Abstract
Objective
A prospective, double-blind, randomized controlled trial to compare the effect of preoperative midazolam or ketamine on the incidence of emergence agitation (EA) following sevoflurane anaesthesia in children.
Methods
Paediatric patients (2–6 years old) undergoing ophthalmic surgery were allocated to receive premedication with either 0.1 mg/kg midazolam or 1 mg/kg ketamine. Incidence of EA and postoperative pain scores were recorded at 10-min intervals in the postanaesthetic care unit (PACU). The use of EA rescue medications (fentanyl or midazolam) was recorded.
Results
The incidence of EA was significantly lower in the ketamine group (n = 33) than the midazolam group (n = 34) at 10 and 20 min after transfer to PACU. There was no significant difference in overall incidence of EA. The frequency of midazolam use as rescue medication was significantly lower in the katamine group than in the midazolam group.
Conclusion
Premedication with ketamine is more effective than midazolam in preventing EA during the early emergence period after sevoflurane anaesthesia in children.
Keywords: Child, ketamine, psychomotor agitation
Introduction
Emergence agitation (EA) comprises restlessness, disorientation, inconsolable crying and cognitive impairment following general anaesthesia, and is frequently observed in preschool-aged children.1 Paediatric patients undergoing ophthalmic surgery may experience severe EA due to visual disturbance.2 Although the pathogenesis of EA remains unclear, factors including inhalation anaesthesia (sevoflurane anaesthesia in particular), pain, surgery and a high level of preoperative anxiety are known to affect EA.1–4 It has been suggested that the rapid and differential elimination of residual inhalation anaesthetics may cause EA in surgical patients,1 but others have shown that rapid awakening is not the cause of EA following sevoflurane anaesthesia in children.5
Children who are more anxious during induction of anaesthesia have higher EA scores on entrance to the recovery room than less anxious children,3 and difficult separation of paediatric patients from their parents is also a risk factor for postoperative EA.6 Standard pharmacological interventions such as propofol, ketamine and fentanyl have been shown to have prophylactic effects in preventing EA in children.7
Midazolam is a commonly used sedative in children that has beneficial effects in reducing paediatric EA. Preoperative oral midazolam decreases both preoperative separation anxiety and the degree of EA observed with sevoflurane anaesthesia.8 Ketamine crosses the blood–brain barrier rapidly and reaches maximal effect in 1 min. Premedication with oral ketamine (6 mg/kg) has been shown to reduce EA in children undergoing adenotonsillectomy under desflurane anaesthesia, without retarding recovery.9 In addition, the administration of 1 mg/kg ketamine intravenously (i.v.), followed by 1 mg/kg per h ketamine infusion during anaesthesia, reduces EA in paediatric strabismus surgery with sevoflurane anaesthesia.10 A combination of i.v. ketamine plus midazolam was more effective than ketamine alone for sedation during a brief painful procedure in children.11
To the best of our knowledge, there are few studies focusing on the effects of intravenous midazolam or ketamine (administered as anaesthetic premedication) on EA following general anaesthesia in children. The aim of the present study was to compare the effects of preoperative midazolam and ketamine i.v. injection on EA after sevoflurane anaesthesia in children.
Patients and methods
Study population
This prospective study recruited patients aged 2–6 years old with American Society of Anesthesiologists physical status 1 or 2, scheduled to undergo elective ophthalmic surgery (procedures <2 h) at Haeundae Paik Hospital, Busan, Republic of Korea, between January 2013 and January 2014. Children with neurological disorders, history of allergy to the study drugs, or respiratory tract infection within the previous 2 weeks were excluded.
Patients were randomly assigned to either the midazolam group (0.1 mg/kg midazolam i.v.) or ketamine group (1.0 mg/kg ketamine i.v.) using a computer generated randomization program (www.random.org).
This prospective study was approved by the institutional review board of Haeundae Paik Hospital, Busan, Republic of Korea, and the parents of each child provided written informed consent prior to enrolment (ClinicalTrials.gov number, NCT02256358).
Anaesthesia
At 1 h before surgery, intravenous access was obtained after applying topical anaesthesia (ANES cream [lidocaine–prilocaine], Tai Guk Pharm Co., Ltd, Seoul, Republic of Korea). After patients arrived in the waiting room (but before premedication), an independent anaesthetist who was not involved in this study assessed the emotional status of each child using a three-point scale (1 = calm, 2 = anxious but not crying, 3 = anxious and crying). A nurse who was blinded to the study conditions administered study drugs (total volume 5 ml) according to group allocation, and the emotional state of each child was then reassessed by the same anaesthetist as before (after premedication).
