Abstract
Objective
To examine the role of microRNA (miR)-205 in proliferation, migration and invasion of nasopharyngeal carcinoma (NPC).
Methods
The human NPC cell line CNE2 was transfected with miR-205 mimic, anti-miR-205 inhibitor or scrambled oligonucleotide (control). Cell proliferation was assessed via MTT assay. Cell migration and invasion were evaluated by transwell migration and Matrigel® invasion assay, respectively. Radiation induced apoptosis was quantified via Caspase-Glo3/7 assay. Apoptotic proteins and epithelial–mesenchymal transition (EMT) proteins were semiquantified by Western blot analysis.
Results
Overexpression of miR-205 increased the proliferation, migration and invasion of CNE2 cells, and decreased radiation-induced apoptosis compared with control cells. MiR-205 overexpression downregulated E-cadherin and upregulated Snail expression via downregulation of PTEN and upregulation of AKT.
Conclusion
MiR-205 plays vital roles in tumourigenesis and tumour progression in NPC, and may be a potential treatment target.
Keywords: AKT, apoptosis, PTEN, metastasis, miRNA, miRNA-205, NPC, EMT
Introduction
Nasopharyngeal carcinoma (NPC) is the most common primary malignancy of the nasopharynx, with a yearly incidence of 25 cases per 100 000 and a high prevalence in southeast Asia and southern China.1 NPC has three subtypes based on degree of differentiation: keratinizing squamous cell carcinoma (SCC) (type 1); nonkeratinizing carcinoma (type 2); undifferentiated carcinoma (type 3).2 Types 2 and 3 NPC are strongly associated with Epstein–Barr virus (EBV).3 In patients with advanced type 3 NPC, the 5-year survival rate is <50%.4 Radiotherapy is the primary treatment for patients with NPC,5 but radioresistance and local recurrence are major limitations to its value. It is therefore important to investigate the molecular mechanisms and identify predictive markers in NPC.
MicroRNAs (miRNAs) are short noncoding RNA molecules that post-transcriptionally regulate gene expression by binding to the 3′-UTR of their target mRNAs.6 MiRNAs regulate many biological functions including tumour development, differentiation, proliferation and apoptosis.7 Expression profiling has shown that miRNA expression is different in NPC tissue compared with normal nasopharyngeal tissue, and aberrant miRNAs are correlated with clinical stage.8 In addition, some studies have demonstrated that EBV-encoded miRNAs play a critical role in the regulation of EBV infection and latency.9
Growing evidence suggests that miRNAs affect NPC carcinogenesis and metastasis by activating various signalling pathways.10 For example, several miRNAs activate c-Myc, which then regulates tumour cell growth and carcinogenesis;11 in addition, MiR-200a regulation of ZEB2 and β-catenin promotes NPC cell migration and invasion.12
The aim of the present study was to examine the roles of miR-205 in the proliferation, migration and invasion of NPC.
Materials and methods
Cell culture
The human NPC cell line CNE2 was obtained from the Cancer Centre of Hangzhou First People’s Hospital, Hangzhou, China. Cells were grown in RPMI1640 medium (Invitrogen™, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin. Cells were cultured at 37℃ in a humidified incubator in 5% carbon dioxide/95% air.
Transfection
MiR-205 mimic and anti-miR-205 inhibitor were purchased from GenePharma (Shanghai, China). When CNE2 cells reached 70% confluence, they were transfected with miR-205 mimic or anti-miR-205 inhibitor using Lipofectamine® 2000 (Invitrogen, USA) according to the manufacturer’s instructions. Scrambled oligonucleotide was used as negative control for the transfection.
MiRNA quantification
At 48 h after transfection, total RNA was extracted from 106 cells using a mirVana® miRNA isolation kit (Ambion, Carlsbad, CA, USA) according to the manufacturer’s instructions, and cDNA was synthesized using a TaqMan® MicroRNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA, USA). Quantitative real-time polymerase chain reaction (qRT–PCR) was performed using the TaqMan® Universal PCR Master Mix. The cycling programme involved preliminary denaturation at 95℃ for 5 min, followed by 35 cycles of denaturation at 95℃ for 30s, annealing at 65℃ for 30 s, and elongation at 70℃ for 60s, followed by a final elongation step at 95℃ for 5 min. Primer sequences were: GAPDH, sense 5′-GTCTCCTCTGACTTCAACAGCG-3′, and antisense 5′- ACCACCCTGTTGCTGTAGCCAA-3′ (GAPDH); and miR-205, sense primer 5’-TTTTCAGACTCC-3’ and antisense primer 5’-CTCTTGTCCTTCATTCCACC-3’ MiR-205 was quantified by reference to GAPDH levels.
