Abstract
Objective
To explore the risk factors associated with postoperative delirium (PD) in elderly patients following total hip arthroplasty (THA) for hip fracture.
Methods
This prospective study enrolled elderly patients (≥ 65 years) with hip fractures who underwent THA under general anaesthesia, and who had a complete set of postoperative observations. Detailed medical history and perioperative characteristics were recorded. During the postoperative period, patients were assessed twice daily for PD using the Confusion Assessment Method.
Results
A total of 572 patients were eligible for inclusion in the study. Of these, 120 patients (21.0%) were diagnosed with PD and 452 patients (79.0%) did not experience PD. Multivariate stepwise logistic regression analyses showed that older age, a history of stroke, lower albumin, higher blood glucose, higher total bilirubin, higher C-reactive protein, longer surgery duration and higher volume of red blood cell transfusions were independent risk factors for PD.
Conclusions
Correcting the modifiable risk factors might help prevent PD. Strategies might include nutritional support, tight blood glucose control, improvement of liver function, preoperative infection control and minimizing surgical injury or blood loss.
Keywords: Postoperative delirium, risk factors, elderly patients, hip fracture
Introduction
Postoperative delirium (PD) is a common complication in elderly patients that is characterized by disorders of consciousness, attention, perception, thinking, memory, mental activity, emotions and sleep-wake cycle.1 This complication usually occurs within 5 days after surgery, especially during the first 24–48 h postoperatively. Studies have demonstrated that the incidence of PD in elderly hip fracture patients is between 20–50%.2,3 PD impairs postoperative recovery, increases the burden of care, prolongs hospitalization time, increases the in-hospitalization mortality rate, and leads to long-term cognitive impairment or even permanent dementia.4 Its pathogenesis is complicated and remains to be elucidated. Emerging hypotheses involve neurotransmitter alterations, decreasing levels of cerebral metabolites, stress and neuroinflammation.5 Elderly patients with a history of hypnotic drug use, preoperative dementia, anxiety and depression are more prone to PD compared with other elderly patients.6,7
Few studies have specifically examined the risk factors relating to perioperative comorbidities and clinical laboratory data for PD in elderly patients with hip fractures; those that have been published were limited by their small sample sizes.8,9 Risk factors for developing delirium after total hip arthroplasty (THA) in elderly hip fracture patients have not been thoroughly investigated. Therefore, the current study was undertaken to investigate the prevalence and perioperative risk factors of PD, including medical history, comorbidities and clinical laboratory data, in elderly patients after THA for hip fracture.
Patients and methods
Patients and setting
This prospective study enrolled consecutive elderly patients with hip fractures who underwent THA under general anaesthesia in the Department of Anaesthesiology and Critical Care Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China between March 2015 and September 2015 and had complete sets of postoperative observations for the first three postoperative days. The following inclusion criteria were applied: (i) American Society of Anesthesiologists physical status I – III;10 (ii) aged ≥ 65 years; (iii) patients agreed to have the THA procedure. The exclusion criteria were as follows: (i) Mini-Mental State Examination (MMSE) score <24 or dementia due to various aetiologies;11 (ii) history of mental illness; (iii) currently using tranquilizers or antidepressants; (iv) duration of anaesthesia > 3 h; (v) patients who underwent secondary surgery or developed severe infectious complications (presence of more than two systemic inflammatory response syndrome criteria was used as a marker of inflammation);12 (vi) unwilling to complete the experimental processes or they had language barriers; (vii) severe hearing or visual impairment; (viii) illiteracy; (ix) alcohol or drug dependence. This study was approved by the Ethics Committee of Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University, Shanghai, China (no. 2015-23) and it was registered with the Chinese Clinical Trial Registry (no. ChiCTR-CPC-15006141). Written informed consent was provided by all study participants.