Patients were transferred to the operating room with monitoring for oxygen saturation, electrocardiogram (ECG), noninvasive blood pressure (BP) and pulse oximetry. General anaesthesia was induced with atropine (0.01 mg/kg), propofol (2 mg/kg) and fentanyl (1 µg/kg). Rocuronium (0.6 mg/kg) was administered to facilitate endotracheal intubation. The Induction Compliance Checklist (ICC) was used to measure anxiety during induction of anaesthesia.12 General anaesthesia was maintained with sevoflurane 2–3 vol% and 50% oxygen in air. End-tidal carbon dioxide (EtCO2) and bispectral index (BIS, Aspect Medical System, Norwood, MA, USA) were maintained at 30–35 mmHg and 40–60, respectively. Surgery began within 20–25 min following administration of premedication.
At the end of surgery, sevoflurane was discontinued and extubation was performed. An anaesthetist who was blinded to study conditions evaluated EA, postoperative pain score, heart rate and oxygen saturation at 10-min intervals for 30 min in the post-anaesthesia care unit (PACU).
Study endpoints
The primary endpoint of the study was the overall incidence of postoperative EA, defined as an Aono’s four-point scale (AFPS) score of ≥3.13 When EA occurred, patients received 0.1 µg/kg fentanyl i.v., regardless of the presence of their parent(s). If EA persisted after two doses of fentanyl, patients received 0.1 mg/kg midazolam i.v. Postoperative pain was assessed using the Children’s and Infants’ Postoperative Pain Scale (CHIPPS).14 The highest EA and CHIPPS score during recovery and the frequency of fentanyl and midazolam administration were recorded.
Postoperative complications (nausea, vomiting, shivering) were noted. Children were transferred to the general ward when they recovered from anaesthesia (modified Aldrete score > 9).15 Duration of anaesthesia, duration of surgery, time to extubation and length of stay in the PACU were recorded for each patient.
Statistical analyses
The sample size calculation was performed based on a pilot study where the between-group difference in incidence of EA was 30%. By calculating a power of 80%, type 1 error of 5% and drop-out rate of 10%, each group required 34 patients.
Data were presented as mean ± SD. Between group comparisons were made with χ2-test for categorical variables, independent samples t-test for continuous variables and Mann–Whitney U-test for rank scales. Spearman’s correlation coefficient was used to investigate associations between AFPS and CHIPPS scores. Statistical analyses were performed using SPSS® version 21.0 (SPSS Inc., Chicago, IL, USA) for Windows®, and MedCalc version 14.12.0 (MedCalc Software bvba, Ostend, Belgium). P-values < 0.05 were considered statistically significant.
Results
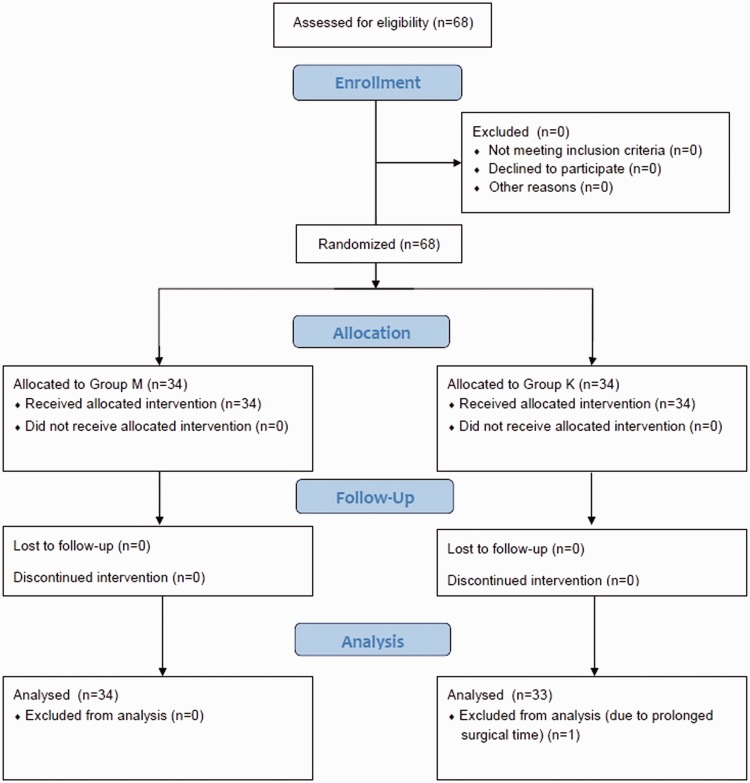
The study randomized 68 patients (32 male/36 female; mean age 4.18 ± 1.33 years; age range 2–6 years). A single patient in the ketamine group was excluded due to extended duration of surgery (>2 h). The final analysis included 34 patients in the midazolam group (16 male/18 female; mean age 4.15 ± 1.40 years; age range 2–6 years) and 33 patients in the ketamine group (16 male/17 female; mean age 4.21 ± 1.32 years; age range 2–6 years). A CONSORT flow diagram for the study is shown Figure 1, and demographic and clinical data are given in Table 1. There were no statistically significant between-group differences in any demographic or clinical parameter. Emotional status scores were significantly lower following premedication in both groups (P < 0.001 for each comparison; Table 1).
