | ||
| Never | 2500 (52) | 596 (47) | |
| Former | 1773 (37) | 475 (38) | |
| Current | 577 (12) | 188 (15) | |
| Median CAC, Agatston Units (IQR) | 0 (78) | 5 (104) | <0.001 |
| Mean CIMT (mm ± SD) | 0.86 ± 0.19 | 0.88 ± 0.19 | 0.005 |
| CAC category, n (%) | <0.001† | ||
| CAC=0 | 2545 (52) | 574 (46) | |
| CAC= 1–99 | 1222 (25) | 368 (29) | |
| CAC=100–399 | 652 (13) | 178 (14) | |
| CAC >400 | 444 (9) | 142 (11) | |
| CIMT category, n (%) | 0.007† | ||
| ≤50th percentile | 2495 (51) | 593 (47) | |
| 51–75th percentile | 1189 (24) | 332 (26) | |
| 76–90th percentile | 709 (15) | 197 (16) | |
| >90th percentile | 470 (10) | 140 (11) | |
p-value for continuous variables was calculated using student’s t-test and for categorical variables using chi-squared test
p-value was calculated using Wilcoxon ranksum test
SD – Standard Deviation
BMI - Body Mass Index
LDL-C - Low Density Lipoprotein-Cholesterol
HDL-C - High Density Lipoprotein-Cholesterol
HTN - Hypertension (defined by JNC VI Criteria)
CAC - Coronary Artery Calcification
CIMT - Carotid Intima-Media Thickness
IQR - Interquartile Range
After a median of 10.2 years follow-up, a total of 382 hard ASCVD and 243 hard CHD events were recorded in the sample overall. Among those with a FH of premature CHD, 104 hard ASCVD and 67 hard CHD events occurred (see Supplement Table 1 for numbers among each race/ethnicity group). In crude analyses, hard ASCVD and hard CHD event rates were higher among those with a FH of premature CHD compared to those without: 8.7 and 5.5 (per 1,000 person years) (p=0.0007), versus 6.0 and 3.7 (p=0.004), respectively.
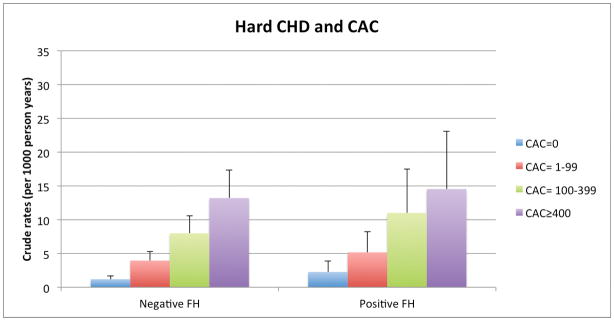
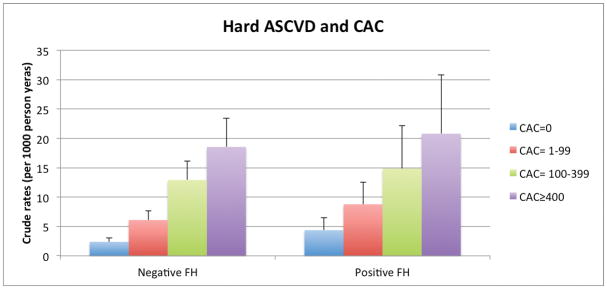
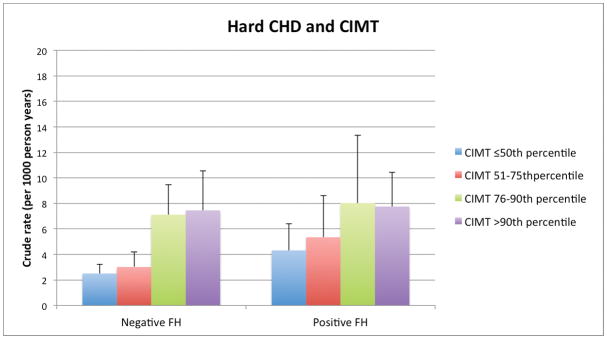
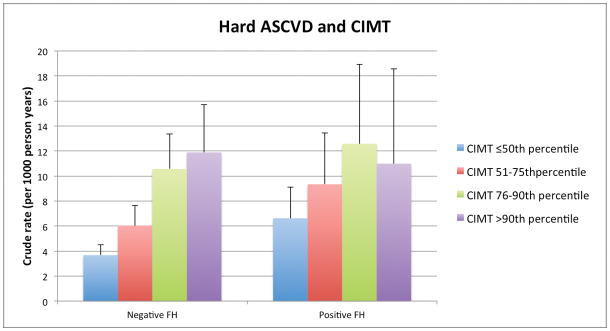
Among persons with and without a FH, there was a graded relationship between both higher CAC and CIMT categories and increasing absolute event rates over follow-up. For example, the lowest crude rate for hard CHD (1.2per 1,000 person-years) was seen in those without a reported FH and without CAC (CAC=0). In contrast, the highest hard CHD event rates occurred in those with a FH of premature CHD and CAC ≥400: 12.1 per 1,000-person years (Figure 1a). Importantly, the incidence rates for both hard ASCVD and hard CHD (per 1,000-person years) were as low as 4.4 and 2.3, respectively, in persons who reported a FH but who had CAC=0. We also found a graded increase in risk for CHD and ASCVD events according to CIMT categories among persons without a FH of premature CHD. However, the relationship between increasing CIMT categories and events was less consistent for those with a FH of premature CHD (Figure 1b).
