Abstract
Objectives
This randomized, prospective double-blind study compared remifentanil with dexmedetomidine for monitored anaesthesia care during minimally invasive corrections of vertebral compression fractures (vertebroplasty or kyphoplasty).
Methods
Patients > 65 years of age with American Society of Anesthesiologists (ASA) classification I–III, scheduled for vertebroplasty or kyphoplasty under monitored anaesthesia care, received remifentanil (i.v. infusion 1–5 µg/kg/h) or dexmedetomidine (loading dose 0.3–0.4 µg/kg followed by i.v. infusion 0.2–1 µg/kg/h) to maintain observer's assessment of alertness/sedation (OAA/S) scale <4 during the procedure.
Results
There were no statistically significant differences in demographic data between the remifentanil (n = 37) and dexmedetomidine groups (n = 38). Patients on dexmedetomidine experienced lower mean arterial pressure (MAP) and heart rate (HR), and higher SpO2 values, than patients on remifentanil. Compared with dexmedetomidine, remifentanil produced more respiratory depression, oxygen desaturation, and reduced the need for additional intraoperative opioids. There were no significant between-group differences in terms of recovery time, investigators’ satisfaction scores, or patients’ overall pain experiences.
Conclusions
During monitored anaesthesia care, dexmedetomidine provides less respiratory depression, lower MAP and HR, but also less analgesic effect than remifentanil in elderly patients undergoing vertebroplasty or kyphoplasty.
Keywords: Remifentanil, dexmedetomidine, vertebroplasy, kyphoplasty, monitored anaesthesia care; elderly
Introduction
Compression fractures of the spine are common causes of disability among the elderly1 and are often treated by the minimally invasive procedures, vertebroplasty or kyphoplasty.2 In these procedures, bone cement is injected percutaneously into the fractured vertebra. Local anaesthesia for vertebroplasty is effective, well tolerated and easy to use. However, for kyphoplasty (which is a more technically complex procedure than vertebroplasty), patients may feel more pain and may move, making the anaesthesia more problematic and so increase its duration.3 Although general anaesthesia for vertebroplasty and kyphoplasty provides a good surgical condition and comfort for both patients and physicians, these spinal procedures are commonly performed in elderly people who have decreased cardiopulmonary function. In addition, these procedures are performed in the prone position, which may increase the risk of anaesthesia-related cardiopulmonary complications. Monitored anaesthesia care, which controls the patients’ anxiety and pain without need for an intervention to maintain respiratory and cardiac function,4 may be more appropriate than general anaesthesia for elderly patients. However, the use of sedatives in patients in the prone position, especially if they are elderly, may induce respiratory depression and make airway management difficult.5,6
Of the sedatives available for monitored anaesthesia care, benzodiazepines, propofol and opioids are most frequently used.7 Propofol and remifentanil are commonly used because of their rapid onset and short duration of action,8,9 and their good tolerability.10 However, interaction and synergism between these drugs may result in oxygen desaturation and hypoxaemia during the procedure.7 Dexmedetomidine is a highly selective α–2 adrenergic agonist with both sedative and analgesic properties that rarely causes respiratory depression.11
To our knowledge, no reported study has compared remifentanil with dexmedetomidine during vertebroplasty or kyphoplasty. Therefore, we compared the effects of remifentanil with dexmedetomidine during monitored anaesthesia care for the two spinal procedures in elderly patients. Treatments were assessed in terms of effects on the patients’ haemodynamic stability and respiratory condition. Following each procedure, we also recorded investigators’ satisfaction scores and patients’ overall pain experience.
Patients and Methods
Patients
This randomized, prospective, double-blind study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital. All patients provided written informed consent. The study was registered retrospectively because during its inception trial registration was not mandatory.
Patients who were scheduled for vertebroplasty or kyphoplasty under monitored anaesthesia care, and had an American Society of Anesthesiologists (ASA) status classification of I–III,12 and were >65 years of age, were randomly assigned to receive remifentanil or dexmedetomidine in equally sized groups using a computer-generated random-numbers programme. Patients with obesity (defined as a body mass index [BMI] > 30 kg/m2), hypotension (systolic blood pressure [BP] < 100 mmHg), bradycardia (heart rate (HR) < 60 beats per min), heart block, baseline oxygen desaturation (SpO2 < 90%), sleep apnoea, asthma, or chronic obstructive pulmonary disease, and those who refused to give informed consent, were excluded from the study.