Cell proliferation
At 48 h after transfection, cell proliferation was assessed using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, as described.13 Briefly, 100 µl of transfected CNE2 cell suspension was inoculated to each well of a 96-well plate at 3 × 104 cells/well. On alternate days, MTT solution (20 µl, 5 mg/ml) was added to each well, and the plates were incubated in the dark for 4 h at 37℃, followed by removal of the culture medium and addition of 100 μl dimethyl sulphoxide. Absorbance was measured at 492 nm, with 655 nm as the reference wavelength. Each group used six parallel wells and all experiments were carried out in triplicate.
Irradiation
At 48 h after transfection, cells were seeded in 24-well plates at density of 1 × 105/well. After incubation for 24 h, cells were irradiated with 0, 2, 5 or 10 Gy. CNE2 cells were cultured for a further 36 h before apoptosis was quantified using a Caspase-Glo3/7 assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Luminescence was measured with a luminometer (ThermoFisher Scientific, Waltham, MA, USA) using a 1 min lag time and 0.5 s/well read-time. Experiments were performed in triplicate.
Cell invasion assay
Cell invasion was quantified by Matrigel® invasion assay. At 48 h after transfection, CNE2 cells (transfected with either miR-205 mimic or anti-miR-205 inhibitor) were placed in the upper chamber of a BD Biocoat® Matrigel® Invasion Chamber (BD Bioscience, San Jose, CA, USA) in 0.5 ml DMEM with 0.1% bovine serum albumin. RPMI1640 containing 5% FBS was added to the lower chamber. Cells were incubated for 24 h then noninvading cells were removed with a cotton swab. Invasive cells were stained by Diff-Quik® (ThermoFisher Scientific) stain and counted via light microscopy. The percentage of invasion was expressed as the ratio of invading cells over the cell number, normalized on day 2 of the growth curve.
Western blotting
At 48 h after transfection, 106 transfected CNE2 cells were lysed with RIPA buffer (Beyotime, Jiangsu, China) then boiled for 5 min. Protein samples were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking with 5% nonfat dry milk in 1 × Tris buffered saline/0.1% Tween-20 (pH 7.4; TBST) at room temperature for 1 h, membranes were incubated overnight at 4℃ with rabbit antihuman antibodies to E-cadherin, Snail, caspase 9, Bcl-2, Bax, AKT and PTEN (all 1:500 dilution; Cell Signaling Technology, Beverly, MA, USA), washed three times with TBST for 10 min at room temperature, then incubated with horseradish peroxidase-conjugated mouse antirabbit secondary antibody (1:1000 dilution; Cell Signaling Technology, USA) for 1 h at room temperature. Membranes were washed three times with TBST for 10 min at room temperature and immunoreactive signals were visualized using an EasySee® Western Blot Kit (TransGen Biotech, Beijing, China). Protein bands were quantified by densitometry (Tanon-1600 gel image system; BioTanon, Shanghai, China).
Statistical analyses
Data were presented as mean ± SD. Between-group comparisons were made using Student’s t-test. Statistical analyses were performed using SPSS® version 11.0 (SPSS Inc., Chicago, IL, USA) for Windows®. P-values < 0.05 were considered statistically significant.