Preoperative registration of medical history and laboratory examinations
During enrolment, all patients were screened and their medical histories were recorded in detail. On the day before surgery, routine laboratory tests were undertaken. These included blood counts, blood chemistries, blood glucose, hepatic function, renal function and erythrocyte sedimentation rate (ESR); each patient underwent an electrocardiogram (ECG) and chest X-radiography examination. Blood samples were collected, treated, stored and tested according to the routine protocols of the Clinical Biochemistry Laboratory of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. In brief, venous blood was collected from the cubital vein in the morning after an overnight fast and 2 ml of whole blood was stored in an anticoagulation tube (BD Vacutainer® Plus plastic whole blood tube [K2 ethylenediaminetetra-acetic acid 7.2 mg, REF 367841]; Becton, Dickinson and Co., Franklin Lakes, NJ, USA). The blood samples were analysed for complete blood cell counts using a Sysmex XE-5000 instrument (Sysmex, Kobe, Japan), C-reactive protein (CRP) using an I-READER (Jokoh, Tokyo, Japan) and ESR using a TEST 1 ESR analysis instrument (Alifax, Padova, Italy) as quickly as possible. An additional 2-ml whole blood sample was stored in a routine blood tube (BD Vacutainer® Tubes [buffered sodium citrate, REF 363095]; Becton, Dickinson and Co.) and tested for coagulation function using a Sysmex CA-7000 instrument (Sysmex). A further 4 ml of whole blood was stored in a routine blood tube (BD Vacutainer® SST II Plus plastic serum tube; Becton, Dickinson and Co.) and serum was separated for use in other biochemical analyses using an R7600-120 autoanalyser (Hitachi, Tokyo, Japan) as quickly as possible. All blood samples were kept temporarily at room temperature.
Anaesthesia and surgery management
All patients underwent THA surgery under the same general anaesthesia regimen. None of the patients was given premedication. Systolic blood pressure, diastolic blood pressure, pulse oxygen saturation, ECG, bispectral index (BIS) and end-tidal CO2 (ETCO2) were continuously monitored during the intraoperative period using a Philips MP30 patient monitor (Philips Healthcare, Best, the Netherlands). Anaesthesia was induced with 0.03 mg/kg midazolam intravenous (i.v.), 0.3 µg/kg sufentanil i.v., 1.5 mg/kg propofol i.v. and 0.1 mg/kg vecuronium i.v. in turn. Anaesthesia was maintained with 1% – 3% sevoflurane inhalation and 2 – 5 mg/kg per h propofol by continuous i.v. infusion; 0.2 µg/kg sufentanil and 2 mg vecuronium were administered by intermittent i.v. to provide analgesia and muscle relaxant effects during the surgery. At the same time, the respiratory parameters of the anaesthesia machine (Fabius® Plus; Dräger, Lubeck, Germany) were modulated to maintain ETCO2 at 30 – 40 mmHg and the depth of anaesthesia was adjusted to keep the BIS value at 40 – 60. Intraoperative blood pressure fluctuations were maintained within ±30% of the baseline value; heart rate was kept at 50 – 100 beats/min by adjusting the anaesthesia depth, transfusion rate, and administration of cardiovascular agents such as 5 mg ephedrine i.v. as and when required. After surgery, all patients received the same treatments for analgesia (8 mg/kg tramadol continuously pumped for 48 h) and infection prevention (2 g cefotiam i.v. infusion administered twice a day for 2 days).
Assessment of postoperative delirium
The preoperative cognitive function was measured using the MMSE on the day before surgery. Delirium was evaluated by the same attending anaesthesiologist twice daily (8:00 h and 20:00 h) preoperatively and for the first three postoperative days. The diagnostic criteria of delirium are described by the Diagnostic and Statistical Manual IV diagnostic criteria13 and the Confusion Assessment Method.14 Delirium assessment contains four evaluation features: (i) acute change or fluctuating course of mental status; (ii) inattention; (iii) altered level of consciousness; (iv) disorganized thinking. A positive diagnosis of delirium required the presence of items (i) and (ii), and either (iii) or (iv). For the analyses, patients were grouped according to the presence or absence of PD.
Data collection
A total of 39 preoperative risk factors that were potentially related to PD development were recorded, including the following: demographic data (age, sex, body mass index [BMI]); general comorbidities (stroke, diabetes mellitus, hypertension, Parkinson’s disease); preoperative electrocardiogram status (atrial fibrillation); duration from hospitalization to surgery; and laboratory examinations (routine blood test, blood electrolytes, hepatic and renal functions, coagulation function, blood glucose and ESR). The following six intraoperative variables were included in the study: surgery duration, anaesthesia duration, blood loss during surgery, red blood cell transfusion usage, plasma transfusion usage and autologous blood transfusion.