Procedures
All patients fasted for 8 h before the procedure and were premedicated with intravenous (i.v.) midazolam 0.02 mg/kg. During the procedure, patients were monitored in the prone position by standard electrocardiogram (ECG), noninvasive BP assessments and pulse oximetry, and received supplemental oxygen (3 l/min) via a nasal cannula.
Study treatments were prepared by one of the authors (S.K.L.) in 20-ml and 50-ml syringes. Initially all patients received an i.v. bolus dose of propofol 0.3 mg/kg. Patients allocated dexmedetomidine received an i.v. loading dose at 0.3–0.4 µg/kg, administered from a 20-ml syringe over 10 min, followed by a continuous i.v. infusion of dexmedetomidine 0.2–1 µg/kg/h from a 50 ml syringe. To maintain the double-blind nature of procedures, patients allocated remifentanil received i.v. saline for the loading dose (administered from a 20-ml syringe over 10 min), followed by a continuous i.v. infusion of remifentanil at 1–5 µg/kg/h from a 50-ml syringe. Levels of patient sedation were checked during the procedure and infusion rates were adjusted to maintain alertness/sedation below 4 on the Observer's assessment of alertness/sedation (OAA/S) scale (Table 1).13 Mean arterial pressure (MAP), heart rate (HR), SpO2, respiratory rate (RR), and adverse effects of the study drugs were recorded by independent assessors (S.K.L.) at every step during the procedure (i.e., T0, baseline; T1, lidocaine infiltration; T2, trocar insertion; T3, cement insertion; T4, skin closure). Ephedrine 5 mg or 10 mg was injected i.v. if systolic BP decreased below 90 mmHg; atropine 0.5 mg was injected i.v. if HR fell below 60 beats per min.
Table 1.
Observer’s assessment of alert/sedation (OAA/S) scale used to assess level of sedation13
| Response scale | Level |
|---|---|
| Responds readily to name spoken in normal tone (awake/alert) | 5 |
| Lethargic response to name spoken in normal tone | 4 |
| Responds only after name spoken loudly or repeatedly | 3 |
| Responds after mild prodding or shaking | 2 |
| Does not respond to mild prodding or shaking (asleep/unrousable) | 1 |
The incidences of oxygen desaturation and respiratory depression were recorded, as were trends of oxygen saturation and RR. Oxygen desaturation was defined as SpO2 < 93% for >10 s; respiratory depression was defined as RR < 12/min. In the event of SpO2 < 90%, patients received oxygen via a facial mask instead of the nasal cannula, oxygen delivery was increased from 3 to 7 l/min and airway assistance manoeuvres were performed. Patients were asked to assess their level of pain during the procedure using a visual analogue scale (VAS) which spanned 0 (no pain) to 10 (worst possible pain). Those who scored VAS ≥ 5 were given additional i.v. fentanyl 0.5–1 µg/kg despite maximal infusion of the study drug. Patients were discharged from the postanaesthesia care unit (PACU) when the postanaesthetic Aldrete recovery score14 was ≥ 9. The duration of PACU stay was recorded and prolonged duration of PACU stay was defined as > 60 min.
Investigators’ satisfaction scores (0; extensive movement and complaints, several interruptions; 1, movements, two interruptions; 2, small movements, no interruptions; 3, no movements, no interruptions) and patients’ overall pain experience (0, no pain; 1, mild; 2, moderate; 3, severe) were also recorded after the procedures by independent assessors.
Statistical analyses
In a previous study, the incidence of oxygen desaturation in the remifentanil group was 40%.3 Therefore, it was estimated that 32 patients in each group were required to detect a difference between treatments, assuming that patients on dexmedetomidine achieve a 75% risk reduction compared with those on remifentanil (α = 0.05, power = 0.8). Assuming a 20% dropout rate, the final sample size was estimated to be 40 patients in each group. Statistical analyses were performed using SPSS® software (version 22.0 for Windows®; IBM, Somers, NY, USA).