Results
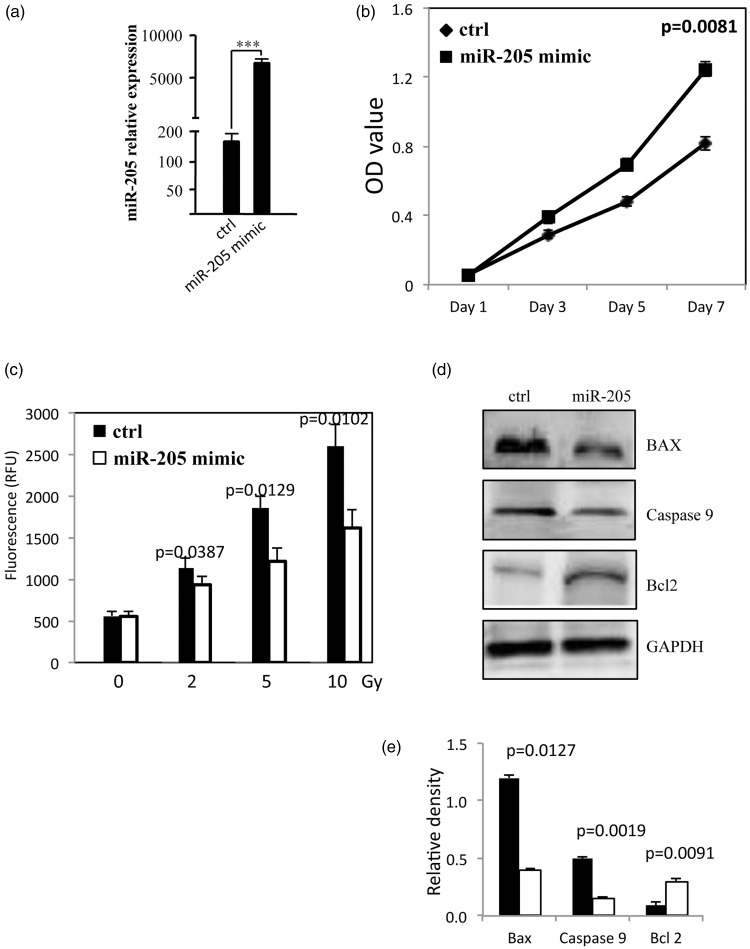
Transfection of CNE2 cells with miR-205 mimic resulted in significantly higher miR-205 expression (P = 0.0002, Figure 1a) and rate of cell proliferation at 7 days after transfection (P = 0.0081; Figure 1b) compared with cells transfected with control oligonucleotide. In addition, CNE2 cells transfected with miR-205 exhibited significantly lower levels of apoptosis after irradiation than control cells (2 Gy irradiation, P = 0.0387; 5 Gy, P = 0.0129; 10 Gy, P = 0.0102; Figure 1c). As shown in Figures 1d and 1e, the radiation-induced (2 Gy) increase in BAX and caspase-9 protein levels was reversed by miR-205 overexpression. In addition, levels of Bcl-2 protein were visibly increased in cells transfected with miR-205 compared with control cells.
Figure 1.
Micro RNA (miR)-205 promotes proliferation and reduces radiation-induced apoptosis in the human nasopharyngeal carcinoma cell line, CNE2. (a) Relative expression of miR-205 in control CNE2 cells and CNE2 cells transfected with miR-205 mimic. (b) Cell proliferation in transfected and control cells (c) Apoptosis (quantified via caspase 3/7 activity) in transfected and control cells treated with 0–10 Gy radiation. (d) Representative Western blot of apoptosis-associated proteins in transfected and control cells treated with 2 Gy radiation (e) Densitometric quantification of apoptosis-associated proteins in transfected and control cells treated with 2 Gy radiation (Black bars denote control and white bars denote miR-205 mimic). All experiments performed in triplicate.