Figure 1.
Figure 1a. Hard ASCVD and CHD Event Rates (per 1000 person-years) with Increasing CAC Score Categories, according to FH of premature CHD status.
Figure 1b. Hard ASCVD and CHD Event Rates (per 1000 person-years) with Increasing CIMT categories, according to FH of premature CHD status.
ASCVD – atherosclerotic cardiovascular disease, CHD – coronary heart disease, FH – family history of premature coronary heart disease, CAC – coronary artery calcium, CIMT – carotid intima-media thickness
Adjusted Hazard Ratios comparing FH status groups
Relative to persons without a FH, those who reported a FH of premature CHD had an adjusted HR for hard ASCVD of 1.35 (95% CI 1.07–1.71) and a HR for hard CHD of 1.41 (95% CI 1.05–1.88), overall. Associations between FH and hard ASCVD events were calculated for each race/ethnicity category: HR= 1.08 (95% CI 0.74–1.56) in whites, 2.09 (95% CI 1.37–3.19) in blacks, 1.34 (95% CI 0.85–2.13) in Hispanics, and 0.95 (95% CI 0.21–4.22) in Chinese. However, there was no interaction between FH and race/ethnicity (p=0.25) in the association with these events. In addition, there was no interaction between FH and age (categorized as <55 or >=55, p=0.05) or sex (p=0.55).
In the mediation analysis, the association for FH diminished slightly, but remained significant, after further adjusting for CAC in the baseline models (HR 1.30 [95% CI 1.03–1.64] for hard ASCVD). For hard CHD, this relationship also remained significant after further adjustment for CAC (HR 1.33, 95% CI: 1.00 to 1.78).
Adjusted Hazard Ratios within FH status groups; according to CAC or CIMT
In the fully adjusted models, increasing HRs for hard ASCVD were seen among higher CAC categories, regardless of the presence or absence of a FH of premature CHD (Table 2). For example, those with a FH had a HR of 2.80 (95% CI 1.44–5.43) in the CAC ≥400 group (relative to CAC=0), in contrast to 3.22 (95% CI 2.15–4.84) in those without a FH. However, there was no statistical interaction between FH and CAC in the association with ASCVD events (p=0.28).
Table 2.
