Abstract
Objective
To investigate the effect of military stress on immune response and Helicobacter pylori stomach infections.
Methods
In this prospective, observational study, the Symptom Checklist-90 questionnaire was completed by military recruits before and following a 3-month basic training programme. H. pylori immunoglobulin (Ig)G levels, C14-urea breath-test values and levels of cortisol, catecholamine, and certain humoral and cellular immune responses were measured before and after the basic training.
Results
For 60 military recruits, somatization, depression and paranoid ideation scores were significantly increased after, compared with before, basic training. Post-training H. pylori IgG detection revealed three additional cases of H. pylori infection. Post-training C14-urea breath-test values were significantly higher compared with before training – thus suggesting higher levels of H. pylori colonization in the stomach. Post-training cortisol and catecholamine levels were increased, while serum IgG levels were decreased; complement component (C)3 and C4 levels remained unchanged. Post-training CD4+ and CD8+ T-cell percentages and the CD4+/CD8+ ratio were significantly reduced compared with before training. Serum interleukin (IL)-2 levels were lower and IL-10 levels were higher following training and there was a significant decrease in the IL-2/IL-10 ratio.
Conclusion
Military stress may reduce humoral and cellular immune responses and may aggravate the severity of H. pylori infection.
Keywords: Military stress, Helicobacter pylori, C14-urea breath test, immune response, stomach infections
Introduction
The army is a distinct occupational group and its members are at high risk of mental health problems. Recruits often undergo military and survival training in extremely harsh environments that make great demands on physical and mental strength. In addition, troops deployed in war zones face near-constant threats from opposing combatants. Such stresses cause psychological and pathophysiological changes,1,2 and have been identified as a primary reason for non-battle casualties in military personnel.3
Helicobacter pylori infection is one of the most common infections in humans worldwide. It is a substantial cause of chronic active gastritis, peptic ulcers and other stomach diseases that are closely correlated with gastric carcinoma and lymphoma.4–6 H. pylori has been categorized by the World Health Organization as a Class I carcinogen.7–9 Emerging epidemiological data show that H. pylori infection (and peptic ulcers resulting from H. pylori infection) are correlated with infectivity of the bacteria, and also with the host’s genetic profile and immune status, in addition to the microenvironment in the gastrointestinal tract.4
Military training has long been associated with an increased incidence of peptic ulcers and related gastric diseases; it is therefore clinically relevant to investigate the impact of military stress on H. pylori infections. Animal models have determined that mental stress can affect the mucosal immune response, thereby increasing H. pylori colonization.10 Such research has also found that psychological stress affects the central nervous system, through the combined action of the hypothalamus, pituitary and adrenal glands (the HPA axis), and the endocrine and immune systems.11 The impact of these factors on H. pylori infections in humans remains unclear, however.
The present study tested the hypothesis that military stress causes emotional and neuroendocrine changes in soldiers that affect their immune response to bacterial infections, including those caused by H. pylori. Military recruits were investigated for H. pylori infection levels and immune response levels before and after undertaking basic military training.
Subjects and methods
Study population
The present prospective observational study included new Chinese male military recruits and was conducted at a military training base in Shijiazhuang City, Hebei Province, China, between December 2012 and February 2013. The recruits were randomly chosen from a group of new soldiers at a training base, using a computer-generated randomization schedule. Inclusion criteria were based on the Candidates Citizen Physical Examination Standards issued by the Ministry of National Defence, China, in 2003. Exclusion criteria comprised previous physical or mental problems (including abnormal liver function, amblyopia or depressive disorders) according to the Candidates Citizen Physical Examination Standards.
The study protocol was approved by the institutional review board of the Bethune International Peace Hospital, and written informed consent was provided by all subjects. The study protocol conformed to ethical guidelines of the Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the Bethune International Peace Hospital human research committee.
Questionnaire
The Symptom Checklist-90 (SCL-90) questionnaire12 assesses nine psychopathology symptoms and is widely used to evaluate a broad range of psychological problems in a variety of settings. All study subjects were asked to complete an SCL-90 questionnaire12 on the day before their military training started and on the day after it ended. They were asked to complete the questionnaire within 15 min. Only questionnaires that were 100% complete were accepted as valid.