Statistical analyses
Logistic regression was used to identify the risk factors for PD. The sample size was calculated using the number of variables for PD after THA. The sample size needed to be equal to 5 to 10 times the number of observed variables according to an empirical formula of sample size estimation. Based on the results of empirical and clinical research,15 45 variables were included in this study, so it was necessary to have a sample size of at least 225 patients in the study.
All statistical analyses were performed using SPSS® version 19.0 (SPSS Inc., Chicago, IL, USA) for Windows®. Continuous variables were expressed as mean ± SD or median (interquartile range). Categorical data were expressed as n of patients. Kolmogorov–Smirnov test was used to test for normality. If continuous variables were not normally distributed, Mann–Whitney U-test was used to compare data. If continuous variables were normally distributed, Student’s t-test was used. Nominal data were analysed using χ2-test. Univariate and multivariate stepwise logistic regression analyses were used to identify the impact of various factors on the development of PD. A P-value < 0.05 was considered statistically significant.
Results
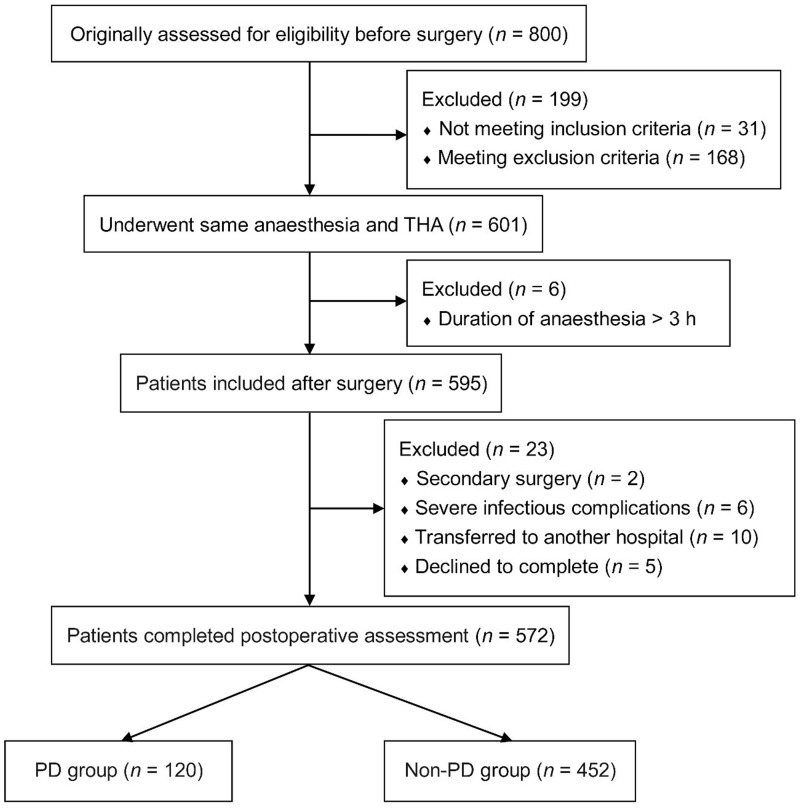
The study originally assessed 800 elderly patients with hip fractures who underwent THA surgery for eligibility for inclusion in the study (Figure 1). Of these, 228 patients were excluded from the final analysis data set: 199 before study enrolment; six immediately following surgery due to the duration of anaesthesia being > 3 h; 23 during the postoperative follow-up period. A total of 572 patients completed the perioperative and postoperative observations, of whom 120 experienced PD (21.0%; 36 [30%] male and 84 [70%] female patients); 452 patients (79.0%; 170 [38%] male and 282 [62%] female patients) were unaffected by PD.
Figure 1.
Flow chart showing progress through the study of elderly patients (n = 800) with hip fractures, evaluated for postoperative delirium (PD) following total hip arthroplasty (THA).
Demographic and clinical characteristics, and the perioperative features of the two groups, are presented in Table 1. Of the 45 risk variables surveyed, 14 factors were significantly higher in the patients who had PD compared with patients without PD: age, history of stroke, atrial fibrillation, white blood cell count, neutrophil proportion, total bilirubin, direct bilirubin, prothrombin time, activated partial thromboplastin time, blood glucose, CRP, surgery duration, intraoperative blood loss and red blood cell transfusion volume (P < 0.05 for all comparisons). Eight factors were significantly lower in patients with PD compared with patients without PD: red blood cell count, platelet count, haemoglobin, haematocrit, calcium, albumin, total protein and albumin/globulin ratio (P < 0.05 for all comparisons).