Haemodynamic and respiratory data were analysed using repeated-measures analysis of variance (ANOVA) and intergroup differences at the same time point were analysed using a two-sample t-test. Investigators’ satisfaction scores and patients’ overall pain experience were assessed using Mann–Whitney U-test. A two-sample t-test was used to analyse recovery time between the two treatment groups. Fisher’s exact test was used to analyse the incidences of adverse effects. Data were presented as mean ± SD or median (interquartile range). P-values < 0.05 were considered to indicate statistical significance.
Results
In total, 80 elderly patients were recruited into the study and five were excluded due to baseline oxygen desaturation. There were no statistically significant differences in demographic or clinical characteristics between patients in the remifentanil group (n = 37) and those in the dexmedetomidine group (n = 38) (Table 2).
Table 2.
Demographic data from the comparison of remifentanil with dexmedetomidine for monitored anaesthesia care in elderly patients during vertebroplasty and kyphoplasty
| Characteristic | Remifentanil | Dexmedetomidine |
|---|---|---|
| Number of patients | 37 | 38 |
| Sex, male: female | 2 : 35 | 4 : 34 |
| Age, years | 77.1 ± 7.4 | 75.4 ± 6.4 |
| Weight, kg | 54.5 ± 9.7 | 57.4 ± 9.8 |
| Height, cm | 151.8 ± 5.7 | 153.5 ± 7.7 |
| Duration of surgery, min | 45.5 ± 9.7 | 45.1 ± 9.0 |
| ASA class | ||
| I | 3 | 2 |
| II | 15 | 14 |
| III | 19 | 22 |
Data presented as mean ± SD or n patients.
ASA: American Society of Anesthesiologists12
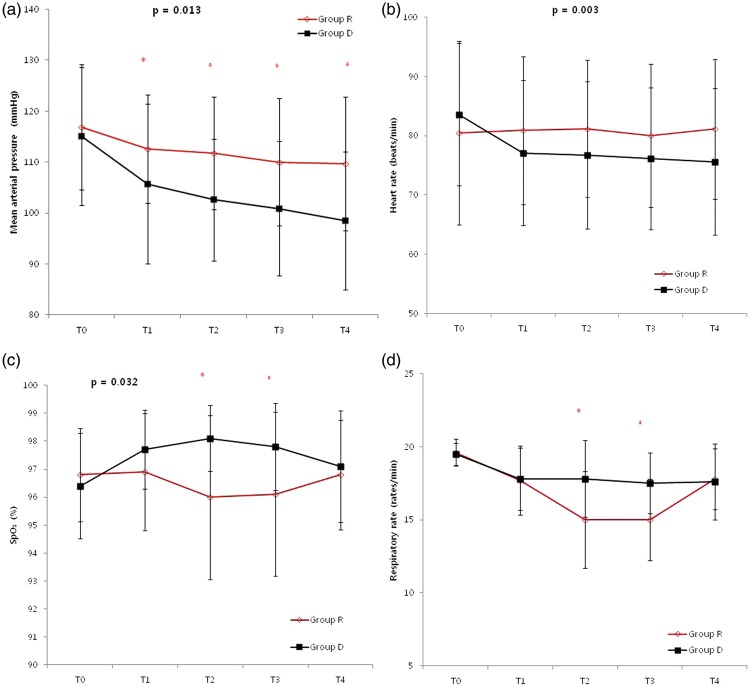
Haemodynamic and respiratory variables at each surgical step are shown in Figure 1. There were no statistically significant intergroup differences in MAP, HR, SpO2, or RR at T0. However, repeated-measures ANOVA showed that over the remaining study period, MAP (Figure 1a; P = 0.013) and HR (Figure 1b; P = 0.003) were statistically significantly lower in the dexmedetomidine group than in the remifentanil group. For MAP, significant differences between treatments were observed at all time points. For SpO2, repeated-measures ANOVA showed that values were statistically significantly lower in the remifentanil group than in the dexmedetomidine group (Figure 1c; P = 0.032), particularly at T2 (96.0 ± 2.9 vs. 98.1 ± 1.2) and T3 (96.1 ± 2.9% vs. 97.8 ± 1.6%). Overall, there was no statistically significant difference in RR during the procedure (Figure 1d; P-value = 0.6), but at T2 and T3, RR was statistically significantly lower in the remifentanil group (15.0 ± 3.3, 15.0 ± 2.8 rpm) than in the dexmedetomidine group (17.8 ± 2.6, 17.5 ± 2.1 rpm) (Figure 1d; P-values ≥0.05.