Measurement of inflammatory blood parameters
Blood samples were obtained at 08:00 h, immediately following morning military training and before breakfast. Venous blood (1 ml) was collected from each subject into procoagulation tubes; it was allowed to clot for 30 min at 37℃. Blood samples were then centrifuged at 1760g for 15 min at 37℃, and the supernatant was transferred into fresh tubes for storage at −20℃ until use.
Serum interleukin (IL)-2 and IL-10 levels were measured using enzyme-linked immunosorbent assay (ELISA) Kits (R&D, Minneapolis, MN, USA) and a DNM-9602 ELISA microplate reader (Prang, Beijing, China), according to each manufacturer’s instructions. Serum levels of immunoglobulin (Ig)G, IgM, IgA, complement component (C)3 and C4 were detected by nephelometry using a BN ProSpec® Analyser and ProSpec® assay reagents (Siemens, Munich, Germany) according to the manufacturer’s instructions.
Measurement of serum hormone levels
Serum concentrations of cortisol and catecholamine were measured within 24 h following serum isolation (using the same serum samples as described for inflammatory blood parameters). Cortisol was measured using the IMMULITE® 2000 automated chemiluminescence immunoassay analyser (Siemens) according to the manufacturer’s instructions. Catecholamine was detected using a Human CA ELISA Kit (Lanji Biotechnology, Shanghai, China) according to the manufacturer’s instructions; and samples were analysed using a DNM-9602 ELISA microplate reader (Perlong, Beijing, China).
Detection of H. pylori
Infection with H. pylori was assessed using two assays: a commercial H. pylori IgG ELISA detection kit (Bell Bio-Engineering, Beijing, China) and a C14-urea breath test. For detection of H. pylori IgG, 5 ml venous blood was drawn into collection tubes without anticoagulant and incubated at 37℃ for 30 min to allow clotting; samples were then centrifuged at 3000g for 15 min at 37℃. The supernatant was collected and stored at 4℃, then measured for H. pylori IgG within 24 h of collection using an ELISA kit (Syno Gene, Taizhou, China). Positive and negative controls were supplied by the manufacturer, and the assay was performed according to the manufacturer’s instructions.
The C14-urea breath test was performed as described previously.13 C14-urea breath-test values have been reported as indicators of the severity of gastric H. pylori infection when values >100 disintegrations per min (dpm) are observed.14,15 For the present study, a C14-urea breath-test value >100 dpm/mmol CO2 was defined as positive and <100 dpm/mmol CO2 as negative. In the morning following an overnight fast, subjects rinsed their mouths prior to the test. Each subject swallowed one capsule containing 2.78 × 10–3 MBq (0.075 × 10–3 mCi) C14 urea with lukewarm water; after 25 min, subjects were asked to exhale through a straw into a CO2 collector. The time taken for the coloured fluid in the CO2 collector to change from purple-red to colourless was measured (this is expected to take between 1 and 3 min). The test was stopped at 3 min, even if the colour was not completely changed. Samples were sealed with caps after adding 4.5 ml of diluted scintillation fluid using pure methanol-rinsed transfer pipettes. Drops of pure methanol were added if the scintillation fluid was not dissolved. Each sample was then labelled on the cap of the bottle. The wall and bottom of the sample bottle were cleaned with paper towels or filter paper without fluorescent brightening agent. Samples were shaken and mixed well, and C14 radiation was measured for 2 min. Dpm was then calculated and data were analysed as mean ± SD dpm values.
Flow cytometry analyses of T-cell subsets
Six parameter flow-cytometric analyses were performed on whole blood samples, according to standard procedures using a panel of monoclonal antibodies. Briefly, 1 ml venous blood was drawn into a K2-ethylenediaminetetra-acetic acid (EDTA)-anticoagulant tube. Immediately following blood collection, 50 μl K2-EDTA anticoagulant blood was mixed with 5 μl multicoloured mouse antihuman CD4-fluorescein isothiocyanate/CD8-Phycoerythrin/CD3-Phycoerythrin-Cy5 (PC5) (clones 13B8.2/B9.11/UCHT1, respectively; BD Bioscience, San José, CA, USA). Following incubation for 45 min at room temperature, the red blood cells were haemolysed by adding 500 μl OPTIYSE C (Beckman Coulter, Pasadena, CA, USA) and incubating for 10 min in a 37℃ water bath. Phosphate buffered saline (1 × PBS, pH7.4) was then added to the final volume of 2 ml and samples were centrifuged at 300 g for 5 min at room temperature. The supernatant was discarded and 500 μl PBS was added. Within 24 h of staining, flow cytometric acquisition was performed on ≥ 100 000 CD3+ T cells on a COULTER® EPICS® XL™ flow cytometer (Beckman Coulter Inc., Brea, CA, USA) driven by Expo32™ ADC software, version 1.2 (Beckman Coulter). The acquired data were analysed using Expo32™ ADC software, version 1.2.
