Abstract
Objective
To investigate the role of zinc-alpha-2-glycoprotein (ZAG) in the early stage of diabetic nephropathy, in patients with type 2 diabetes mellitus (T2DM).
Methods
This cross-sectional observational study recruited patients with longstanding T2DM and healthy control subjects. Patients with T2DM were further stratified based on their urine albumin–creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR). Serum and urine concentrations of ZAG were determined using an enzyme-linked immunosorbent assay.
Results
Eighty patients with T2DM and 20 healthy control subjects were enrolled in the study. Mean ± SD concentrations of ZAG in serum and urine were both significantly higher in patients with T2DM (serum: 38.29 ± 22.72 mg/l; urine: 53.64 ± 29.48 mg/g) compared with concentrations in healthy control subjects (serum: 21.61 ± 8.83 mg/l; urine: 28.17 ± 10.64 mg/g). Serum ZAG concentration was positively correlated with serum creatinine and eGFR. Urine ZAG concentration was positively correlated with UACR. Urine concentration of ZAG in the higher eGFR group was higher than that in the normal eGFR group (41.26 ± 13.67 versus 32.05 ± 8.55 mg/g, respectively).
Conclusion
These preliminary findings suggest that ZAG might be a potentially useful biomarker for early diagnosis of diabetic nephropathy in patients with T2DM.
Keywords: Zinc-alpha-2-glycoprotein, diabetic nephropathy, albuminuria, biomarker
Introduction
Diabetes mellitus continues to be an important clinical problem throughout the world.1 One of the factors associated with mortality and morbidity in patients with diabetes is diabetic nephropathy.2 Currently, the most popular method of detecting the early signs of nephropathy in patients with diabetes is the measurement of microalbuminuria.3 However, pathological abnormalities have been reported to occur before the onset of microalbuminuria.4 Interestingly, in chronic cases of diabetic nephropathy, renal function correlates better with the degree of tubulointerstitial injury rather than with glomerular lesions, suggesting that researchers should look for tubular biomarkers in order to identify patients with diabetic nephropathy.5 There has been a growing interest in identifying alternative biomarkers that might provide a sensitive and rapid means of detecting the progression of diabetic nephropathy. In this regard, biomarkers that reflect tubular damage have been proposed by various investigators.6–8
Zinc-alpha-2-glycoprotein (ZAG) is a 41–43 kDa glycoprotein assigned to the major histocompatibility complex class I family of proteins.9,10 ZAG is present in a variety of epithelia and is secreted into many body fluids.11 ZAG is known to stimulate lipolysis through stimulation of adenylate cyclase in a guanosine triphosphate-dependent process via binding through the β3 adrenoreceptor.9 ZAG was suggested to be involved in various biological processes including regulation of melanin production by melanocytes, prostate and bladder cancer, cachexia, obesity and inhibition of cell proliferation.12–19 ZAG might be involved in the pathogenesis of obesity-related metabolic disorders in humans as ZAG was correlated with glucose, creatinine and uric acid in patients with metabolic syndrome.20 Russell and Tisdale21 found that recombinant human ZAG counters some of the metabolic features of the diabetic state in ob/ob mice, including a reduction in plasma insulin levels associated with an increased retention of insulin by the pancreas, and an improved response in the glucose tolerance test due to increased glucose use. Proteomic analyses found that urine ZAG increased specifically in patients with diabetes and may be used as a biomarker for the specific and accurate clinical analysis of diabetic nephropathy.22,23
Immunohistochemical analyses have shown that ZAG is expressed mainly in the tubules of the human kidney.24 This present study hypothesized that the urine concentrations of ZAG might increase earlier in the progression of diabetic nephropathy, before microalbuminuria becomes evident. If this is the case, then urine ZAG might be a novel biomarker for the early identification of diabetic nephropathy. This study aimed to determine the role of ZAG in the early diagnosis of diabetic nephropathy by investigating the concentrations of urine ZAG in patients with diabetes mellitus, stratified according to their levels of albuminuria and kidney function.