Figure 1.
CONSORT flow diagram for a study that compared the effect of preoperative midazolam or ketamine on the incidence of emergence agitation following sevoflurane anaesthesia in children.
Table 1.
Demographic and clinical characteristics of patients included in a study to compare the effect of preoperative midazolam or ketamine on the incidence of emergence agitation following sevoflurane anaesthesia in children undergoing ophthalmic surgery, stratified by study drug.
| Characteristic | Midazolam group n = 34 | Ketamine group n = 33 |
|---|---|---|
| Age, years | 4.15 ± 1.40 | 4.21 ± 1.32 |
| Sex, male/female | 16/18 | 16/17 |
| Height, cm | 107.52 ± 11.59 | 106.56 ± 9.36 |
| Weight, kg | 18.84 ± 4.36 | 18.27 ± 3.93 |
| Duration of anaesthesia, min | 69.12 ± 12.46 | 66.06 ± 15.09 |
| Duration of surgery, min | 34.41 ± 12.48 | 32.42 ± 9.61 |
| Time to extubation, min | 17.06 ± 3.7 | 15.76 ± 5.61 |
| Duration of PACU stay, min | 39.26 ± 10.45 | 38.94 ± 13.68 |
| EtCO2 at extubation | 0.29 ± 0.11 | 0.28 ± 0.09 |
| Emotional statusa | ||
| Before study drug, 1/2/3 | 3/27/4 | 1/28/4 |
| After study drug, 1/2/3 | 32/2/0b | 33/0/0b |
| ICC, 0/1/2 | 29/2/3 | 31/2/0 |
Data presented as mean ± SD or n patients.
EtCO2, end-tidal carbon dioxide concentration; PACU, postanaesthesia care unit; ICC, induction compliance checklist.
Evaluated using a 3-point scale (1 = calm, 2 = anxious but not crying, 3 = anxious and crying).
No statistically significant between group differences (P ≥ 0.05; χ2-test for categorical variables, independent samples t-test for continuous variables).
P < 0.001 vs before study drug within group; independent samples t-test.
Data regarding the incidence of EA in the PACU are given in Table 2. There was no statistically significant between-group difference in the overall incidence of EA (0–30 min after arrival in PACU). The incidence of EA was significantly lower in the ketamine group than in the midazolam group at 10 min and 20 min after arrival in the PACU (P = 0.011 and P = 0.042, respectively).
Table 2.
Incidence of emergence agitationa following sevoflurane anaesthesia in children undergoing ophthalmic surgery, stratified by study drug.
| Time after arrival at PACU, min | Midazolam group n = 34 | Ketamine group n = 33 |
|---|---|---|
| 0 | 8 (23.5) | 8 (24.2) |
| 10 | 6 (17.6) | 0 (0.0)* |
| 20 | 4 (11.8) | 0 (0.0)* |
| 30 | 0 (0.0) | 0 (0.0) |
| Overall (0–30) | 15 (44.2) | 11 (33.3) |
Data presented as n (%) of patients.
Aono’s four-point scale score ≥3.13
PACU, postanaesthesia care unit.
P < 0.05 versus midazolam group; χ2-test.
Table 3 shows data regarding the frequency and timing of fentanyl and midazolam injection for treatment of EA. There was no statistically significant between-group difference in the frequency of fentanyl use. The frequency of midazolam use was significantly lower in the ketamine group than in the midazolam group (P = 0.042; Table 3). A total of four patients in the midazolam group required both fentanyl and midazolam. No patient in the ketamine group required both drugs.
Table 3.