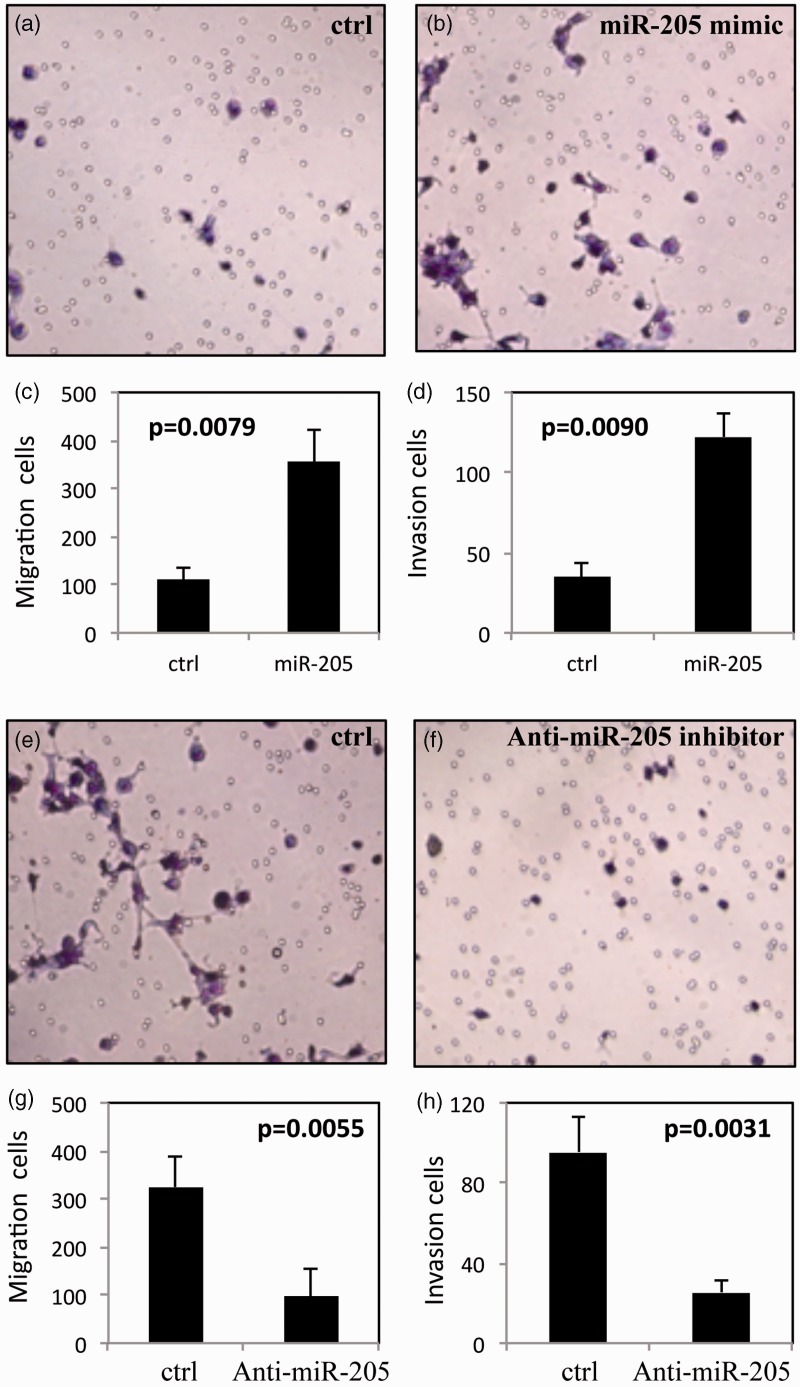
Figure 2.
Micro RNA (miR)-205 increases cell migration and invasion in the human nasopharyngeal carcinoma cell line, CNE2. (a, b) Representative light photomicrographs of Matrigel® invasion assay in (a) control cells and (b) cells transfected with miR-205 mimic. (c) Migration and (d) invasion of control cells and cells transfected with miR-205 mimic. (e, f) Representative light photomicrographs of Matrigel® invasion assay in (e) control cells and (f) cells transfected with anti-miR-205 inhibitor. (g) Migration and (h) invasion of control cells and cells transfected with anti-miR-205 inhibitor. All experiments performed in triplicate.
Migration and invasion were significantly enhanced in CNE2 cells transfected with miR-205 mimic compared with control cells (migration, P = 0.0079; invasion, P = 0.0090; Figures 2a–d). In contrast, transfection with anti-miR-205 inhibitor significantly inhibited migration and invasion compared with control cells (migration, P = 0.0055; invasion, P = 0.0031; Figures 2e–h).
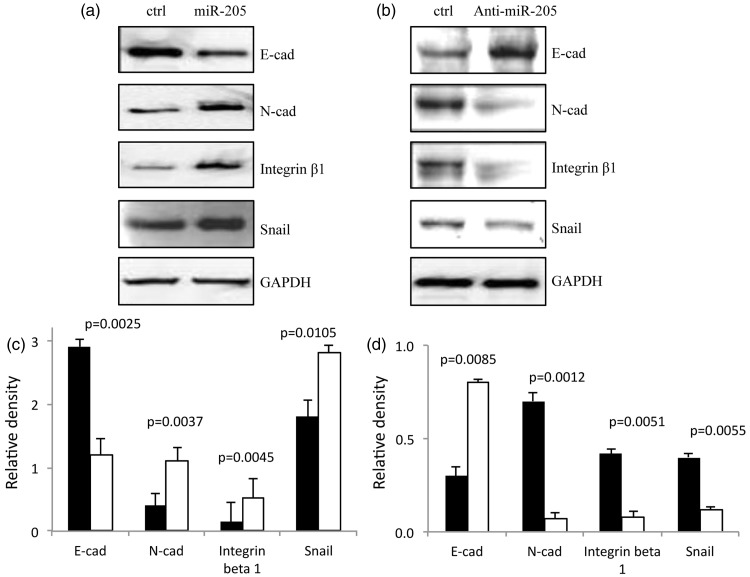
Western blot analysis of epithelial–mesenchymal transition (EMT) proteins is shown in Figure 3. E-cadherin levels were visibly decreased and N-cadherin and Snail levels were visibly increased in CNE2 cells transfected with miR-205 mimic, compared with control cells (Figures 3a and 3c) (P < 0.05). In contrast, levels of E-cadherin were increased, and N-cadherin and Snail were decreased, in CNE2 cells transfected with anti miR-205 inhibitor, compared with control cells (Figures 3b and 3d) (P < 0.05).