Patients and methods
Study population
This cross-sectional observational study enrolled consecutive patients with type 2 diabetes mellitus (T2DM) attending the Department of Nephrology, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China between January and December 2014. All patients with T2DM (diagnostic criteria: glycosylated haemoglobin ≥6.5%, fasting blood glucose ≥7 mmol/l or oral glucose tolerance test 2-h blood glucose ≥11.1 mmol/l)25 had a longstanding history of diabetes (>10 years, to ensure sufficient duration of exposure to diabetes)and an estimated glomerular filtration rate (eGFR) >60 ml/min per 1.73 m2, as assessed by the Modification of Diet in Renal Disease (MDRD) equation.26 Patients with T2DM were excluded when renal diseases attributable to other causes were suspected. Therefore, exclusion criteria included the presence of haematuria, renal insufficiency of unexplained origin, urinary tract infection and history of rapidly progressive renal failure, glomerulonephritis and polycystic kidney disease. According to the urine albumin–creatinine ratio (UACR),27 to investigate the role of urine ZAG concentration in the early stages of diabetic nephropathy, patients with T2DM were stratified into a normal albuminuria group (UACR < 30 mg/g), microalbuminuria group (30 mg/g ≤ UACR < 300 mg/g) and macroalbuminuria group (UACR ≥ 300 mg/g). The normal albuminuria group was further divided into a normal eGFR group (eGFR < 120 ml/min per 1.73 m2) and a higher eGFR group (eGFR ≥ 120 ml/min per 1.73 m2).
The study also recruited healthy volunteers who were attending a clinic for routine examination at The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China.
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Dalian Medical University (no. 2014-86). All study participants provided written informed consent before they were recruited into the study.
Anthropometric and biochemical measurements
Standard anthropometric (height, weight, body mass index [BMI]), clinical (systolic and diastolic blood pressures) and laboratory biochemical analyses were performed. All participants were required to fast for 12 h overnight before their blood and urine samples were taken. Blood (5 ml) was drawn under aseptic conditions from the cubital vein in the morning after the overnight fast. Serum (2–3 ml) was separated in a –4℃ centrifuge at 3000 g for 20 min (GDXL-16D; Kaihang Instrument Company, Changzhou, China). Urine samples (10 ml) were collected in the morning after the overnight fast. Serum and urine samples were stored at −80℃ until processed. Serum samples were analysed for total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, glucose, high-sensitivity C-reactive protein (hsCRP), creatinine and blood urea nitrogen (BUN), using an automated biochemical analyser (ADVIA® 1800 Clinical Chemistry System; Erlangen, Germany). Urine samples were analysed for albuminuria and urine creatinine using an automated biochemical analyser (ADVIA® 1800 Clinical Chemistry System). UACR were calculated by dividing the concentration of urine albumin by the concentration of urine creatinine; eGFR was calculated using the formula of MDRD.26 Serum and urine concentrations of ZAG were determined with a commercially available enzyme-linked immunosorbent assay (ELISA; BioVendor – Laboratorní Medicína, Brno, Czech Republic) according to the manufacturer’s instructions. The concentration of urine ZAG (mg/g) was calculated by dividing the urine concentration of ZAG by the concentration of urine creatinine. The minimum detectable concentration was 0.1 mg/l for ZAG. Intra- and interassay coefficients of variation for all ELISA were <10 % and <15 %, respectively.
Statistical analyses
All statistical analyses were performed using the SPSS® statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for Windows®. All data were presented as mean ± SD. Spearman’s rank correlation coefficient analysis was used to establish the association between ZAG concentrations and the other parameters. The comparison of ZAG serum and urine concentrations among the different groups was performed by either Student’s t-test or one-way analysis of variance. A P-value < 0.05 was considered to be statistically significant.