Frequency and timing of administration of fentanyl and midazolam for treatment of emergence agitationa following sevoflurane anaesthesia in children undergoing ophthalmic surgery, stratified by study drug.
| Parameter | Midazolam group n = 34 | Ketamine group n = 33 |
|---|---|---|
| Total fentanyl dose | NS | |
| 0 | 21 | 23 |
| 1 | 4 | 6 |
| 2 | 9 | 4 |
| Total midazolam dose | P = 0.042 | |
| 0 | 30 | 33 |
| 1 | 4 | 0 |
| 2 | 0 | 0 |
| Timing of doses after arrival in PACU | ||
| 0–10 min | ||
| Fentanyl, one dose | 3/4 | 4/6 |
| Fentanyl, two doses | 9/9 | 3/4 |
| 10–20 min | ||
| Fentanyl, one dose | 1/4 | 2/6 |
| Fentanyl, two doses | 0/9 | 1/4 |
| Midazolam, one dose | 4/4 | – |
| 20–30 min | – | – |
Data presented as n patients.
NS, not statistically significant (P ≥ 0.05; χ2-test).
PACU, postanaesthesia care unit.
Aono’s four-point scale score ≥3.13
There was a significant positive correlation between peak CHIPPS and peak AFPS scores in the total study population (r = 0.816, P < 0.001). There were no statistically significant between-group differences in CHIPPS at any time point (Table 4). No complications were observed in either group.
Table 4.
Children’s and Infants’ Postoperative Pain Scale (CHIPPS)14 scores following sevoflurane anaesthesia in children undergoing ophthalmic surgery, stratified by study drug.
| Time after arrival at PACU, min | Midazolam group n = 34 | Ketamine group n = 33 |
|---|---|---|
| 0 | 0 (0–9) | 0 (0–8) |
| 10 | 0 (0–9) | 0 (0–8) |
| 20 | 0 (0–10) | 0 (0–4) |
| 30 | 0 (0–2) | 0 (0–4) |
Data presented as median (range).
PACU, postanaesthesia care unit.
No statistically significant between group differences (P ≥ 0.05; Mann–Whitney U-test).
Discussion
This randomized, double-blind study investigated the effects of intravenous midazolam or ketamine as premedication on the incidence of EA, in paediatric patients undergoing ophthalmic surgery with sevoflurane anaesthesia. The administration of ketamine as premedication decreased both the occurrence of EA during the early emergence period (10–20 min) and the use of rescue drugs compared with midazolam premedication. Midazolam and ketamine had similar effects at reducing preanaesthesia separation anxiety in the present study.
Emergence agitation is observed in around 40% of preschool-aged children following sevoflurane anaesthesia;13 it causes discomfort and can lead to critical problems during the early recovery period. Inconsolable crying or restlessness is the most commonly observed manifestation during the initial 10 min of recovery.6 Premedication with ketamine significantly decreased the incidence of EA during the first 20 min of recovery after sevoflurane anaesthesia, compared with midazolam premedication, in the present study.
Midazolam is widely used as a premedication to decrease anxiety in children prior to surgery, but its effects on EA are unclear. Premedication with oral midazolam has been shown to decrease the incidence of EA without delaying discharge from the PACU.16 In addition, intravenous administration of a subhypnotic dose of midazolam (0.05 mg/kg), in addition to fentanyl before discontinuation of sevoflurane, was also found to be effective in decreasing EA.17 In contrast, however, others found that intravenous midazolam did not reduce the incidence of EA.18,19 A meta-analysis of pharmacological prevention of EA in children indicated that midazolam was ineffective in the prevention of EA in this context.7
Ketamine is a traditional intraoperative anaesthetic agent and is a useful premedication for sedating fearful children in the surgical waiting room.20 The NMDA-antagonistic properties of ketamine may be important in attenuating sensitization and opioid tolerance, and have sparked interest in the use of subanaesthetic doses for pain control.21 Ketamine has pre-emptive analgesic effects and decreases the development of EA following adenotonsillectomy in children.22,23 These data are consistent with the results of our study, which demonstrated that premedication with i.v. ketamine compared with midazolam decreased the incidence of EA in children undergoing ophthalmic surgery. Low-dose ketamine as an adjuvant to opioids or local anaesthetics may perform a substantial function in the reduction of acute postoperative pain and may also reduce the dose of rescue analgesics.24
Premedication with i.v. ketamine was more effective than i.v. midazolam in reducing the occurrence of EA in the present study. The elimination half-life of ketamine (2.5–2.8 h) is longer than that of midazolam (1.7–2.6 h).25 The co-administration of ketamine with an opioid such as alfentanil has been shown to enhance the distribution and clearance of ketamine, and to increase the distribution of ketamine into the brain.21 These properties of ketamine may contribute to its superior effect on EA prevention, compared with midazolam.