Table 1.
Association between risk factors and postoperative delirium (PD) in elderly patients (n = 572) with hip fractures who underwent total hip arthroplasty.
| Variable | Non-PD group n = 452 | PD group n = 120 | Statistical analysis |
|---|---|---|---|
| Age, years | 76.00 (72.00–80.00) | 82.00 (76.00–86.00) | P < 0.001a |
| Sex, male/female | 170/282 | 36/84 | NSb |
| BMI, kg/m2 | 22.42 ± 1.98 | 22.08 ± 1.80 | NSc |
| MMSE | 26.10 (25.3–27.2) | 26.00 (25.4–26.8) | NSa |
| Duration from hospitalization to surgery, days | 3.69 ± 1.59 | 3.62 ± 1.72 | NSc |
| History of hypertension | 204 | 52 | NSb |
| History of Parkinson’s disease | 2 | 2 | NSb |
| History of stroke | 22 | 24 | P < 0.001b |
| Atrial fibrillation | 0 | 16 | P < 0.001b |
| WBC, ×109/l | 6.40 (5.20–7.40) | 8.00 (5.50–10.93) | P = 0.003a |
| RBC, ×1012/l | 4.29 (3.86–4.66) | 4.00 (3.35–4.35) | P = 0.003a |
| Platelet count, 109/l | 204.74 ± 51.06 | 184.65 ± 53.13 | P < 0.001c |
| Haemoglobin, g/l | 125.00 (115.00–133.00) | 116.50 (104.25–129.00) | P = 0.006a |
| Haematocrit, % | 37.60 (35.30–40.20) | 35.00 (30.60–38.78) | P = 0.002a |
| Neutrophils, % | 63.10 ± 12.64 | 72.06 ± 13.53 | P < 0.001c |
| Potassium, mmol/l | 3.98 ± 0.41 | 4.01 ± 0.43 | NSc |
| Sodium, mmol/l | 141.71 ± 3.03 | 141.25 ± 2.64 | NSc |
| Chlorine, mmol/l | 103.53 ± 3.72 | 103.02 ± 3.08 | NSc |
| Calcium, mmol/l | 2.23 ± 0.19 | 2.18 ± 0.11 | P = 0.004c |
| Phosphorus, mmol/l | 1.07 ± 0.13 | 1.06 ± 0.15 | NSc |
| Magnesium, mmol/l | 0.91 ± 0.07 | 0.91 ± 0.07 | NSc |
| ALT, U/l | 20.65 ± 13.05 | 18.77 ± 10.36 | NSc |
| AST, U/l | 22.11 ± 9.15 | 22.35 ± 8.18 | NSc |
| BUN, mmol/l | 5.91 ± 2.20 | 6.34 ± 2.13 | NSc |
| Cr, µmol/l | 63.98 ± 27.69 | 69.55 ± 27.59 | NSc |
| BUN/Cr ratio | 0.10 ± 0.03 | 0.09 ± 0.02 | NSc |
| Albumin, g/l | 43.00 (40.00–45.00) | 39.00 (35.25–41.75) | P < 0.001a |
| Total protein, g/l | 67.27 ± 5.48 | 63.38 ± 6.22 | P < 0.001c |
| Albumin/globulin ratio | 1.70 (1.50–2.00) | 1.60 (1.40–1.98) | P = 0.013a |
| GGT, U/l | 29.63 ± 21.32 | 26.70 ± 33.29 | NSc |
| Total bilirubin, µmol/l | 10.45 (8.70–13.50) | 14.00 (9.13–18.58) | P = 0.008a |
| Direct bilirubin, µmol/l | 3.50 (2.70–4.90) | 4.20 (3.38–5.78) | P = 0.005a |
| PT, s | 10.96 ± 1.52 | 11.58 ± 0.96 | P < 0.001c |
| APTT, s | 26.75 ± 4.14 | 28.56 ± 7.07 | P < 0.001c |
| Fibrinogen, g/l | 2.97 (2.56–3.31) | 2.96 (2.68–3.57) | NSa |
| Blood glucose, mmol/l | 5.28 (4.85–6.07) | 6.38 (5.35–7.87) | P < 0.001a |
| Cystatin C, mg/l | 1.20 (1.00–1.40) | 1.15 (1.00–1.48) | NSa |
| CRP, mg/l | 1.93 (1.15–3.22) | 8.45 (3.71–15.23) | P = 0.034a |
| ESR, mm/h | 26.85 ± 20.20 | 29.10 ± 17.67 | NSc |
| Anaesthesia duration, min | 90.00 (80.00–105.00) | 90.00 (75.00–120.00) | NSa |
| Surgery duration, min | 62.50 (60.00–80.00) | 72.50 (60.00–90.00) | P = 0.003a |
| Intraoperative blood loss, ml | 349.34 ± 178.03 | 403.33 ± 197.01 | P = 0.004c |
| Red blood cell transfusion, ml | 0 (0–400.00) | 400.00 (0–400.00) | P < 0.001a |
| Plasma transfusion, ml | 30.97 ± 103.88 | 26.67 ± 93.25 | NSc |
| Autologous blood transfusion, ml | 7.96 ± 43.95 | 12.00 ± 58.81 | NSc |
Continuous variables expressed as mean ± SD or median (interquartile range). Categorical data expressed as n patients.