Results
A total of 20 healthy control subjects (12 male and eight female; mean ± SD age, 51.6 ± 8.6 years) and 80 patients with T2DM (42 male and 38 female; mean ± SD age 56.7 ± 9.5 years) were enrolled in this study. Demographic and clinical characteristics of the two groups are presented in Table 1. Mean serum concentrations of ZAG were significantly higher in patients with T2DM compared with healthy control subjects (P < 0.01). According to Spearman’s rank correlation coefficient analysis, the serum ZAG concentration was positively correlated with serum creatinine (r = 0.275, P = 0.034) and eGFR (r = 0.262, P = 0.042), but not with glucose, cholesterol, triglycerides, HDL-C, LDL-C, hsCRP or BMI.
Table 1.
Demographic and clinical characteristics of patients with type 2 diabetes mellitus (n = 80) and healthy control subjects (n = 20) who participated in a study to determine the role of zinc-alpha-2-glycoprotein (ZAG) in the early diagnosis of diabetic nephropathy.
| Characteristic | Healthy control subjects n = 20 | Patients with type 2 diabetes mellitus n = 80 |
|---|---|---|
| Age, years | 51.6 + 8.6 | 56.7 + 9.5 |
| Sex, male/female | 12/8 | 42/38 |
| Fasting blood glucose, mmol/l | 5.61 + 0.29 | 9.09 + 2.67a |
| Blood urea nitrogen, mmol/l | 5.24 + 0.65 | 7.71 + 3.87a |
| Creatinine, µmol/l | 75.75 + 18.79 | 85.22 + 27.32a |
| Body mass index, kg/m2 | 25.34 + 3.21 | 26.27 + 3.35 |
| Cholesterol, mmol/l | 4.48 + 0.44 | 4.93 + 0.96b |
| Triglycerides, mmol/l | 1.30 + 0.64 | 1.77 + 0.90b |
| HDL-C, mmol/l | 1.19 + 0.20 | 1.11 + 0.24 |
| LDL-C, mmol/l | 2.78 + 0.30 | 2.90 + 0.67b |
| hsCRP, mg/l | 1.26 + 1.17 | 1.99 + 1.36b |
| eGFR, ml/min per 1.73 m2 | 109.51 + 19.13 | 105.51 + 39.19 |
| Serum ZAG, mg/l | 21.61 + 8.83 | 38.29 + 22.72a |
| Urine ZAG, mg/gc | 28.17 + 10.64 | 53.64 + 29.48a |
Data presented as mean ± SD.
P < 0.01, bP < 0.05, compared with control group; Student’s t-test.
Level of urine ZAG (mg/g) calculated by dividing the urine concentration of ZAG by the concentration of urine creatinine.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate.
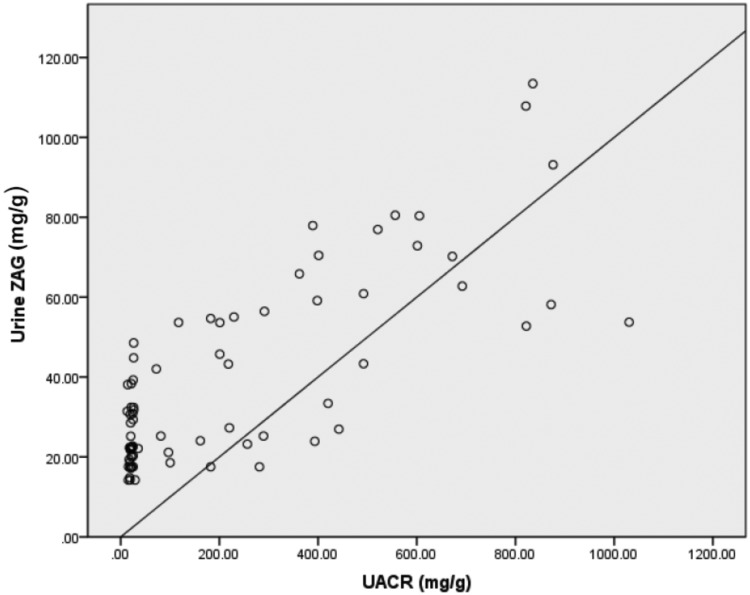
Mean urine concentrations of ZAG were significantly higher in patients with T2DM compared with healthy control subjects (P < 0.01) (Table 1). According to Spearman’s rank correlation coefficient analysis, the urine ZAG concentration was positively correlated with albuminuria (r = 0.824, P < 0.01) (Figure 1). There was no relationship between urinary ZAG concentration and eGFR, serum creatinine, BMI, hsCRP and age.