Multivariable-adjusted* hazards ratios (95% confidence interval) for the association of CAC and CIMT with hard ASCVD events, stratified by family history of premature CHD
| Hazard Ratios (95% CI) | Hard ASCVD Events | |||
|---|---|---|---|---|
| Negative FH | Positive FH | Negative FH | Positive FH | |
| (n= 4863) | (n= 1262) | (n=278) | (n=104) | |
|
|
|
|||
| CAC 0 | 1 (ref) | 1 (ref) | 60 | 25 |
| CAC 1–99 | 1.75 (1.22–2.50) | 1.64 (0.94–2.87) | 72 | 30 |
| CAC 100–399 | 2.78 (1.91–4.06) | 2.45 (1.31–4.58) | 76 | 24 |
| CAC >400 | 3.23 (2.15–4.86) | 2.80 (1.44–5.43) | 70 | 25 |
| p-value for interaction | 0.28 | |||
| CIMT ≤50th percentile | 1 (ref) | 1 (ref) | 90 | 38 |
| CIMT 51–75th percentile | 0.93 (0.67–1.29) | 1.18 (0.71–1.95) | 69 | 29 |
| CIMT 76–90th percentile | 1.27 (0.90–1.80) | 1.30 (0.74–2.28) | 69 | 23 |
| CIMT >90th percentile | 1.11 (0.75–1.63) | 0.76 (0.39–1.50) | 50 | 14 |
| p-value for interaction | 0.21 | |||
| ln (CAC+1) | 1.20 (1.13–1.26) | 1.17 (1.07–1.29) | ||
| p-value for interaction | 0.28 | |||
| Continuous CIMT | 1.54 (0.82–2.89) | 0.89 (0.32–2.49) | ||
| p-value for interaction | 0.21 | |||
Adjusted for age, sex, race, MESA site, cigarette smoking, hypertension, diabetes, BMI, LDL-C, HDL-C, cholesterol lowering medications
Continuous CAC units are log-transformed Agatston Units, continuous CIMT units are per millimeter change in thickness
ASCVD – atherosclerotic cardiovascular disease
FH – family history of premature coronary heart disease
CHD – coronary heart disease
CAC – coronary artery calcium
CIMT – carotid intima-media thickness
BMI – body mass index
LDL-C – low density lipoprotein-cholesterol
HDL-C - high density lipoprotein-cholesterol
In contrast to CAC, hazard ratios for hard ASCVD demonstrated only marginal increases among higher CIMT categories and these associations did not meet statistical significance, either in the presence or absence of a FH of premature CHD. For persons with CIMT >90th percentile, those without a FH had a HR of 1.11 (95% CI 0.75–1.63), relative to the lowest CIMT category) compared to 0.76 (95% CI 0.39–1.50) in those with a FH. There was also no interaction between CIMT (either by percentile category or by quartile) with ASCVD events based on FH status (p=0.21) (Table 2, Supplement Table 2).
Results for hard CHD were qualitatively similar to those for hard ASCVD in fully adjusted models (Table 3). Notably, the HR for hard CHD within each CAC group was higher in those without a FH of premature CHD. For example, the HR for hard CHD was 3.85 (95% CI 1.65–9.02, compared to CAC=0) in those with CAC ≥400 and a FH versus 4.87 (95% CI 2.88–8.24) in those without. However, in addition to the higher absolute risk in the group with both CAC=0 and a FH compared to those with CAC=0 and no FH, there was also higher relative risk in the CAC=0 and FH of premature CHD group (relative to the CAC=0 and negative FH group), with a HR for hard CHD of 1.54 (95% CI 0.79–3.01) for the comparison. In contrast, CIMT (by percentile group or quartile) was not associated with hard CHD (Table 3, Supplement Table 3). Similar to hard CVD, there was also no interaction between CAC or CIMT and FH status (p=0.49 and p=0.51, respectively). All HRs for both events were consistent with the categorical results above when CAC and CIMT were modeled continuously (Tables 2 and 3).
Table 3.