Statistical analyses
Data were expressed as mean ± SD, or n % (qualitative data). Results were analysed using SPSS® software, version 13.0 (SPSS Inc., Chicago, IL, USA). Student’s paired t-tests were used to examine any differences between results before and after military training. McNemar’s test was applied to compare differences between the rate of H. pylori antibody positivity before and after military training. P < 0.05 was considered to be statistically significant.
Results
A total of 60 male military recruits were included in the present study, with a mean age of 18.8 ± 1.7 years (range, 18–23 years). All subjects were educated to at least high school or undergraduate college level.
SCL-90 scores
Prior to the start of their basic military training, the new military recruits’ SCL-90 factor scores were not significantly different from those of nonmilitary subjects with no specific stress factors (data not shown).16 Following basic military training, recruits had significantly higher SCL-90 scores in terms of somatization, depression and paranoid ideation (all P < 0.05; Table 1) compared with their scores before training. There were no significant before or after training differences in other itemized factor scores, including obsessive-compulsive behaviour, interpersonal sensitivity, anxiety, hostility, phobic anxiety or psychosis.
Table 1.
Symptom checklist-90 (SCL-90) questionnaire scores in male subjects aged 18.8 ± 1.7 years from a military base in Shijiazhuang City, Hebei Province, China, prior to and following a 3-month basic training programme.
| Data set |
|||
|---|---|---|---|
| SCL-90 factor | Before military training n = 60 | After military training n = 60 | Statistical significance |
| Somatization | 1.27 ± 0.32 | 1.64 ± 0.63 | P < 0.001 |
| Obsessive–compulsive behaviour | 1.54 ± 0.46 | 1.66 ± 0.51 | NS |
| Interpersonal sensitivity | 1.46 ± 0.46 | 1.57 ± 0.48 | NS |
| Depression | 1.35 ± 0.40 | 1.64 ± 0.65 | P = 0.006 |
| Anxiety | 1.65 ± 0.47 | 1.74 ± 0.49 | NS |
| Hostility | 1.29 ± 0.46 | 1.42 ± 0.44 | NS |
| Phobic anxiety | 1.15 ± 0.29 | 1.21 ± 0.32 | NS |
| Paranoid ideation | 1.29 ± 0.55 | 1.58 ± 0.61 | P = 0.008 |
| Psychosis | 1.51 ± 0.40 | 1.66 ± 0.50 | NS |
Data presented as mean ± SD.
NS, no statistically significant between-group difference (P > 0.05; Student’s paired t-test).
H. pylori positivity rates
Helicobacter pylori IgG ELISA results were similar before (26/60 [43.3%]) and after (29/60 [48.3%]) basic military training, and showed that there were three new H. pylori-positive subjects following training. Using the C14-urea breath-test criteria (>100 dpm/mmol CO2 indicating a positive and <100 dpm/mmol CO2 a negative result), the H. pylori infection rates were 46.7% (28/60) prior to training and 48.3% (29/60) post-training. Mean C14-urea breath-test values were significantly higher after military training than before training (470.85 ± 691.27 dpm versus 222.95 ± 405.32 dpm; P < 0.01).
Serum hormone levels
Serum cortisol levels were significantly higher in military recruits after basic military training (18.69 ± 5.98 µg/dl) compared with before training (8.28 ± 2.43 µg/dl; P < 0.01). Serum catecholamine levels were also significantly higher in military recruits after basic military training (320.76 ± 123.75 pg/ml) than before training (276.64 ± 156.89 pg/ml; P < 0.01).