Figure 1.
Spearman’s rank correlation coefficient analysis of the association between urine zinc-alpha-2-glycoprotein (ZAG) concentration and urine albumin–creatinine ratio (UACR) in patients with type 2 diabetes mellitus (n = 80) who participated in this study to determine the role of ZAG in the early diagnosis of diabetic nephropathy. r = 0.824, P < 0.01.
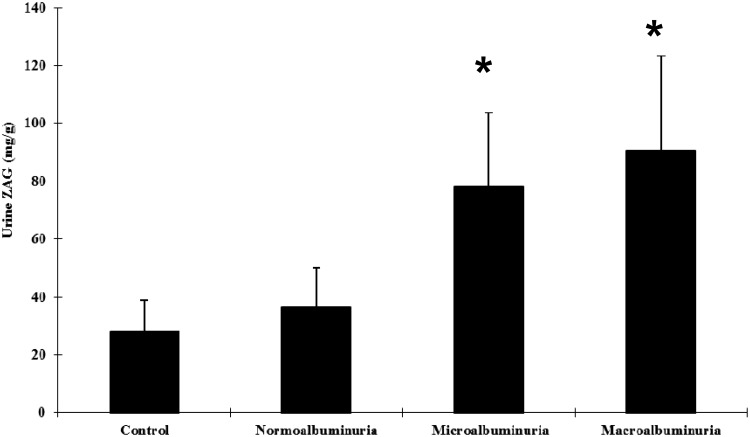
According to the UACR, patients with T2DM were stratified into a normal albuminuria group (UACR < 30 mg/g, n = 40), microalbuminuria group (30 mg/g ≤ UACR < 300 mg/g, n = 20) and macroalbuminuria group (UACR ≥ 300 mg/g, n = 20). There was no significant difference in mean ± SD urine ZAG concentrations between patients in the normal albuminuria group and healthy control subjects. Mean ± SD urine concentrations of ZAG in patients in the microalbuminuria (78.15 ± 25.52 mg/g) and macroalbuminuria groups (90.68 ± 32.57 mg/g) were almost two-to-three-fold higher compared with those in healthy control subjects (P < 0.01) (Figure 2).
Figure 2.
Urine zinc-alpha-2-glycoprotein (ZAG) concentrations in patients with type 2 diabetes mellitus (n = 80), stratified according to urine albumin–creatinine ratio (UACR) into a normal albuminuria group (UACR < 30 mg/g, n = 40), microalbuminuria group (30 mg/g ≤ UACR < 300 mg/g, n = 20), and macroalbuminuria group (UACR ≥ 300 mg/g, n = 20), compared with healthy control subjects (n = 20). Data presented as mean ± SD. *P < 0.01 compared with healthy control group; one-way analysis of variance.