Multivariable-adjusted* hazards ratios (95% confidence interval) for the association of CAC and CIMT with hard CHD events stratified by family history of premature CHD
| Hazard Ratios (95% CI) | Hard CHD Events | |||
|---|---|---|---|---|
| Negative FH | Positive FH | Negative FH | Positive FH | |
| (n= 4863) | (n= 1262) | (n=176) | (n=67) | |
|
|
|
|||
| CAC 0 | 1 (ref) | 1 (ref) | 30 | 13 |
| CAC 1–99 | 2.35 (1.46–3.78) | 1.93 (0.91–4.10) | 47 | 18 |
| CAC 100–399 | 3.54 (2.14–5.85) | 3.52 (1.58–7.84) | 48 | 18 |
| CAC >400 | 4.87 (2.88–8.24) | 3.85 (1.65–9.02) | 51 | 18 |
| p-value for interaction | 0.49 | |||
| CIMT ≤50th percentile | 1 (ref) | 1 (ref) | 62 | 25 |
| CIMT 51–75th percentile | 0.70 (0.46–1.08) | 1.02 (0.54–1.94) | 35 | 17 |
| CIMT 76–90th percentile | 1.20 (0.79–1.84) | 1.29 (0.64–2.60) | 47 | 15 |
| CIMT >90th percentile | 0.94 (0.58–1.52) | 0.87 (0.38–1.96) | 32 | 10 |
| p-value for interaction | 0.51 | |||
| ln (CAC+1) | 1.25 (1.17–1.35) | 1.22 (1.09–1.37) | ||
| p-value for interaction | 0.39 | |||
| Continuous CIMT | 1.30 (0.59–2.90) | 1.20 (0.35–4.13) | ||
| p-value for interaction | 0.60 | |||
Adjusted for age, sex, race, MESA site, cigarette smoking, hypertension, diabetes mellitus, BMI, LDL-C, HDL-C, cholesterol lowering medications
Continuous CAC units are log-transformed Agatston Units, continuous CIMT units are per millimeter change in thickness
CHD – coronary heart disease
FH – family history of premature coronary heart disease
CAC – coronary artery calcium
CIMT – carotid intima-media thickness
BMI – body mass index
LDL-C – low density lipoprotein-cholesterol
HDL-C - high density lipoprotein-cholesterol
Addition of CAC vs CIMT for discrimination of incident ASCVD and CHD events
The addition of CAC to the base model comprising the variables from the PCE for ASCVD risk estimation led to an increase in the Harrell’s C-statistic for hard CHD from 0.74 to 0.77 (p=0.0005), while the addition CIMT was not significant (p=0.97). Similar results for hard ASCVD were obtained when either CAC or CIMT were added to the base model [base AUC = 0.75; base plus CAC = 0.77 (p=0.0004) and base plus CIMT = 0.75 (p=0.70)] (Figure 2). Results were similar for those without FH (supplement).
Figure 2.
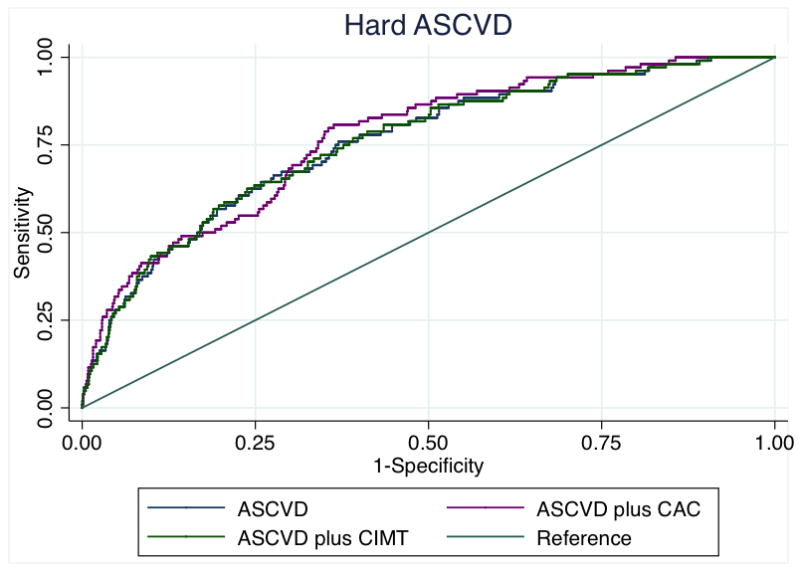
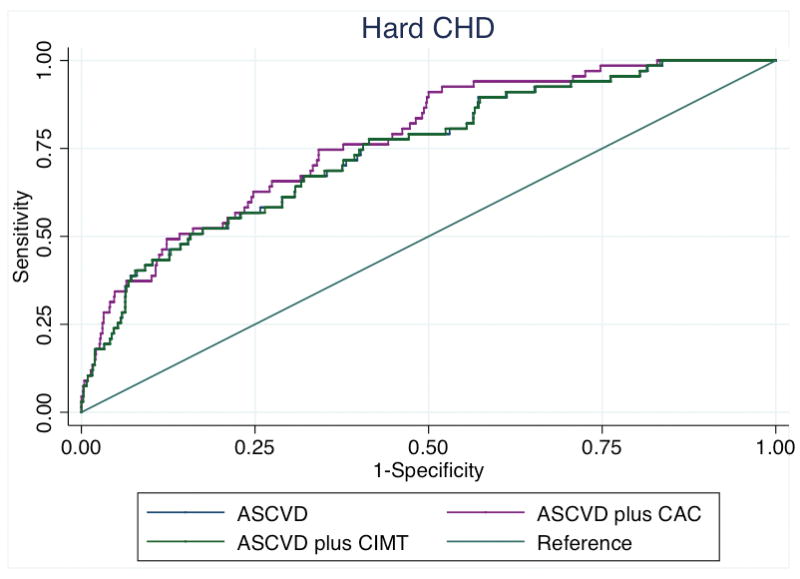
Figure 2a. Receiver operating characteristic curves showing area under the curve for incident hard ASCVD among those with a family history of premature CHD.