Figure 3.
Micro RNA (miR)-205 regulates levels of epithelial-to-mesenchymal (EMT) markers in the human nasopharyngeal carcinoma cell line, CNE2. Representative Western blot of EMT markers in (a) control CNE2 cells and CNE2 cells transfected with miR-205 mimic, and (b) control CNE2 cells and CNE2 cells transfected with anti-miR-205 inhibitor. Densitometric quantification (relative to GAPDH levels) of EMT markers in (c) control CNE2 cells and CNE2 cells transfected with miR-205 mimic, and (d) control CNE2 cells and CNE2 cells transfected with anti-miR-205 inhibitor. Black bars denote control and white bars denote miR-205 mimic (c) and anti-miR-205 inhibitor (d). All experiments performed in triplicate.
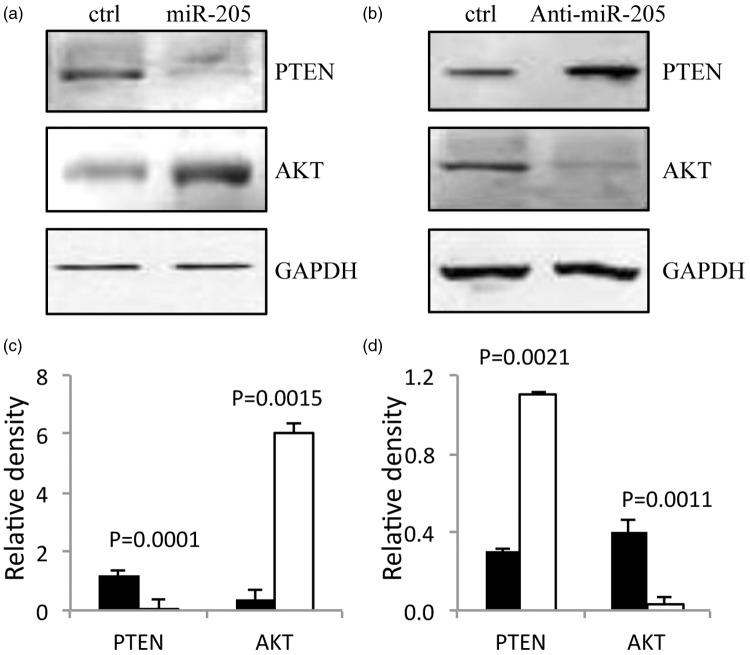
Western blot analysis of the signalling proteins AKT and PTEN is shown in Figure 4. Transfection with miR-205 mimic resulted in increased AKT and decreased PTEN levels compared with control cells (Figures 4a) and 4c) (P < 0.05). In contrast, transfection with anti miR-205 inhibitor resulted in decreased AKT and increased PTEN levels compared with control cells (Figures 4b and d) (P < 0.05).
Figure 4.
Micro RNA (miR)-205 reduces PTEN and increases AKT levels in the human nasopharyngeal carcinoma cell line, CNE2. Representative Western blot of PTEN and AKT in (a) control CNE2 cells and CNE2 cells transfected with miR-205 mimic, and (b) control CNE2 cells and CNE2 cells transfected with anti-miR-205 inhibitor. Densitometric quantification (relative to GAPDH levels) of AKT and PTEN in (c) control CNE2 cells and CNE2 cells transfected with miR-205 mimic, and (d) control CNE2 cells and CNE2 cells transfected with anti-miR-205 inhibitor. Black bars denote control and white bars denote miR-205 mimic (c) and anti-miR-205 inhibitor (d). All experiments performed in triplicate.