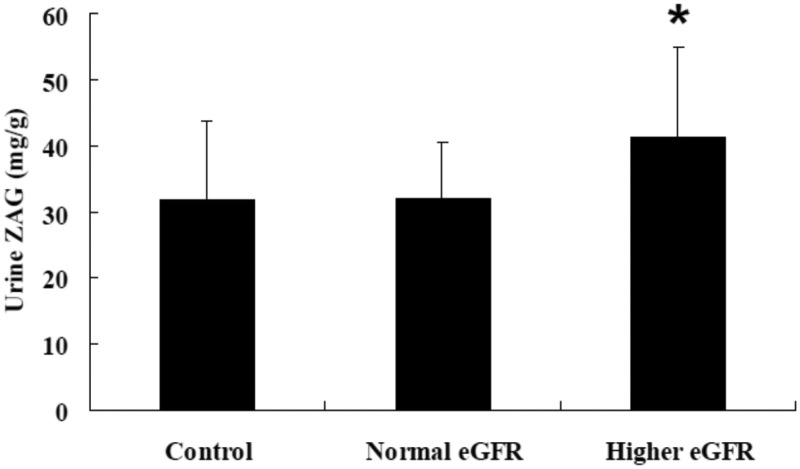
Patients with T2DM in the normal albuminuria group (UACR < 30 mg/g, n = 40) were further stratified into a normal eGFR group (eGFR < 120 ml/min per 1.73 m2, n = 20) and a higher eGFR group (eGFR ≥ 120 ml/min per 1.73 m2, n = 20). Mean ± SD urine concentrations of ZAG were significantly increased in patients with T2DM and a higher eGFR compared with patients with T2DM and a normal eGFR (41.26 ± 13.67 mg/g versus 32.05 ± 8.55 mg/g, respectively; P < 0.05) (Figure 3). There was no significant difference between patients with T2DM with a normal eGFR and healthy control subjects.
Figure 3.
Urine zinc-alpha-2-glycoprotein (ZAG) concentrations in patients with type 2 diabetes mellitus in a normal albuminuria group (n = 40), stratified according to estimated glomerular filtration rate (eGFR) into a normal eGFR group (eGFR < 120 ml/min per 1.73 m2, n = 20) and a higher eGFR group (eGFR ≥ 120 ml/min per 1.73 m2, n = 20) compared with healthy control subjects (n = 20). Data presented as mean ± SD. *P < 0.05 compared with normal eGFR group; one-way analysis of variance.
Discussion
Microalbuminuria is considered to be the earliest clinical manifestation of the onset of diabetic nephropathy.3 Diabetic nephropathy affects all of the cellular components in the glomeruli and renal tubular interstitium.4 As glomerular damage usually results in proteinuria, much research has been undertaken on glomerular damage in patients with T2DM.28 However, some patients with diabetes can experience a decrease in eGFR and may progress to end-stage renal disease without having any significant albuminuria.29 Similarly, some patients with microalbuminuria have advanced renal pathological changes for which therapy is less effective than one might usually expect for those with early stage disease.28,29 The correlation between albuminuria and eGFR has been found to be weak and urinary albumin lacks both sensitivity and specificity to detect early stages of diabetic nephropathy.29
The proximal tubules in the kidneys are particularly susceptible to diabetes-associated injury as they are subjected to prolonged exposure to various metabolic and haemodynamic perturbations.5In chronic cases of diabetic nephropathy, renal function correlates better with the degree of tubulointerstitial injury than the degree of glomerular lesions,5,30,31 suggesting that research should perhaps focus on tubular biomarkers to predict renal damage in patients with early diabetic nephropathy. Several tubular urinary biomarkers have been investigated, such as neutrophil-gelatinase associated lipocalin, kidney injury molecule 1 and liver-fatty acid-binding protein.7
In this present study, urine concentrations of ZAG were significantly increased in patients with T2DM compared with healthy control subjects. The urine concentration of ZAG correlated positively with UACR and presented earlier than albuminuria, as demonstrated by the heightened urine ZAG concentrations in patients who had normal albuminuria but who were in the higher eGFR group. These novel findings suggest that increased urine ZAG concentrations might reflect renal damage earlier than microalbuminuria in patients with diabetic nephropathy and that it might be a potential new biomarker of this diabetic complication.
Jain et al.22 showed that ZAG is one of the additional proteins identified in urine samples from patients with diabetes who also have microalbuminuria. These proteins could potentially be used as biomarkers for the specific and accurate clinical analyses of diabetic nephropathy. A proteomic study speculated that increased urinary ZAG concentrations might be related to the pathogenesis of a nonalbuminuric variant of diabetic nephropathy.32 As ZAG is mainly expressed in the proximal convoluted and straight tubules,24 the changes in urine ZAG concentrations observed in the present study might be indicative of the tubular damage that is present in the earlier stages of diabetic nephropathy, ahead of those that result in microalbuminuria.