CD4+and CD8+ T-cell levels
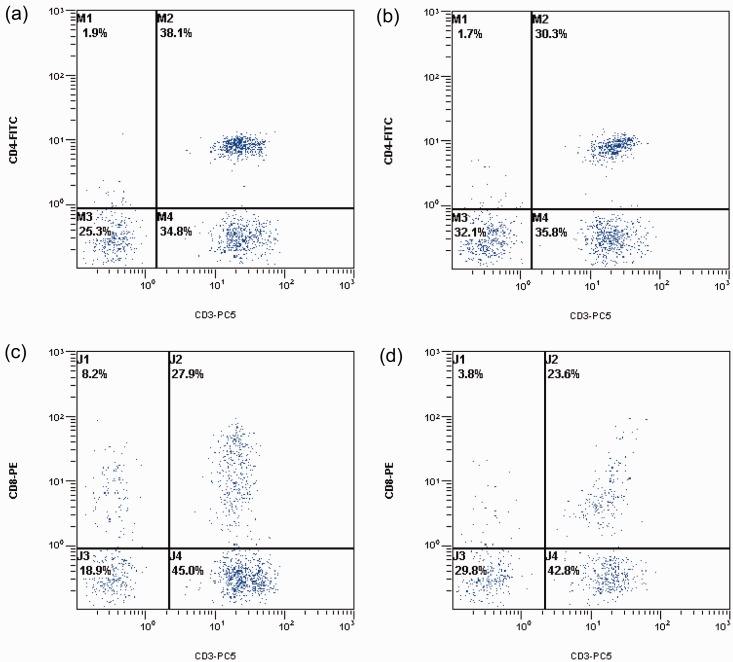
The percentage of CD4+ T cells decreased from 38.00 ± 9.62% before training to 35.39 ± 5.67% after training (P < 0.05; Figure 1a, 1b). In addition, the percentage of CD8+ T cells decreased from 26.78 ± 5.07% before training to 25.71 ± 5.11% after training (P < 0.05; Figures 1C, 1D). In addition, the CD4+/CD8+ ratio decreased from 1.48 ± 0.27 before military training to 1.37 ± 0.25 after training (P < 0.05; data not shown).
Figure 1.
Flow cytometry scatter plots showing CD4+ and CD8+ T cells isolated from male subjects aged 18.8 ± 1.7 years from a military base in Shijiazhuang City, Hebei Province, China, prior to and following a 3-month basic training programme. (A) Number of CD4+ cells before military training commenced; (B) Number of CD4+ cells following military training; (C) Number of CD8+ cells before military training commenced; (D) Number of CD8+ cells following military training. CD4-FITC, CD4+ fluorescein isothiocyanate-stained cells; CD8-PE, CD8+ phycoerythrin-stained cells; CD3-PC5, CD3+ phycoerythrin-Cy5-stained cells; M1–4 and J1–4 represent arbitrary labelling of the four quadrants for analysis.
Serum IL-2 and IL-10 levels
Serum IL-2 levels decreased from 35.04 ± 13.77 pg/ml in subjects before basic military training commenced to 30.89 ± 13.85 pg/ml after training (P < 0.01), while IL-10 levels increased from 11.02 ± 3.32 pg/ml to 13.26 ± 3.86 pg/ml (before and after training, respectively; P < 0.01). The IL-2/IL-10 ratio was significantly reduced from 3.26 ± 1.66 before training commenced to 2.66 ± 1.63 after training (P < 0.01).
Serum IgG and complement levels
Serum IgG, IgA and IgM levels were 12.81 ± 1.76 g/l, 1.98 ± 0.53 g/l and 1.96 ± 0.54 g/l, respectively, in military recruits before military training commenced; after training, all significantly decreased to 11.91 ± 1.91 g/l, 1.82 ± 0.54 g/l and 1.92 ± 0.52 g/l, respectively (all P < 0.05). In contrast, complement C3 and C4 levels did not change significantly after, compared with before, military training (C3, 1.13 ± 0.22 g/l before versus 1.12 ± 0.25 g/l after training; C4, 0.26 ± 0.079 g/l before versus 0.27 ± 0.080 g/l after training).