Mann–Whitney U-test; bχ2-test; cStudent’s t-test.
BMI, body mass index; MMSE, Mini-Mental State Examination; WBC, white blood cell count; RBC, red blood cell count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; GGT, gamma glutamyltranspeptidase; PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; NS, no significant between-group difference (P ≥ 0.05).
Multivariate stepwise logistic regression analyses were performed to determine the risk factors for PD in this elderly patient population. Age (odds ratio [OR] 1.109; 95% confidence interval [CI] 1.058, 1.163; P < 0.001), albumin (OR 0.898; 95% CI 0.834, 0.966; P = 0.004), blood glucose (OR 1.228; 95% CI 1.082, 1.394; P = 0.001), history of stroke (OR 5.618; 95% CI 2.277, 13.862; P < 0.001), total bilirubin (OR 1.077; 95% CI 1.036, 1.121; P < 0.001), CRP (OR 1.028; 95% CI 1.011, 1.045; P = 0.001), surgery duration (OR 1.010; 95% CI 1.001, 1.019; P = 0.026) and red blood cell transfusions (OR 1.001; 95% CI 1.000, 1.003; P = 0.039) were found to be independent risk factors (Table 2).
Table 2.
Multivariate stepwise logistic regression analysis of risk factors for postoperative delirium in elderly patients (n = 572) with hip fractures who underwent total hip arthroplasty.
| Variable | Odds ratio (95% CI) | Statistical analysis |
|---|---|---|
| Age, years | 1.109 (1.058, 1.163) | P < 0.001 |
| Albumin, g/l | 0.898 (0.834, 0.966) | P = 0.004 |
| Blood glucose, mmol/l | 1.228 (1.082, 1.394) | P = 0.001 |
| History of stroke | 5.618 (2.277, 13.862) | P < 0.001 |
| Total bilirubin, µmol/l | 1.077 (1.036, 1.121) | P < 0.001 |
| CRP, mg/l | 1.028 (1.011, 1.045) | P = 0.001 |
| Surgery duration, min | 1.010 (1.001, 1.019) | P = 0.026 |
| Red cell transfusion, ml | 1.001 (1.000, 1.003) | P = 0.039 |
CI, confidence interval; CRP, C-reactive protein.
Discussion
Postoperative delirium is a serious and frequent complication in elderly patients with hip fractures who have undergone THA.16 To the best of our knowledge, this present investigation is the first prospective study to measure the prevalence of PD and the risk factors associated with PD in elderly patients with hip fractures undergoing THA in a large sample size. In the present study, the prevalence of PD after THA was 21.0%, which was similar to the rate reported by a previous study.2 As studies have found that preoperative dementia, anxiety, depression and nutritional deprivation are associated with PD,17,18 this condition is thought to be associated with multiple risk factors. In the current study, multivariate stepwise logistic regression analyses indicated that older age, lower albumin, a history of stroke, higher blood glucose, higher total bilirubin, higher CRP, longer duration of surgery, and a higher volume of red blood cell transfusions were independent risk factors of PD in elderly patients following THA for hip fracture.