The pathophysiological role of ZAG in renal tubules remains unknown. ZAG expression is increased in the proximal tubular cells of aged mice.33 The addition of recombinant ZAG to primary tubular epithelial cell cultures decreased proliferation, whereas knockdown of ZAG increased cell proliferation.33 In vivo, systemic small interference RNA increased the rate of tubular epithelial cell proliferation after renal ischaemia/reperfusion in aged mice, but also increased parenchymal fibrosis.33 It is unclear whether the ZAG found in the urine is filtrated through the glomeruli or actively secreted by the tubular epithelial cells. The present study found that the concentration of ZAG in urine was higher than that in serum, especially in patients with T2DM, which suggests that the increased urine concentrations of ZAG were mainly due to increased ZAG secretion by tubular epithelial cells. Further research is required to determine whether this is the case.
This present study had a number of limitations. First, the cross-sectional observational design did not allow for the determination of a cause–effect relationship between urine concentrations of ZAG and diabetic nephropathy. Secondly, the absence of renal biopsies prevented both the accurate diagnosis of diabetic nephropathy and the immunohistochemical evaluation of the expression levels of ZAG in the kidney, which in turn meant that the source of the elevated urinary concentrations of ZAG could not be determined. Thirdly, the lack of a sample-size calculation was a further limitation, which may impact on the conclusions drawn. Large-scale prospective studies and animal experiments are needed to comprehensively understand the potential pathophysiological role of ZAG in diabetic nephropathy.
In conclusion, this present study provides preliminary clinical evidence supporting the pathophysiological role of ZAG in diabetic nephropathy. The strong positive association between urinary ZAG concentration and UACR, and the earlier appearance of urine ZAG compared with albuminuria, suggest that ZAG might be a potentially useful biomarker for the early diagnosis of diabetic nephropathy, in patients with T2DM.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This work was supported by a grant from the Technology Plan Projects for Liaoning Province, China (no. 2013225002). The sponsor had no role in any of the following: design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. The corresponding author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas Sixth edition, https://www.idf.org/sites/default/files/EN_6E_Atlas_Full_0.pdf (2013, accessed 6 January 2016).
- 2.Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am 2013; 97: 1–18. [DOI] [PubMed] [Google Scholar]
- 3.Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, et al. Diabetic kidney disease: from physiology to therapeutics. J Physiol 2014; 592: 3997–4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol 2007; 27: 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis 2005; 12: 177–186. [DOI] [PubMed] [Google Scholar]
- 6.Tramonti G, Kanwar YS. Review and discussion of tubular biomarkers in the diagnosis and management of diabetic nephropathy. Endocrine 2013; 43: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matheson A, Willcox MD, Flanagan J, et al. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev 2010; 26: 150–171. [DOI] [PubMed] [Google Scholar]
- 8.Fu WJ, Xiong SL, Fang YG, et al. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine 2012; 41: 82–88. [DOI] [PubMed] [Google Scholar]
- 9.Hassan MI, Waheed A, Yadav S, et al. Zinc alpha 2-glycoprotein: a multidisciplinary protein. Mol Cancer Res 2008; 6: 892–906. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy MW, Heikema AP, Cooper A, et al. Hydrophobic ligand binding by Zn-alpha 2-glycoprotein, a soluble fat-depleting factor related to major histocompatibility complex proteins. J Biol Chem 2001; 276: 35008–35013. [DOI] [PubMed] [Google Scholar]
- 11.Poortmans JR, Schmid K. The level of Zn-alpha 2-glycoprotein in normal human body fluids and kidney extract. J Lab Clin Med 1968; 71: 807–811. [PubMed] [Google Scholar]