Discussion
Stress is a nonspecific adaptive bodily response that can be stimulated by a variety of internal and external events or social and psychological factors. It is recognized as an important cause of serious health problems. Psychological stress has been demonstrated to inhibit immune function, increase the body’s susceptibility to infection and play a substantial role in a variety of diseases.17,18 Anecdotal evidence and clinical observation have also suggested that exposure to psychological stress can affect disease outcomes in immune-related disorders, such as viral/bacterial infections and chronic autoimmune diseases.17,19,20 Interactions between the components of the HPA axis that produce cortisol and catecholamine, and which affect the sympathetic nervous system, also impact on immune regulation.21
Epidemiological data show that the presence of H. pylori infection is closely correlated with the immune response and microenvironment in the gastrointestinal tract.4,9 H. pylori infection is a substantial cause of numerous gastrointestinal diseases that are closely associated with gastric carcinoma and lymphoma.6 In patients with gastric ulcers, a synergistic relationship between H. pylori infection and stress is associated with ulcer recurrence,22–24 but this relationship remains unclear. In one study, H. pylori colonization was significantly higher in the stomachs of psychologically stressed BALB/c mice than in those of control mice, and the increased H. pylori colonization was thought to be mediated by a stress-related decrease in the mucosal immune response.10
The present study found that the stress of military training was associated with an increase in several SCL-90 scores along with cortisol and catecholamine levels, both of which are markers of stress.20,21 Following basic training, the H. pylori IgG ELISA assay revealed three new cases of H. pylori infection, and C14-urea breath-test levels were significantly increased. High C14 urea levels have been shown to indicate heightened levels of gastric H. pylori bacteria,14,15 and may have occurred because stress-induced activation of the endocrine system (indicated by upregulation of cortisol and catecholamines) caused impaired immune function and resulted in a higher vulnerability to infection, including H. pylori infection.25–27 The natural acquisition or spontaneous elimination of H. pylori appears to be very rare in adults,28 but under abnormal physiological conditions (such as military stress), H. pylori infections could be enhanced.
The T lymphocytes are critical to the immune response by protecting the body from external pathogens.29 In the military recruits included in the present study, there were fewer peripheral blood CD4+ and CD8+ T lymphocytes after military training compared with before training. CD4+ T lymphocytes are also called helper T cells (TH cells); these cells enhance the activity of other immune cells by releasing T-cell cytokines.30 CD4+ T cells are essential for B-cell antibody class switching, activation and growth of cytotoxic T cells, and maximizing the bactericidal activity of phagocytes such as macrophages.31 CD8+ T cells are also called suppressor T cells and have the ability to downregulate nonself antigens, including antigens to viral or bacterial infections.32 The reductions in CD4+ and CD8+ T cells following basic military training in the present study suggest that the stress of such training impaired immune function.
The CD4+/CD8+ ratio is also a reflection of immune system health. The present study findings showed that basic military training was associated with reduced CD4+/CD8+ ratios, suggesting that such training may attenuate the cellular immune response of new recruits. These results concur with reports showing that CD4+/CD8+ infiltration of the gastric mucosa is correlated with H. pylori infection and symptoms in patients with chronic gastritis.33,34
Proliferating CD4+ cells develop into the two major types of effector T cells: TH1 and TH2. TH1 cells are triggered by IL-2 and IL-12; TH2 cells are triggered by IL-4, IL-5, IL-10 and IL-13. TH1 and TH2 cells are the most important antagonists to viral/bacterial infections.35 In the present study, basic military training was associated with decreased serum IL-2 levels and increased serum IL-10 levels, resulting in a significant reduction in the IL-2/IL-10 ratio after, compared with before, training. This change in the IL-2/IL-10 ratio may have resulted in a disturbance in the TH1/TH2 population, which may be associated with immune dysfunction and may contribute to military stress-induced aggravation of H. pylori infection.