Discussion
Studies have shown that miR-205 can act as either a tumour suppressor or an oncogene depending on the tumour type.14–16 Overexpression of miR-205 increased proliferation and inhibited radiation-induced apoptosis of CNE2 cells in the present study. Consistent with our findings, others have shown that miR-205 promotes growth, metastasis and chemoresistance in nonsmall cell lung cancer17 and is upregulated in NPC.18 There is evidence to suggest that miR-205 induces radioresistance in NPC cells via activation of PTEN.19,20 Taken together, these data suggest that miR-205 plays a critical role in NPC development and treatment.
Aggressive tumours are characterized by local invasion and distant metastasis. These processes include cell proliferation, changes in cell morphology and destruction of the extracellular matrix.21–23 EMT proteins play vital roles in tumour progression, invasion and metastasis,24 and the PTEN pathway is involved in EMT signalling in NPC.25–27 The tumour suppressor PTEN is crucial for multiple cellular processes including cell proliferation, apoptosis and cell migration,28,29 and negatively regulates AKT signalling.24 AKT controls multiple biological processes including cell proliferation, differentiation, apoptosis, glucose metabolism, and tumourigenesis,30 and promotes cell proliferation, survival and metastasis in NPC via inactivation of FKHR and BAD.31,32 In accordance with the findings of others,19 overexpression of miR-205 downregulated PTEN and upregulated AKT levels, in the present study.
Migration and invasion of CNE2 cells were enhanced by overexpression of miR-205 in the present study. In addition, mIR-205 overexpression resulted in downregulation of E-cadherin and upregulation of Snail proteins. E-cadherin, a member of the cadherin superfamily, is a calcium-dependent transmembrane glycoprotein involved in maintaining cell polarity and normal structure by strengthening intercellular adhesion.33 Downregulation of E-cadherin is a marker of EMT changes in epithelial cells.34 The zinc-finger transcription factor Snail is a prominent inducer of EMT that has been shown to inhibit E-cadherin expression35,36 and PTEN transcription.37 Snail is also associated with radioresistance,37 maintenance of a stem cell-like phenotype38 and poor prognosis.39 It is possible that elevated levels of Snail may contribute to PTEN downregulation in the present study.
In conclusion, our findings suggest that miR-205 promotes NPC cell proliferation, invasion and EMT, and inhibits radiation induced apoptosis. MiR-205 plays critical roles in the progression of NPC via regulation of PTEN and AKT signalling, and may represent a molecular treatment target for NPC.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by Award to Young Physician Scientist in Hangzhou First People’s Hospital, Hangzhou, China (2011020103).
References
- 1.Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer 2011; 30: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer 1993; 71: 2689–2697. [DOI] [PubMed] [Google Scholar]
- 3.Gullo C, Low WK, Teoh G. Association of Epstein-Barr virus with nasopharyngeal carcinoma and current status of development of cancer-derived cell lines. Ann Acad Med Singapore 2008; 37: 769–777. [PubMed] [Google Scholar]
- 4.Chang JT, Ko JY, Hong RL. Recent advances in the treatment of nasopharyngeal carcinoma. J Formos Med Assoc 2004; 103: 496–510. [PubMed] [Google Scholar]
- 5.Feng XP, Yi H, Li MY, et al. Identification of biomarkers for predicting nasopharyngeal carcinoma response to radiotherapy by proteomics. Cancer Res 2010; 70: 3450–3462. [DOI] [PubMed] [Google Scholar]
- 6.Felekkis K, Touvana E, Stefanou Ch, et al. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010; 14: 236–240. [PMC free article] [PubMed] [Google Scholar]
- 7.Ha TY. MicroRNAs in human diseases: from cancer to cardiovascular disease. Immune Netw 2011; 11: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Chen JX, Fu XP, et al. microRNA expression profiling of nasopharyngeal carcinoma. Oncol Rep 2011; 25: 1353–1363. [DOI] [PubMed] [Google Scholar]
- 9.Iizasa H, Wulff BE, Alla NR, et al. Editing of Epstein-Barr virus-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem 2010; 285: 33358–33370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He ML, Luo MX, Lin MC, et al. MicroRNAs: potential diagnostic markers and therapeutic targets for EBV-associated nasopharyngeal carcinoma. Biochim Biophys Acta 2012; 1825: 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Luo J, Xiao J, Tao Z, et al. Detection of c-myc gene expression in nasopharyngeal carcinoma by nonradioactive in situ hybridization and immunohistochemistry. Chin Med J (Engl) 1997; 110: 229–232. [PubMed] [Google Scholar]