Figure 1.
Changes in (a) mean arterial pressure (MAP), (b) heart rate (HR), (c) oxygen desaturation (SpO2) and (d) respiratory rate (RR) in patients >65 years of age receiving remifentanil and dexmedetomidine during vertebroplasty or kyphoplasty (T0, baseline; T1, lidocaine infiltration; T2, trocar insertion; T3, cement insertion; T4, skin closure). Variables of MAP, HR and SpO2 showed significant difference over time between both groups, using repeated-measures analysis of variance (ANOVA). Intergroup differences at the same time point were analysed using a two-sample t-test (*P < 0.05)
Adverse effects of the study drugs included oxygen desaturation, respiratory depression, nausea/vomiting, additional fentanyl administration, hypotension and prolonged recovery time (Table 3). The incidences of oxygen desaturation and respiratory depression were higher with remifentanil than with dexmedetomidine (P = 0.026 for oxygen desaturation, and P < 0.0001 for respiratory depression). In the remifentanil group, no patients complained of pain during the procedure, whereas seven patients in the dexmedetomidine group complained of pain (VAS ≥ 5) and received additional fentanyl. The difference between the two treatments was statistically significant (P = 0.012). The incidences of hypotension and prolonged duration of PACU stay were higher with dexmedetomidine than with remifentanil, but intra- or postoperative nausea or vomiting rates were higher with remifentanil (four reports) than dexmedetomidine (no reports), but the differences were not statistically significant. The two patients in the dexmedetomidine group who developed hypotension received ephedrine (one patient received 5 mg; the other received 10 mg) to maintain systolic BP > 90 mmHg. No patient required atropine for bradycardia.
Table 3.
Adverse effects of remifentanil and dexmedetomidine for monitored anaesthesia care in the elderly during vertebroplasty and kyphoplasty
| Adverse effects | Remifentanil (continuous i.v. infusion of 1–5 µg/kg/h) (n = 37) | Dexmedetomidine (i.v. loading dose at 0.3–0.4 µg/kg followed by a continuous i.v. infusion of 0.2–1 µg/kg/h) (n = 38) | Statistical significancea |
|---|---|---|---|
| Oxygen desaturation | 13 (35.1) | 5 (13.2) | P = 0.026 |
| Respiratory depression | 15 (40.0) | 2 (5.3) | P < 0.0001 |
| Nausea/vomiting | 4 (10.8) | 0 | NS |
| Additional fentanyl administration | 0 | 7 (18.4) | P = 0.012 |
| Hypotension | 0 | 2 (5.3) | NS |
| Prolonged duration of PACU stay | 0 | 1 (2.6) | NS |
Data presented as n (%) patients.
Fisher’s exact test.
NS: not statistically significant (P > 0.05).
No statistically significant between-group differences were detected in the investigators’ satisfaction scores (for both treatments, median score was 2 [interquartile range, 2–3]), patients’ overall pain experience (remifentanil median score,1 [range 1–1]; dexmedetomidine median score, 1 [range 1–2]), or duration of PACU stay (21.49 ± 8.57 min, 21.84 ± 11.59 min).
Discussion
In this study, haemodynamic variables, adverse effects and other parameters were compared in elderly patients undergoing vertebroplasty or kyphoplasty under monitored anaesthesia care using remifentanil or dexmedetomidine. The findings showed that patients who received remifentanil were less likely to require additional opioids for pain control. However, compared with patients on dexmedetomidine, they had higher MAP and HR, lower SpO2, and more respiratory depression.