Recent research has found that advanced age may assist in defining patients at increased risk for PD.19 Elderly patients have both a reduced ability to respond to stress and to adapt to an abnormal metabolism, and this dysfunctional state is accompanied by the loss of central cholinergic neurons.20 In addition, disturbances to a wide variety of neurotransmitter systems with increasing age may be the major pathological cause of PD.21 These changes appear to make the elderly more prone to PD.
Evidence suggests that undernutrition (defined as a low BMI and protein malnutrition) negatively impacts on health-related quality of life and postoperative outcomes.22,23 A multivariate logistic regression analysis demonstrated that malnutrition was an independent preoperative risk factor for PD after coronary artery bypass grafting.18 Elderly patients with hip fracture are often prone to metabolic disorders, low calorie consumption, impaired nutrition and concomitant cerebrovascular disease leading to impaired nutritional intake.24 The findings of the present study appear to support this as a lower albumin concentration was associated with the risk of PD.
Preoperative cerebral infarction is a significant risk factor for delirium after coronary artery bypass graft surgery.25 Very old hip fracture patients (age ≥ 80 years) with a medical history of stroke were more likely to develop PD compared with elderly patients < 80 years.26 These findings can be explained by the fact that stroke is a major contributor to dementia in later years and one of the main pathophysiological mechanisms in vascular dementia.27 Furthermore, preoperative cerebral infarction might be exacerbated during the perioperative period by embolisms induced by the hip fracture or THA, as suggested by observations in patients who underwent off-pump coronary artery bypass grafting surgery.28 The present study demonstrated that a history of stroke was a risk factor for PD in elderly patients (aged ≥ 65 years) after THA.
Glucose control during the perioperative period is an area associated with considerable controversy and debate among clinicians with much of the research being conducted in cardiac surgery.29 Moreover, the majority of clinicians advocate tight blood glucose control. Research has confirmed that delirium after cardiac surgery was independently associated with fasting glucose level, and the fasting glucose level of patients with PD was higher than that of patients without PD.29 Short- and long-term mortality were shown to independently correspond with baseline plasma glucose in a cohort of cardiac surgery patients.30 An abnormal serum glucose level was one of the causative factors in the development of postoperative psychiatric disorders in patients undergoing general thoracic surgery.31 A study showed that the initial, mean, and maximal intraoperative blood glucose concentrations were significantly higher in patients experiencing complications including death after cardiac surgery.32 Another study indicated that intraoperative tight blood glucose control using hyperinsulinaemic normoglycaemia increases delirium after cardiac surgery.33 In the present study, a higher baseline blood glucose level was an independent risk factor for PD in elderly patients undergoing THA for hip fracture. As a consequence, we would recommend tight blood glucose control during the perioperative and postoperative periods.
Plasma cholinesterase activity can be a useful biomarker to identify those elderly patients with a heightened risk of developing PD after THA.34 Plasma cholinesterase activity correlated positively with calcium and haemoglobin, and negatively with total bilirubin and international normalized ratio.34 Impaired liver function can affect the development of PD after liver resection for hepatocellular carcinoma.35 As various intracorporal bioactive substances are synthesized, secreted and excreted by the liver, and a variety of drugs (e.g. anaesthetics) are metabolized in the liver, liver function is closely related to neurological status.6 This present study showed that elderly patients with preoperative decreased albumin and increased total bilirubin were at a heightened risk of experiencing PD.
Surgical trauma can lead to activation of the immune system and hyperactivity of the hypothalamic–pituitary–adrenal axis, subsequently resulting in disorders of acetylcholine, norepinephrine, 5-hydroxytryptamine and other neurotransmitters in the central nervous system (CNS), which ultimately might result in brain dysfunction after surgery.36 A high cortisol level after surgery is linked to PD.37 Peripheral inflammatory cytokines caused by surgery could enter into the CNS via the transport receptors in the blood–brain barrier, subsequently activating microglia to produce an inflammatory response.38 Inflammatory cytokines could interfere with the connection and transmission functions of synapses.39 Multivariate logistic regression analysis confirmed the relationship between an elevated CRP value and PD following vascular surgery.40 A lower intraoperative haemoglobin level and excessive haemorrhaging during surgery were significant variables for the development of PD in a multivariate analysis of patients following surgery for oral cancer.41 The present study demonstrated that increased CRP, a longer duration of surgery, and a higher red blood cell transfusion volume were risk factors for the development of PD.