Immunoglobulins and complements are important mediators of humoral immunity.36,37 After basic military training, IgG, IgA, and IgM levels all significantly decreased compared with before training in the present study. This suggests impaired humoral immunity, which may contribute to increased H. pylori colonization. In contrast, complement C3 and C4 were not affected by basic military training. This may be because complement proteins are not directly affected by antigens; they autoregulate and are the most constant mediators in the immune response.38
The present study has a number of limitations. First, the sample size was relatively small. Secondly, the H. pylori IgG ELISA test only reflects the presence of infection, whereas C14-urea breath-test values may reflect the intensity of the level of colonization in subjects already infected.14,15,39 The two methods used to detect H. pylori infection in the present study are not as precise as real-time polymerase chain reaction analysis of H. pylori DNA from gastric mucosal specimens; however, the invasiveness of gastric mucosa sampling and limited time allotted for military training prohibited the performance of gastroscopic examinations. Thirdly, 3 months of living in close contact with other recruits may have led to new H. pylori infections; precautions were taken, however to minimize risk of cross contamination: sharing of eating utensils and materials for washing, shaving, teeth brushing and clothes was strictly prohibited; and daily indoor sanitation and regular air disinfection procedures were performed. In addition, future research is needed to evaluate the generation of free-oxide radicals during training in military recruits. Such research may provide important additional information that could strengthen the link between heightened stress levels and negative effects in the gastrointestinal tract.
In conclusion, the results of the present study show that military stress, occurring as a result of basic military training, may reduce both cellular and humoral immunity, and may aggravate H. pylori infections in new recruits. The severity of H. pylori infection can increase the risk of peptic ulcers and their complications, such as gastrointestinal haemorrhage and perforation; these can potentially compromise the ability of military personnel to carry out their duties correctly. Although the impact of all forms of military stress on host immunity and H. pylori infections needs further investigation, the present study suggests that immune-boosting or anti-H. pylori agents may help soldiers to better withstand military stress.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research was supported by the Natural Science Foundation of Hebei Province (H2014505009).
References
- 1.Yu XZ, Liu HF, Sun ZX. Investigation of the effect of military stress on the prevalence of functional bowel disorders. World J Gastroenterol 2012; 18: 3004–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor MK, Padilla GA, Stanfill KE, et al. Effects of dehydroepiandrosterone supplementation during stressful military training: a randomized, controlled, double-blind field study. Stress 2012; 15: 85–96. [DOI] [PubMed] [Google Scholar]
- 3.Macgregor AJ, Tang JJ, Dougherty AL, et al. Deployment-related injury and posttraumatic stress disorder in US military personnel. Injury 2013; 44: 1458–1464. [DOI] [PubMed] [Google Scholar]
- 4.Backert S, Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter 2011; 16 Suppl 1: 19–25. [DOI] [PubMed] [Google Scholar]
- 5.Dubois A. Spiral bacteria in the human stomach: the gastric helicobacters. Emerg Infect Dis 1995; 1: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall BJ, Windsor HM. The relation of Helicobacter pylori to gastric adenocarcinoma and lymphoma: pathophysiology, epidemiology, screening, clinical presentation, treatment, and prevention. Med Clin North Am 2005; 89: 313–344. [DOI] [PubMed] [Google Scholar]
- 7.Roesler BM, Rabelo-Gonçalves EM, Zeitune JM. Virulence factors of helicobacter pylori: a review. Clin Med Insights Gastroenterol 2014; 7: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watari J, Chen N, Amenta PS, Fukui H, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 2014; 20: 5461–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepulveda AR. Helicobacter, Inflammation, and Gastric Cancer. Curr Pathobiol Rep 2013; 1: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo G, Jia KR, Shi Y, et al. Psychological stress enhances the colonization of the stomach by Helicobacter pylori in the BALB/c mouse. Stress 2009; 12: 478–485. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Xu Z, Gao Y, et al. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PloS One 2012; 7: e51148–e51148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bech P, Bille J, Møller SB, et al. Psychometric validation of the Hopkins Symptom Checklist (SCL-90) subscales for depression, anxiety, and interpersonal sensitivity. J Affect Disord 2014; 160: 98–103. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Zou X, Liu YF, et al. Association between helicobacter pylori infection and chronic idiopathic neutropenia. J Huazhong Univ Sci Technolog Med Sci 2013; 33: 353–356. [DOI] [PubMed] [Google Scholar]