- 12.Xia H, Cheung WK, Sze J, et al. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and beta-catenin signaling. J Biol Chem 2010; 285: 36995–37004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maioli E, Torricelli C, Fortino V, et al. Critical appraisal of the MTT assay in the presence of rottlerin and uncouplers. Biol Proced Online 2009; 11: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Tang S, Le SY, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One 2008; 3: e2557–e2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sempere LF, Christensen M, Silahtaroglu A, et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res 2007; 67: 11612–11620. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer A, Jung M, Mollenkopf HJ, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer 2010; 126: 1166–1176. [DOI] [PubMed] [Google Scholar]
- 17.Lei L, Huang Y, Gong W. miR-205 promotes the growth, metastasis and chemoresistance of NSCLC cells by targeting PTEN. Oncol Rep 2013; 30: 2897–2902. [DOI] [PubMed] [Google Scholar]
- 18.Chen HC, Chen GH, Chen YH, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer 2009; 100: 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qu C, Liang Z, Huang J, et al. MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle 2012; 11: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Wang S, Liu Q, et al. SZ-685C exhibits potent anticancer activity in both radiosensitive and radioresistant NPC cells through the miR-205-PTEN-Akt pathway. Oncol Rep 2013; 29: 2341–2347. [DOI] [PubMed] [Google Scholar]
- 21.Hainaut P, Plymouth A. Targeting the hallmarks of cancer: towards a rational approach to next-generation cancer therapy. Curr Opin Oncol 2013; 25: 50–51. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 23.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer 2013; 4: 66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 2005; 24: 7443–7454. [DOI] [PubMed] [Google Scholar]
- 25.Lin Z, Wan X, Jiang R, et al. Epstein-Barr virus-encoded latent membrane protein 2A promotes the epithelial-mesenchymal transition in nasopharyngeal carcinoma via metastatic tumor antigen 1 and mechanistic target of rapamycin signaling induction. J Virol 2014; 88: 11872–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, Li S, Xie D, et al. CD44 regulates epithelial-mesenchymal transition and metastasis in nasopharyngeal cancer cells. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2013; 27: 250–254. [in Chinese, English Abstract]. [PubMed] [Google Scholar]
- 27.Cai LM, Lyu XM, Luo WR, et al. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene 2015; 34: 2156–2166. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Liu Y, Du Y, et al. The predictive role of phosphatase and tensin homolog (PTEN) loss, phosphoinositol-3 (PI3) kinase (PIK3CA) mutation, and PI3K pathway activation in sensitivity to trastuzumab in HER2-positive breast cancer: a meta-analysis. Curr Med Res Opin 2013; 29: 633–642. [DOI] [PubMed] [Google Scholar]
- 29.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 2009; 4: 127–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 2007; 129: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yip WK, Leong VC, Abdullah MA, et al. Overexpression of phospho-Akt correlates with phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal carcinoma. Oncol Rep 2008; 19: 319–328. [PubMed] [Google Scholar]
- 32.Jiang H, Gao M, Shen Z, et al. Blocking PI3K/Akt signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol Rep 2014; 32: 559–566. [DOI] [PubMed] [Google Scholar]
- 33.Chaw SY, Majeed AA, Dalley AJ, et al. Epithelial to mesenchymal transition (EMT) biomarkers–E-cadherin, beta-catenin, APC and Vimentin–in oral squamous cell carcinogenesis and transformation. Oral Oncol 2012; 48: 997–1006. [DOI] [PubMed] [Google Scholar]
- 34.Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol 2006; 172: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Shi J, Chai K, et al. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets 2013; 13: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2: 84–89. [DOI] [PubMed] [Google Scholar]
- 37.Escrivà M, Peiró S, Herranz N, et al. Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol 2008; 28: 1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W, Lv R, Qi W, et al. Snail contributes to the maintenance of stem cell-like phenotype cells in human pancreatic cancer. PLoS One 2014; 9: e87409–e87409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin NR, Jeong EH, Choi CI, et al. Overexpression of snail is associated with lymph node metastasis and poor prognosis in patients with gastric cancer. BMC Cancer 2012; 12: 521–521. [DOI] [PMC free article] [PubMed] [Google Scholar]