The induction dose of midazolam is 0.05–0.15 mg/kg and the dose of midazolam used in this study (0.1 mg/kg) has been established as the optimum for preanaesthetic anxiolysis.26 Administration of 1 mg/kg ketamine prior to entering the operating room was shown to decrease separation anxiety, degree of postoperative pain, and the incidence of EA following paediatric ophthalmic surgery under desflurane anaesthesia.20 In contrast, low-dose ketamine (0.5 mg/kg) had no effect on postoperative pain, additional analgesic requirement, or postoperative nausea and vomiting during painful ophthalmic surgery.27 Based on these data, we chose to use 0.1 mg/kg midazolam or 1 mg/kg ketamine in our present study.
The current study has some limitations. First, there is broad variability in the definitions and analysis tools related to EA. The Pediatric Anesthesia Emergence Delirium (PAED) scale is a reliable tool for the assessment of EA in children,28 but relies partly on the assessment of postoperative eye contact. The ophthalmic nature of the surgery in our study meant that we were unable to evaluate eye contact, therefore we used a simple AFPS score to measure EA. Secondly, ethical concerns prevented the inclusion of a placebo group. Thirdly, the role of pain in EA remains debatable and it is difficult to distinguish between signs of EA and postoperative pain.1 There was no significant between-group difference in postoperative pain score in our study, however. Our experience suggested that visual disturbance and postoperative pain complicated our assessment of EA.
In conclusion, premedication with 0.1 mg/kg midazolam or 1 mg/kg ketamine lowers preoperative anxiety. Premedication with ketamine was more effective than midazolam in the prevention of early postoperative EA, and reduced the requirement for rescue medication in children after sevoflurane anaesthesia.
Acknowledgements
The authors would like to thank Sung Rok Kim, an Anaesthetist, Haeundae Paik Hospital, and Hyeon Ju Na, a recovery room nurse, Haeundae Paik Hospital, Busan, Republic of Korea, for their involvement.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by the 2013 Inje University research grant.
References
- 1.Dahmani S, Delivet H, Hilly J. Emergence delirium in children: an update. Curr Opin Anaesthesiol 2014; 27: 309–315. [DOI] [PubMed] [Google Scholar]
- 2.Aouad MT, Nasr VG. Emergence agitation in children: an update. Curr Opin Anaesthesiol 2005; 18: 614–619. [DOI] [PubMed] [Google Scholar]
- 3.Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg 2004; 99: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 4.Weldon BC, Bell M, Craddock T. The effect of caudal analgesia on emergence agitation in children after sevoflurane versus halothane anesthesia. Anesth Analg 2004; 98: 321–326. [DOI] [PubMed] [Google Scholar]
- 5.Oh AY, Seo KS, Kim SD, et al. Delayed emergence process does not result in a lower incidence of emergence agitation after sevoflurane anesthesia in children. Acta Anaesthesiol Scand 2005; 49: 297–299. [DOI] [PubMed] [Google Scholar]
- 6.Cole JW, Murray DJ, McAllister JD, et al. Emergence behaviour in children: defining the incidence of excitement and agitation following anaesthesia. Paediatr Anaesth 2002; 12: 442–447. [DOI] [PubMed] [Google Scholar]
- 7.Dahmani S, Stany I, Brasher C, et al. Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br J Anaesth 2010; 104: 216–223. [DOI] [PubMed] [Google Scholar]
- 8.Lapin SL, Auden SM, Goldsmith LJ, et al. Effects of sevoflurane anaesthesia on recovery in children: a comparison with halothane. Paediatr Anaesth 1999; 9: 299–304. [DOI] [PubMed] [Google Scholar]
- 9.Kararmaz A, Kaya S, Turhanoglu S, et al. Oral ketamine premedication can prevent emergence agitation in children after desflurane anaesthesia. Paediatr Anaesth 2004; 14: 477–482. [DOI] [PubMed] [Google Scholar]