Figure 2b. Receiver operating characteristic curves showing area under the curve for incident hard CHD among those with a family history of premature CHD.
ASCVD – atherosclerotic cardiovascular disease, CHD – coronary heart disease, FH – family history of premature coronary heart disease, CAC – coronary artery calcium, CIMT – carotid intima-media thickness
*CIMT and CAC were modelled continuously
Discussion
In this community-based multi-ethnic cohort, FH of premature CHD was an independent risk factor for both hard ASCVD and hard CHD events, even with adjustment for baseline subclinical atherosclerosis burden. Nonetheless, we demonstrate that CAC testing is more effective than CIMT at stratifying absolute and relative risk for both ASCVD and CHD in those with a FH of premature CHD. In addition, almost half of those individuals reporting a positive FH had zero CAC and were at very low absolute risk for events over a median of 10 years of follow-up. Furthermore, the addition of CAC added significant prognostic information for discrimination for CHD events in persons with a FH of premature CHD, whereas the addition of CIMT did not.
Adjusted ASCVD risk comparing persons with and without a FH of premature coronary heart disease
In this modern multiethnic cohort, FH of premature CHD is an independent risk factor for events over 10 years. While there was a trend for higher relative risk among blacks with a FH of premature CHD (with weaker findings for other ethnicities- Supplement Table 1), we did not find interaction, suggesting that the associations between FH status and outcomes were statistically similar by race.
In addition, and motivated by recent reports (23), we also examined whether CAC mediated the effect of FH on subsequent ASCVD. Confirming prior research, we found no significant reduction in the impact of FH on ASCVD risk when CAC was added to our models, suggesting that, although CAC is able to stratify risk in both the presence and absence of FH, the effect of a positive FH on events does not appear to be mediated through CAC. In line with this finding, other investigators have reported that non-calcified coronary plaque (not captured by CAC) is more prevalent among those with a FH, particularly in persons <55 years of age (24). Thus, our findings are additive to a recent report that demonstrated an increased relative risk (despite a very low absolute risk) for cardiovascular events among intermediate risk subjects with any FH and zero CAC (we note that this report did not examine persons with premature FH specifically) (25).
ASCVD risk within the premature FH group; stratified by subclinical atherosclerosis
Our results may also inform risk prognostication and treatment decisions in persons reporting a FH of premature CHD. Specifically, we found a wide range of absolute event rates based on CAC values. In addition, nearly one half of individuals (46%) with a FH of premature CHD in this study were at very low 10-year cardiovascular risk due to the absence of CAC. This finding may be explained, at least in part, by the fact that not all individuals with a FH will share the same familial risk factors – genes or lifestyle – as their affected family members. Additionally, there are often errors in the self-reporting of FH, which could lead to misclassification. Furthermore, the addition of CAC to a model comprised of variables from the PCE improved discrimination of incident CHD.