- 12.Bing C, Bao Y, Jenkins J, et al. Zinc-alpha2 glycoprotein, a lipid mobilizing factor is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc Natl Acad Sci USA 2004; 101: 2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olofsson LE, Olsson B, Lystig T, et al. Preliminary report: Zn-alpha2-glycoprotein genotype and serum levels are associated with serum lipids. Metabolism 2010; 59: 1316–1318. [DOI] [PubMed] [Google Scholar]
- 14.Philipp A, Kralisch S, Bachmann A, et al. Serum levels of the adipokine zinc-α2-glycoprotein are increased in chronic hemodialysis. Metabolism 2011; 60: 669–672. [DOI] [PubMed] [Google Scholar]
- 15.Qu F, Ying X, Guo W, et al. The role of Zn-alpha2 glycoprotein in sperm motility is mediated by changes in cyclic AMP. Reproduction 2007; 134: 569–576. [DOI] [PubMed] [Google Scholar]
- 16.Hale LP. Zinc alpha-2-glycoprotein regulates melanin production by normal and malignant melanocytes. J Invest Dermatol 2002; 119: 464–470. [DOI] [PubMed] [Google Scholar]
- 17.Hale LP, Price DT, Sanchez LM, et al. Zinc alpha-2-glycoprotein is expressed by malignant prostatic epithelium and may serve as a potential serum marker for prostate cancer. Clin Cancer Res 2001; 7: 846–853. [PubMed] [Google Scholar]
- 18.He N, Brysk H, Tyring SK, et al. Zinc-alpha(2)-glycoprotein hinders cell proliferation and reduces cdc2 expression. J Cell Biochem Suppl 2001; Suppl 36: 162–169. [PubMed]
- 19.Yeung DC, Lam KS, Wang Y, et al. Serum zinc-alpha2-glycoprotein correlates with adiposity, triglycerides, and the key components of the metabolic syndrome in Chinese subjects. J Clin Endocrinol Metab 2009; 94: 2531–2536. [DOI] [PubMed] [Google Scholar]
- 20.Stejskal D, Karpísek M, Reutová H, et al. Determination of serum zinc-alpha-2-glycoprotein in patients with metabolic syndrome by a new ELISA. Clin Biochem 2008; 41: 313–316. [DOI] [PubMed] [Google Scholar]
- 21.Russell ST, Tisdale MJ. Antidiabetic properties of zinc-alpha2-glycoprotein in ob/ob mice. Endocrinology 2010; 151: 948–957. [DOI] [PubMed] [Google Scholar]
- 22.Jain S, Rajput A, Kumar Y, et al. Proteomic analysis of urinary protein markers for accurate prediction of diabetic kidney disorder. J Assoc Physicians India 2005; 53: 513–520. [PubMed] [Google Scholar]
- 23.Varghese SA, Powell TB, Budisavljevic MN, et al. Urine biomarkers predict the cause of glomerular disease. J Am Soc Nephrol 2007; 18: 913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tada T, Ohkubo I, Niwa M, et al. Immunohistochemical localization of Zn-alpha2-glycoprotein in normal human tissues. J Histochem Cytochem 1991; 39: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 25.IDF Clinical Guidelines Task Force. Global Guideline for Type 2 diabetes. Brussels: International Diabetes Federation, 2005, https://www.idf.org/webdata/docs/IDF%20GGT2D.pdf (accessed 17 December 2015).
- 26.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 27.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am J Kidney Dis 1999; 33: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 28.Halimi JM. The emerging concept of chronic kidney disease without clinical proteinuria in diabetic patients. Diabetes Metab 2012; 38: 291–297. [DOI] [PubMed] [Google Scholar]
- 29.Fu WJ, Li BL, Wang SB, et al. Changes of the tubular markers in type 2 diabetes mellitus with glomerular hyperfiltration. Diabetes Res Clin Pract 2012; 95: 105–109. [DOI] [PubMed] [Google Scholar]
- 30.Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 2012; 74: 351–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielsen SE, Andersen S, Zdunek D, et al. Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int 2011; 79: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 32.Lim SC, Liying DQ, Toy WC, et al. Adipocytokine zinc α2 glycoprotein (ZAG) as a novel urinary biomarker for normo-albuminuric diabetic nephropathy. Diabet Med 2012; 29: 945–949. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt R, Marlier A, Cantley LG. Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 2008; 19: 2375–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]