In conclusion, this present study found that older age, a history of stroke, lower albumin, higher blood glucose, higher total bilirubin, higher CRP, longer surgery duration, and greater volumes of red blood cell transfusions were independent risk factors for PD in elderly patients undergoing THA for hip fracture. These risk factors can be used to identify patients at high risk of PD. Moreover, correcting the modifiable risk factors may help prevent PD. Strategies might include nutritional support, tight blood glucose control, improvement of liver function, preoperative infection control, and minimizing surgical injury and blood loss.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This work was supported by Shanghai Municipal Commission of Health and Family Planning Foundation for Key Developing Disciplines (2015ZB0103) and Shanghai Jiao Tong University Biomedical Engineering Cross Research Foundation (YG2015MS15). The content is solely the responsibility of the authors.
References
- 1.Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry 2008; 165: 803–812. [DOI] [PubMed] [Google Scholar]
- 2.Wang NY, Hirao A, Sieber F. Association between intraoperative blood pressure and postoperative delirium in elderly hip fracture patients. PLoS One 2015; 10: e0123892–e0123892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zywiel MG, Hurley RT, Perruccio AV, et al. Health economic implications of perioperative delirium in older patients after surgery for a fragility hip fracture. J Bone Joint Surg Am 2015; 97: 829–836. [DOI] [PubMed] [Google Scholar]
- 4.Skrobik Y. Delirium prevention and treatment. Anesthesiol Clin 2011; 29: 721–727. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 2013; 21: 1190–1222. [DOI] [PubMed] [Google Scholar]
- 6.Chen YL, Lin HC, Lin KH, et al. Low hemoglobin level is associated with the development of delirium after hepatectomy for hepatocellular carcinoma patients. PLoS One 2015; 10: e0119199–e0119199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai S, Xu L, Zhang L, et al. Preoperative risk factors of postoperative delirium after transurethral prostatectomy for benign prostatic hyperplasia. Int J Clin Exp Med 2015; 8: 4569–4574. [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski CJ, Trenerry MR, Cook DJ, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesth Analg 2011; 112: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 9.Westhoff D, Witlox J, Koenderman L, et al. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J Neuroinflammation 2013; 10: 122–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Society of Anesthesiologists. ASA Physical Status Classification System, www.asahq.org/resources/clinical-information/asa-physical-status-classification-system (accessed 15 October 2014).
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 12.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992; 20: 864–874. [PubMed]
- 13.Widiger TA, Frances AJ, Pincus HA, et al. Diagnostic and statistical manual of mental disorders: DSM-IV, 4th ed Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
- 14.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–948. [DOI] [PubMed] [Google Scholar]
- 15.Zhang WY, Wu WL, Gu JJ, et al. Risk factors for postoperative delirium in patients after coronary artery bypass grafting: A prospective cohort study. J Crit Care 2015; 30: 606–612. [DOI] [PubMed] [Google Scholar]
- 16.Freter S, Dunbar M, Koller K, et al. Risk of pre-and post-operative delirium and the Delirium Elderly At Risk (DEAR) tool in hip fracture patients. Can Geriatr J 2015; 4: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Castro SM, Ünlü Ç, Tuynman JB, et al. Incidence and risk factors of delirium in the elderly general surgical patient. Am J Surg 2014; 208: 26–32. [DOI] [PubMed] [Google Scholar]
- 18.Ringaitienė D, Gineitytė D, Vicka V, et al. Impact of malnutrition on postoperative delirium development after on pump coronary artery bypass grafting. J Cardiothorac Surg 2015; 10: 74–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raats JW, van Eijsden WA, Crolla RM, et al. Risk factors and outcomes for postoperative delirium after major surgery in elderly patients. PLoS One 2015; 10: e0136071–e0136071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson BD, Robertson TJ. Postoperative delirium after hip fracture. J Bone Joint Surg Am 2006; 88: 2060–2068. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein JH, Timberger M, Reich DL, et al. Central nervous system dysfunction after noncardiac surgery and anesthesia in the elderly. Anesthesiology 2007; 106: 622–628. [DOI] [PubMed] [Google Scholar]