Current guidelines recommend that providers consider the assessment of additional ‘novel’ risk factors (which are not contained in the PCE equation for ASCVD risk estimation) to guide statin allocation and help inform the patient-provider risk discussion, particularly when the decision to proceed with preventive pharmacotherapy such as statins remains uncertain (8). Of relevance to this analysis, the assessment of either FH of premature CHD or CAC was endorsed by the guideline committee to help reclassify risk in uncertain situations (level IIb of evidence). However, as mentioned above, a potential limitation to using FH of premature CHD alone is that penetrance can vary enormously and FH is by nature self-reported and, thus, subject to misclassification due to reporting error (26).
In this context, our results suggest that, for those reporting a FH of premature CHD and in whom preventive treatment decisions are uncertain, CAC may be a useful adjunctive test prior to commencing lifelong statin therapy (8). Further, aspirin use for primary ASCVD prevention is endorsed for those considered higher risk (10-y risk >10%) (Class IIa evidence) (27), although it is frequently prescribed to low risk individuals on the basis of FH status (a practice that is not guideline driven). However, the use of aspirin in low-risk populations may be outweighed by an increased risk of bleeding (28) and our results also suggest that CAC may also be a useful adjunctive test prior to commencing aspirin in persons with a FH of premature CHD.
Study Limitations
The results of our study should be interpreted in the context of several limitations. First, this is an observational study and our conclusions are hypothesis generating. Accurate family history assessment can be limited by reporting errors (29). Because of high specificity and comparatively lower sensitivity, some subjects with a positive FH are falsely classified in the group of subjects without FH, which may result in underestimation of the risk associated with a FH of premature CHD. Secondly, details on FH of premature CHD were only assessed at MESA exam 2 and could introduce selection bias due to the fact that some MESA participants died from an ASCVD or CHD related event after visit 1, when CAC and CIMT imaging were obtained, but before visit 2, and were excluded from the analysis. However, to perform our analyses using visit one FH data would have required that we use family history of “any” CHD (regardless of age of onset) as the exposure variable. Given the prevalence of CHD in the U.S., the presence of family history of “any” CHD is common (43% of the MESA sample), is non-specific, and is far less likely to reflect the underlying exposure of interest in our analysis (shared genetic risk factors for CHD among family members). In this context, we used an asynchronous analytic approach (visit one imaging data and visit two FH data). We believe this is justified for a number of reasons; 1) very few people died between visits one and two (150 of 6814 participants); 2) of those who did die, the proportion with a positive family history of any CHD was statistically similar to the proportion of those with a family history of any CHD among the persons included in our analysis; 3) the time between visits was short (median time 1.6 years) making it unlikely that family history data (which are generally fixed) differed between visits for the vast majority of participants; and 4) we also performed sensitivity analyses using the crude visit one family history data and the results were all qualitatively similar (Supplement Tables 4 and 5).
Conclusion
CAC as a prognostic test appears to perform equally well in persons with and without a FH. Current prevention guidelines endorse (Class II, Level B recommendation) the assessment of a FH of premature CHD to guide ASCVD prevention treatment strategies (6). However, in MESA, we found that the risk of ASCVD and CAC distribution was heterogeneous in those reporting a positive family history, with almost half demonstrating zero CAC. We also demonstrate that CAC effectively stratifies absolute and relative risk in persons with a FH, whereas CIMT does not. Thus, prior to starting lifelong statin and/or aspirin therapy, it may be reasonable to consider additional CAC testing to enhance ASCVD risk stratification in those who report a family history of premature CHD.
Supplementary Material
Acknowledgments
The authors would like to thank the other investigators, the staff, and the participants of the MESA Study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa.nhlbi.org.
Footnotes
Disclosures
Drs. Patel, Al Rifai, Blaha, Post, Polak, Bluemke, Sheuner, Kronmal, Blumenthal, and Nasir have no disclosures to report. Dr. McEvoy is supported by the Pollin Cardiovascular Prevention Fellowship. Dr. Budoff is a consultant for General Electric.