- 10.Kawaraguchi Y, Miyamoto Y, Fukumitsu K, et al. The effect of ketamine on reducing postoperative agitation after sevoflurane anesthesia in pediatric strabismus surgery. Masui 2002; 51: 1343–1348. [in Japanese, English Abstract]. [PubMed] [Google Scholar]
- 11.Dilli D, Dallar Y, Sorguç NH. Comparison of ketamine plus midazolam versus ketamine for sedation in children during lumbar puncture. Clin J Pain 2009; 25: 349–350. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed MI, Farrell MA, Parrish K, et al. Preoperative anxiety in children risk factors and non-pharmacological management. Middle East J Anesthesiol 2011; 21: 153–164. [PubMed] [Google Scholar]
- 13.Aono J, Ueda W, Mamiya K, et al. Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys. Anesthesiology 1997; 87: 1298–1300. [DOI] [PubMed] [Google Scholar]
- 14.Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth 2000; 10: 303–318. [DOI] [PubMed] [Google Scholar]
- 15.Aldrete JA. Modifications to the postanesthesia score for use in ambulatory surgery. J Perianesth Nurs 1998; 13: 148–155. [DOI] [PubMed] [Google Scholar]
- 16.Ko YP, Huang CJ, Hung YC, et al. Premedication with low-dose oral midazolam reduces the incidence and severity of emergence agitation in pediatric patients following sevoflurane anesthesia. Acta Anaesthesiol Sin 2001; 39: 169–177. [PubMed] [Google Scholar]
- 17.Chen J, Li W, Hu X, et al. Emergence agitation after cataract surgery in children: a comparison of midazolam, propofol and ketamine. Paediatr Anaesth 2010; 20: 873–879. [DOI] [PubMed] [Google Scholar]
- 18.Cohen IT, Drewsen S, Hannallah RS. Propofol or midazolam do not reduce the incidence of emergence agitation associated with desflurane anaesthesia in children undergoing adenotonsillectomy. Paediatr Anaesth 2002; 12: 604–609. [DOI] [PubMed] [Google Scholar]
- 19.Breschan C, Platzer M, Jost R, et al. Midazolam does not reduce emergence delirium after sevoflurane anesthesia in children. Paediatr Anaesth 2007; 17: 347–352. [DOI] [PubMed] [Google Scholar]
- 20.Jeong WJ, Kim WY, Moon MG, et al. The effect of ketamine on the separation anxiety and emergence agitation in children undergoing brief ophthalmic surgery under desflurane general anesthesia. Korean J Anesthesiol 2012; 63: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reves JG, Glass P, Lubarsky DA, et al. Intravenous anesthetics. In: Miller RD. (ed). Miller’s anesthesia, 17th ed Philadelphia: Churchill Livingstone Elsevier, 2010, pp. 742–747. [Google Scholar]
- 22.Eghbal MH, Taregh S, Amin A, et al. Ketamine improves postoperative pain and emergence agitation following adenotonsillectomy in children. A randomized clinical trial. Middle East J Anesthesiol 2013; 22: 155–160. [PubMed] [Google Scholar]
- 23.Elshammaa N, Chidambaran V, Housny W, et al. Ketamine as an adjunct to fentanyl improves postoperative analgesia and hastens discharge in children following tonsillectomy - a prospective, double-blinded, randomized study. Paediatr Anaesth 2011; 21: 1009–1014. [DOI] [PubMed] [Google Scholar]
- 24.Bell RF, Dahl JB, Moore RA, et al. Peri-operative ketamine for acute post-operative pain: a quantitative and qualitative systematic review (Cochrane review). Acta Anaesthesiol Scand 2005; 49: 1405–1428. [DOI] [PubMed] [Google Scholar]
- 25.Reves JG, Glass P, Lubarsky DA, et al. Intravenous anesthetics. In: Miller RD. (ed). Miller’s anesthesia, 17th ed Philadelphia: Churchill Livingstone Elsevier, 2010, pp. 721–721. [Google Scholar]
- 26.Reves JG, Glass P, Lubarsky DA, et al. Intravenous anesthetics. In: Miller RD. (ed). Miller’s anesthesia, 17th ed Philadelphia: Churchill Livingstone Elsevier, 2010, pp. 739–739. [Google Scholar]
- 27.Abdolahi M, Soltani HA, Montazeri K, et al. Preemptive low-dose of ketamine does not effective on anesthetic consumption, perioperative analgesic requirement and postoperative pain, nausea and vomiting in painful ophthalmic surgery. J Res Med Sci 2013; 18: 583–587. [PMC free article] [PubMed] [Google Scholar]
- 28.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 2004; 100: 1138–1145. [DOI] [PubMed] [Google Scholar]