Few studies have examined vertebroplasty or kyphoplasty under monitored anaesthesia care. Mohr et al.15 reported that i.v. piritramide and midazolam during kyphoplasty were well tolerated and useful for their sedative and analgesic properties; Della Puppa et al.16 reported that continuous i.v. infusion of propofol and intermittent fentanyl injection were well tolerated and effective during vertebroplasty or kyphoplasty.
Remifentanil is known to be useful for spinal procedures under monitored anaesthesia care: its continuous infusion has been shown to provide better analgesia than intraosseous lidocaine alone or intermittent fentanyl injection during some spinal procedures.,17,18 Dexmedetomidine has been reported to be an effective and well tolerated sedative in patients undergoing a variety of procedures (orthopaedic, ophthalmic, plastic, vascular stent, breast biopsies, hernias, arteriovenous fistulae and excision of lesions) under monitored anaesthesia care.19 However, the use of dexmedetomidine in spinal procedures has not been reported; nor has a comparison of the drug with remifentanil been documented. For this study, the chosen maintenance i.v. doses of remifentanil (1–5 µg/kg/h) and dexmedetomidine (0.2–1 µg/kg/h) were based on previous studies where they have been reported to provide adequate sedation.7,20–23 Although there was no difference in patient overall pain experience between groups, the need for additional opioids during the procedure was higher in those on dexmedetomidine than in those on remifentanil, indicating that dexmedetomidine alone did not effectively control pain for patients undergoing vertebroplasty or kyphoplasty. Ryu et al.7 reported that patients who underwent flexible bronchoscopy under continuous infusion of dexmedetomidine required more frequent administration of topical anaesthesia than those undergoing the procedure on remifentanil. In addition, in another study, dexmedetomidine lacked the analgesic effects of alfentanil at a plasma concentration that produced mild-to-severe sedation.24 Although dexmedetomidine is known to have strong analgesic properties,11 it appears to be weaker than opioids in this respect. The results from this current study suggest that continuous infusion with dexmedetomidine alone does not appear to be a satisfactory option in spinal procedures.
All patients who underwent vertebroplasty or kyphoplasty in this study were elderly and would have been expected to have reduced lung capacity. Furthermore, the procedures were performed in the prone position, which results in thoracic compression and decreased functional residual capacity which also may have contributed to deoxygenation.5 Additionally, depending on the number of treated vertebrae, fat and bone marrow or cement embolism may have also contributed to decreased arterial PO2.5 Moreover, sedatives may worsen oxygenation and increase right ventricular afterload due to hypercapnia,3 emphasizing the anaesthesiologist’s role in maintaining adequate patient respiration during monitored anaesthesia care.
Overall, patients’ SpO2 was >95%, and there was no statistical difference between the two groups in RR. However, RR values at T2 and T3 were lower in patients receiving remifentanil than in those receiving dexmedetomidine, leading to a greater incidence of respiratory depression and oxygen desaturation. Although remifentanil appears to suppress patient respiration more than dexmedetomidine,11 its depressive effects may be alleviated by increased oxygen delivery or verbal stimuli.
Both MAP and HR were lower with dexmedetomidine than remifentanil, probably due to the sympatholytic action25 and suppression of catecholamine levels by dexmedetomidine.26 Studies that recorded haemodynamic variables following these treatments showed similar findings.23,27,28 Although in this current study, the analgesic effect of dexmedetomidine was lower than that of remifentanil, dexmedetomidine had a relatively greater effect on haemodynamic variables than remifentanil. Hypotension and bradycardia are known haemodynamic effects of dexmedetomidine and are associated with loading dose and infusion time.29 However, in this current study, MAP was maintained over 60 mmHg and the two patients who developed hypotension on dexmedetomidine were readily treated with ephedrine 5–10 mg. These results suggest that careful monitoring of haemodynamic changes is advised when using dexmedetomidine in elderly patients because they are more likely to have comorbidities compared with younger patients.
There was no statistical difference between the groups in investigators’ satisfaction scores or patients’ overall pain experience. Unlike general anaesthesia, most patients under monitored anaesthesia care are conscious and response to painful stimuli, so they tend to move and cause disturbances in situations of insufficient analgesia. However, most patients underwent the procedures with few movements and no disturbance. This might have been due to the additional fentanyl injections in patients receiving dexmedetomidine despite its lesser analgesic properties.