References
- 1.Wilhelmsen L, Wedel H, Tibblin G. Multivariate analysis of risk factors for coronary heart disease. Circulation. 1973;48:950–8. doi: 10.1161/01.cir.48.5.950. [DOI] [PubMed] [Google Scholar]
- 2.Rose G. Familial patterns in ischemic heart disease. Br J Prev Soc Med. 1964;18:75–80. doi: 10.1136/jech.18.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 2001;104:393–98. doi: 10.1161/hc2901.093115. [DOI] [PubMed] [Google Scholar]
- 4.Ranthe MF, Carstensen L, Oyen N, Tfelt-Hansen J, Christiansen M, McKenna WJ, Wohlfahrt J, Melbye M, Boyd HA. Family history of premature death and risk of early onset cardiovascular disease. J Am Coll Cardiol. 2012;60:814–21. doi: 10.1016/j.jacc.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Bensen JT, Liese AD, Rushing JT, Province M, Folsom AR, Rich SS, Higgins M. Accuracy of proband reported family history: the NHLBI Family Heart Study (FHS) Genet Epidemiol. 1999;17:141–150. doi: 10.1002/(SICI)1098-2272(1999)17:2<141::AID-GEPI4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Familial risk assessment for early-onset coronary heart disease. Genet Med. 2006;8:525–531. doi: 10.1097/01.gim.0000232480.00293.00. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell CJ. Barriers and Opportunities for the Use of Family History Information in Risk Prediction and Prevention. Circulation. 2004;110:2074–76. doi: 10.1161/01.CIR.0000145539.77021.AC. [DOI] [PubMed] [Google Scholar]
- 8.Goff DC, Jr, Lloyd-Jones DM, Bennet G, Coady D, D’Agostino RB, Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheuner MT, Setodji CM, Pankow JS, Blumenthal RS, Keeler E. General Cardiovascular Risk Profile identifies advanced coronary artery calcium and is improved by family history: the multiethnic study of atherosclerosis. Circ Cardiovasc Genet. 2010;3:97–105. doi: 10.1161/CIRCGENETICS.109.894527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, Engström G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Hofman A, Holewijn S, Ikeda A, Kavousi M, Kitagawa K, Kitamura A, Koffijberg H, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O’Leary DH, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Witteman JC, Moons KG, Bots ML. Common Carotid Intima-Media Thickness Measurements in Cardiovascular Risk Prediction: A Meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 12.Newman AB, Naydeck BL, Ives DG, Boudreau RM, Sutton-Tyrrell K, O’Leary DH, Kuller LH. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–92. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB., Sr Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–21. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–8. [PubMed] [Google Scholar]
- 17.Joint National Committee: The sixth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1997;157:2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 18.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 19.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 20.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–7. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 21.O’Leary DH, Polak JF, Wolfson SK, Jr, Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Healthy Study. CHD collaborative Research Group. Stroke. 1991;22:1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 22.Seshan VE, Gönen M, Begg CB. Comparing ROC curves derived from regression models. Stat Med. 2013;32:1483–93. doi: 10.1002/sim.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paixao AR, Berry JD, Neeland IJ, Ayers CR, Rohatgi A, de Lemos JA, Khera A. Coronary Artery Calcification and Family History of Myocardial Infarction in the Dallas Heart Study. JACC Cardiovasc Imaging. 2014;7:679–86. doi: 10.1016/j.jcmg.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Kral BG, Becker LC, Vaudya D, Yanek LR, Qayyum R, Zimmerman SL, Dey D, Berman DS, Moy TF, Fishman EK, Becker DM. Noncalcified Coronary Plaque Volumes in Healthy People with a Family History of Early Onset Coronary Artery Disease. Circ Cardiovasc Imaging. 2014;7:446–53. doi: 10.1161/CIRCIMAGING.113.000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen R, Budoff M, McClelland RL, Sillau S, Burke G, Blaha M, Szklo M, Uretsky S, Rozanski A, Shea S. Significance of a Positive Family History for Coronary Heart Disease in Patients With a Zero Coronary Artery Calcium Score (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2014;114:1210–4. doi: 10.1016/j.amjcard.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kee F, Tiret L, Robo JY, Nicaud V, McCrum E, Evans A, Cambien F. Reliability of reported family history of myocardial infarction. Br Med J. 1993;307:1528–30. doi: 10.1136/bmj.307.6918.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antithrombotic Trialists’ (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury MJ, Flanders WD. Bias in using family history as a risk factor in case-control studies of disease. Epidemiology. 1995;6:511–519. doi: 10.1097/00001648-199509000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.