- 22.Chermesh I, Hajos J, Mashiach T, et al. Malnutrition in cardiac surgery: food for thought. Eur J Prev Cardiol 2014; 21: 475–483. [DOI] [PubMed] [Google Scholar]
- 23.van Venrooij LM, de Vos R, Zijlstra E, et al. The impact of low preoperative fat-free body mass on infections and length of stay after cardiac surgery: a prospective cohort study. J Thorac Cardiovasc Surg 2011; 142: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 24.Chu CS, Liang CK, Chou MY, et al. Short-Form Mini Nutritional Assessment as a useful method of predicting the development of postoperative delirium in elderly patients undergoing orthopedic surgery. Gen Hosp Psychiatry 2016; 38: 15–20. [DOI] [PubMed] [Google Scholar]
- 25.Otomo S, Maekawa K, Goto T, et al. Pre-existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2013; 17: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luger MF, Müller S, Kammerlander C, et al. Predictors of postoperative cognitive decline in very old patients with hip fracture: a retrospective analysis. Geriatr Orthop Surg Rehabil 2014; 5: 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knopman DS. Cerebrovascular disease and dementia. Br J Radiol 2007; 80: S121–S127. [DOI] [PubMed] [Google Scholar]
- 28.Omiya H, Yoshitani K, Yamada N, et al. Preoperative brain magnetic resonance imaging and postoperative delirium after off-pump coronary artery bypass grafting: a prospective cohort study. Can J Anaesth 2015; 62: 595–602. [DOI] [PubMed] [Google Scholar]
- 29.Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, et al. Complex assessment of the incidence and risk factors of delirium in a large cohort of cardiac surgery patients: a single-center 6-year experience. Biomed Res Int 2013; 2013: 835850–835850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, et al. Detailed insight into the impact of postoperative neuropsychiatric complications on mortality in a cohort of cardiac surgery subjects: a 23,000-patient-year analysis. J Cardiothorac Vasc Anesth 2014; 28: 448–457. [DOI] [PubMed] [Google Scholar]
- 31.Ozyurtkan MO, Yildizeli B, Kuşçu K, et al. Postoperative psychiatric disorders in general thoracic surgery: incidence, risk factors and outcomes. Eur J Cardiothorac Surg 2010; 37: 1152–1157. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi GY, Nuttall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc 2005; 80: 862–866. [DOI] [PubMed] [Google Scholar]
- 33.Saager L, Duncan AE, Yared JP, et al. Intraoperative tight glucose control using hyperinsulinemic normoglycemia increases delirium after cardiac surgery. Anesthesiology 2015; 122: 1214–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerejeira J, Batista P, Nogueira V, et al. Low preoperative plasma cholinesterase activity as a risk marker of postoperative delirium in elderly patients. Age Ageing 2011; 40: 621–626. [DOI] [PubMed] [Google Scholar]
- 35.Yoshimura Y, Kubo S, Shirata K, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World J Surg 2004; 28: 982–986. [DOI] [PubMed] [Google Scholar]
- 36.Cerejeira J, Batista P, Nogueira V, et al. The stress response to surgery and postoperative delirium: evidence of hypothalamic-pituitary-adrenal axis hyperresponsiveness and decreased suppression of the GH/IGF-1 Axis. J Geriatr Psychiatry Neurol 2013; 26: 185–194. [DOI] [PubMed] [Google Scholar]
- 37.Kazmierski J, Banys A, Latek J, et al. Mild cognitive impairment with associated inflammatory and cortisol alterations as independent risk factor for postoperative delirium. Dement Geriatr Cogn Disord 2014; 38: 65–78. [DOI] [PubMed] [Google Scholar]
- 38.Beloosesky Y, Hendel D, Weiss A, et al. Cytokines and C-reactive protein production in hip-fracture-operated elderly patients. J Gerontol A Biol Sci Med Sci 2007; 62: 420–426. [DOI] [PubMed] [Google Scholar]
- 39.Campbell IL. Transgenic mice and cytokine actions in the brain: bridging the gap between structural and functional neuropathology. Brain Res Brain Res Rev 1998; 26: 327–336. [DOI] [PubMed] [Google Scholar]
- 40.Pol RA, van Leeuwen BL, Izaks GJ, et al. C-reactive protein predicts postoperative delirium following vascular surgery. Ann Vasc Surg 2014; 28: 1923–1930. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa T, Saito I, Takeda D, et al. Risk factors associated with postoperative delirium after surgery for oral cancer. J Craniomaxillofac Surg 2015; 43: 1094–1098. [DOI] [PubMed] [Google Scholar]