- 14.Sheu BS, Lee SC, Yang HB, et al. Quantitative result of 13C urea breath test at 15 minutes may correlate with the bacterial density of H. pylori in the stomach. Hepatogastroenterology 1999; 46: 2057–2062. [PubMed] [Google Scholar]
- 15.Vincent P, Michaud L, Martin de Lasalle E, et al. 13C-urea breath test and gastric mucosal colonization by Helicobacter pylori in children: quantitative relation and usefulness for diagnosis of infection. Helicobacter 1999; 4: 233–237. [DOI] [PubMed] [Google Scholar]
- 16.Feng Z, Yang G, Ren H, et al. Personality, coping style and mental health status of military personnel in military stress. Journal of the Fourth Military Medical University 2004; 25: 2079–2082. [Google Scholar]
- 17.Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun 2007; 21: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 18.Melinder C, Udumyan R, Hiyoshi A, et al. Decreased stress resilience in young men significantly increases the risk of subsequent peptic ulcer disease - a prospective study of 233 093 men in Sweden. Aliment Pharmacol Ther 2015; 41: 1005–1015. [DOI] [PubMed] [Google Scholar]
- 19.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol 2006; 1: 421–427. [DOI] [PubMed] [Google Scholar]
- 20.Kanno T, Iijima K, Abe Y, et al. Peptic ulcers after the Great East Japan earthquake and tsunami: possible existence of psychosocial stress ulcers in humans. J Gastroenterol 2013; 48: 483–490. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 2006; 8: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto N, Sakagami T, Fukuda Y, et al. Influence of Helicobacter pylori infection on development of stress-induced gastric mucosal injury. J Gastroenterol 2000; 35: 332–340. [DOI] [PubMed] [Google Scholar]
- 23.Budzynski J, Klopocka M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J Gastroenterol 2014; 20: 5212–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahm KB, Lee KJ, Kim YS, et al. Augmented eradication rates of Helicobacter pylori by new combination therapy with lansoprazole, amoxicillin, and rebamipide. Dig Dis Sci 1998; 43: 235–240. [DOI] [PubMed] [Google Scholar]
- 25.Black PH. Central nervous system-immune system interactions: psychoneuroendocrinology of stress and its immune consequences. Antimicrob Agents Chemother 1994; 38: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang L, Del Ben KS, Rehm KE, et al. Effects of acute stress-induced immunomodulation on TH1/TH2 cytokine and catecholamine receptor expression in human peripheral blood cells. Neuropsychobiology 2012; 65: 12–19. [DOI] [PubMed] [Google Scholar]
- 27.Sandal GM, Grønningsæter H, Eriksen HR, et al. Personality and Endocrine Activation in Military Stress Situations. Military Psychology 1998; 10: 45–61. [Google Scholar]
- 28.Xia HH, Talley NJ. Natural acquisition and spontaneous elimination of Helicobacter pylori infection: clinical implications. Am J Gastroenterol 1997; 92: 1780–1787. [PubMed] [Google Scholar]
- 29.Kononova TE, Urazova OI, Novitski VV, et al. [T-lymphocyte-helper type 17 mediated regulation of antibacterial (antituberculosis) immunity]. Mol Biol (Mosk) 2013; 47: 883–890. [In Russian, English abstract]. [PubMed] [Google Scholar]
- 30.Lapierre P, Lamarre A. Regulatory T cells in autoimmune and viral chronic hepatitis. J Immunol Res 2015; 2015: 479703–479703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phetsouphanh C, Xu Y, Zaunders J. CD4 T cells mediate both positive and negative regulation of the immune response to HIV infection: Complex role of T follicular helper cells and regulatory T cells in pathogenesis. Front Immunol 2015; 6: 681–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raphael I, Nalawade S, Eagar TN, et al. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015; 74: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu AP, Zhang SS, Zha QL, et al. Correlation between CD4, CD8 cell infiltration in gastric mucosa, Helicobacter pylori infection and symptoms in patients with chronic gastritis. World J Gastroenterol 2005; 11: 2486–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang KX, Chen L. Helicobacter pylori L-form and patients with chronic gastritis. World J Gastroenterol 2004; 10: 1306–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Infante-Duarte C, Kamradt T. Th1/Th2 balance in infection. Springer Semin Immunopathol 1999; 21: 317–338. [DOI] [PubMed] [Google Scholar]
- 36.Appledorn DM, McBride A, Seregin S, et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther 2008; 15: 1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rus H, Cudrici C, Niculescu F. The role of the complement system in innate immunity. Immunol Res 2005; 33: 103–112. [DOI] [PubMed] [Google Scholar]
- 38.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol 2007; 171: 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20: 280–322. [DOI] [PMC free article] [PubMed] [Google Scholar]