Previous studies that have compared recovery time of remifentanil with that of dexmedetomidine have yielded conflicting results. Ryu et al.7reported that patients who underwent flexible brochoscopy sedated by a bolus i.v. dose of 0.2 µg/kg dexmedetomidine followed by its continuous i.v. infusion 0.4–2 µg/kg/h showed longer recovery times than those on remifentanil. Likewise, Park et al.23 reported that patients undergoing cataract surgery had a longer recovery time after 0.5 µg/kg dexmedetomidine over 10 min followed by i.v. infusion at 0.2 µg/kg/h than those on remifentanil. However, Na et al.21 reported that patients’ sedation scores were well maintained during cataract surgery with dexmedetomidine 0.6 µg/kg/h with no loading dose; in these patients, discharge was not delayed. In the present study, the relatively low loading dose of dexmedetomidine (0.3–0.4 µg/kg) and the low continuous i.v. infusion (0.2–1 µg/kg/h) did not induce profound sedation. In addition, the prolongation of recovery time due to oversedation was not statistically significant between the two study groups.
A limitation of this study was the lack of continuous monitoring of partial pressure of oxygen or carbon dioxide during the procedures. Although pulse oximetry and RR monitoring were convenient methods to assess oxygenation, intra-arterial blood gas analysis or end-tidal CO2 monitoring would have been more sensitive methods to detect respiratory depression and oxygenation. However, serial arterial blood gas analysis would perhaps have been too invasive for these elderly patients undergoing monitored anaesthesia care. In addition, end tidal CO2 monitoring via a nasal cannula or facial mask in the prone position is sometimes inaccurate because of impaired sample collection.
In conclusion, both remifentanil and dexmedetomidine are well tolerated for monitored anaesthesia care in elderly patients undergoing vertebroplasty or kyphoplasty. Compared with remifentanil, dexmedetomidine induced less respiratory depression and produced a lower MAP, and HR. Therefore, dexmedetomidine may be an attractive choice for monitored anaesthesia care during vertebroplasty and kyphoplasty in elderly patients. However, the drug was less effective in inducing analgesia than remifentanil and was associated with more frequent need for intraoperative opioids.
Acknowledgements
We would like to acknowledge the support of medical staff at the Spine Centre (particularly Drs Seok Woo Kim and Yong Chan Kim), and residents at Hallym University Sacred Heart Hospital, for their co-operation during patient recruitment.
Footnotes
This clinical trial is registered at ClinicalTrials.gov. (NCT02476981)
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 1998; 128: 793–800. [DOI] [PubMed] [Google Scholar]
- 2.Cauley JA, Hochberg MC, Lui LY, et al. Long-term risk of incident vertebral fractures. JAMA 2007; 298: 2761–2767. [DOI] [PubMed] [Google Scholar]
- 3.Luginbühl M. Percutaneous vertebroplasty, kyphoplasty and lordoplasty: implication for the anesthesiologist. Curr Opin Anaesthesiol 2008; 21: 504–513. [DOI] [PubMed] [Google Scholar]
- 4.Sen J, Sen B. A comparative study on monitored anesthesia care. Anesth Essays Res 2014; 8: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uemura A, Numaguchi Y, Matsusako M, et al. Effect on partial pressure of oxygen in arterial blood in percutaneous vertebroplasty. AJNR Am J Neuroradiol 2007; 28: 567–569. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KH. Safe sedation and hypnosis using dexmedetomidine for minimally invasive spine surgery in a prone position. Korean J Pain 2014; 27: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu JH, Lee SW, Lee JH, et al. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth 2012; 108: 503–511. [DOI] [PubMed] [Google Scholar]
- 8.Davies L, Mister R, Spence DP, et al. Cardiovascular consequences of fibreoptic bronchoscopy. Eur Respir J 1997; 10: 695–698. [PubMed] [Google Scholar]
- 9.Glass PS, Hardman D, Kamiyama Y, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil. Anesth Analg 1993; 77: 1031–1040. [DOI] [PubMed] [Google Scholar]
- 10.Holas A, Krafft P, Marcovic M, et al. Remifentanil, propofol or both for conscious sedation during eye surgery under regional anaesthesia. Eur J Anaesthesiol 1999; 16: 741–748. [DOI] [PubMed] [Google Scholar]
- 11.Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics: part I. crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 2004; 101: 1066–1076. [DOI] [PubMed] [Google Scholar]
- 12.Miller RD, Cohen NH, Eriksson LI, et al. Miller’s Anesthesia, 8th ed Philadelphia: Elsevier, 2015, pp. 1144–1144. [Google Scholar]
- 13.Patki A, Shelgaonkar VC. A comparison of equisedative infusions of propofol and midazolam for conscious sedation during spinal anesthesia – a prospective randomized study. J Anaesthesiol Clin Pharmacol 2011; 27: 47–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Miller RD, Cohen NH, Eriksson LI, et al. Miller’s Anesthesia, 8th ed Philadelphia: Elsevier, 2015, pp. 2942–2942. [Google Scholar]
- 15.Mohr M, Pillich D, Kirsch M, et al. Percutaneous balloon kyphoplasty with the patient under intravenous analgesia and sedation: a feasibility study. AJNR Am J Neuroradiol 2011; 32: 649–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Puppa A, Andreula C, Frass M. Assisted sedation: a safe and easy method for pain-free percutaneous vertebroplasty. Minerva Anestesiol 2008; 74: 57–62. [PubMed] [Google Scholar]
- 17.Dauri M, Coniglione F, Faria S, et al. Continuous i.v. infusion of remifentanil and intraosseous lidocaine provide better analgesia than intraosseous lidocaine alone in percutaneous vertebroplasty of osteoporotic fractures. Br J Anaesth 2009; 103: 901--902. [DOI] [PubMed]
- 18.Hwang KI, Lee HY, Shim KD, et al. Analgesia-based sedation using remifentanil during percutaneous endoscopic lumbar discectomy. Korean J Anesthesiol 2006; 50: 36--41.
- 19.Candiotti KA, Bergese SD, Bokesch PM, et al. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg 2010; 110: 47–56. [DOI] [PubMed] [Google Scholar]
- 20.Alhashemi JA. Dexmedetomidine vs midazolam for monitored anaesthesia care during cataract surgery. Br J Anaesth 2006; 96: 722–726. [DOI] [PubMed] [Google Scholar]
- 21.Na HS, Song IA, Park HS, et al. Dexmedetomidine is effective for monitored anesthesia care in outpatients undergoing cataract surgery. Korean J Anesthesiol 2011; 61: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: a double-blinded randomized controlled trial. J Anesth 2013; 27: 211–217. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Kwon JY. Remifentanil or dexmedetomidine for monitored anesthesia care during cataract surgery under topical anesthesia. Korean J Anesthesiol 2012; 63: 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angst MS, Ramaswamy B, Davis MF, et al. Comparative analgesic and mental effects of increasing plasma concentration of dexmedetomidine and alfentanil in humans. Anesthesiology 2004; 101: 744–752. [DOI] [PubMed] [Google Scholar]
- 25.Bekker A, Sturaitis M, Bloom M, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg 2008; 107: 1340–1347. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Singh A. Dexmedetomidine induced catecholamine suppression in pheochromocytoma. J Nat Sci Biol Med 2014; 5: 182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özcan AA, Özyurt Y, Saraçoğlu A, et al. Dexmedetomidine versus remifentanil for controlled hypotensive anesthesia in functional endoscopic sinus surgery. Turk J Anesth Reanim 2012; 40: 257–261. [Google Scholar]
- 28.Gümüş F, Şinikoğlu SN, Erkalp K, et al. The analgesic and hemodynamic effects of dexmedetomidine and remifentanil during chest tube removal. Turkish J Thorac Cardiovasc Surg 2013; 21: 966–971. [Google Scholar]
- 29.Miller RD, Cohen NH, Eriksson LI, et al. Miller’s Anesthesia, 8th ed Philadelphia: Elsevier, 2015, pp. 858–858. [Google Scholar]