Abstract
Actinomycosis is a rare disorder caused by an anaerobic gram-positive bacillus (Actinomyces), predominantly by the Actinomyces israelii species. Only 20% of cases show an abdominal manifestation, the appendix and ileocecal valve being the most frequent locations. Definitive diagnosis is based on microbiological cultures, microscopy or macroscopy examination. Nevertheless, histological examination of the percutaneous biopsy and blood microbiological cultures are rarely positives. Preoperative diagnosis is hampered by the lack of specific clinical and imaging manifestations, which often mimic malignancy. The rate of preoperative diagnosis is less than 10%, however, the outcome is excellent, with a low mortality rate. The authors describe the case of a patient who was diagnosed with primary hepatic actinomycosis only by a histological examination of the surgical specimen of left hepatectomy extended to segments V and VIII, for suspected malignant lesion. This case demonstrates the difficulties in diagnosing hepatic actinomycosis.
Keywords: Actinomyces, Granulomatous Disease, Actinomycosis, Liver Diseases
Resumo
A actinomicose é uma entidade clínica rara, causada por uma bactéria anaeróbia gram-positiva (Actinomyces), predominantemente da espécie Actinomyces israelii. Apenas em 20% dos casos apresenta manifestação abdominal, sendo o apêndice e a válvula ileocecal as localizações mais frequentes. Os autores descrevem o caso de um doente em que foi feito o diagnóstico de actinomicose hepática primária apenas pelo exame histológico da peça cirúrgica de hepatectomia esquerda alargada aos segmentos V e VIII, por suspeita de lesão maligna. Este caso demonstra a dificuldade diagnóstica da actinomicose hepática. O diagnóstico pré-operatório é dificultado pela falta de manifestações clínicas e imagiológicas específicas, muitas vezes simulando doença maligna. Para além disso, as culturas e o exame histológico de biópsia percutânea raramente são positivos. A taxa de diagnóstico pré-operatório é inferior a 10%, contudo o prognóstico é bom, apresentando uma taxa de mortalidade de cerca de 7,6%.
Palavras-chave: Actinomyces, Actinomicose, Doença Granulomatosa, Doenças do Fígado
1. Introduction
Actinomycosis is a rare chronic, granulomatous infection caused by gram-positive bacilli of the genus Actinomyces. There are 13 different species, but only 6 are associated with human disease. The most common pathogenic specie in humans is Actinomyces israelli.1 These microorganisms are prominent among the commensal flora of the oropharynx, gastrointestinal tract and female genital tract.2 Actinomycosis is most frequently associated with cervicofacial infection. Only 20% of the cases present abdominal manifestations, the appendix and the ileocecal region being the most common affected sites.3 Liver involvement occurs in 15% of abdominal infections representing 5% of all cases of actinomycosis,4 and it is usually secondary to other intraabdominal infection.5 The insidious course with nonspecific symptoms makes the diagnosis of this condition a challenging.
2. Case report
A 38-year-old man was referred to the Gastroenterology Department for a history of epigastric pain refractory to proton pump inhibitor therapy. He reported a 2 years history of night sweats and weight loss (12% of body weight). The patient denied other dyspeptic complaints, hematemesis or melena and systemic symptoms, including fever, malaise and anorexia. His past medical history included duodenal ulcer treated with proton pump inhibitor, 3 years ago. No history of previous surgeries. He was medicated with omeprazole 20 mg qd on regular basis. The physical examination was normal. Laboratory tests evidenced normocytic/normochromic anemia (Hb 12 g/dl, normal: 14–18), leukocytosis (15.21 × 103/uL, normal: 4–11.5) with neutrophilia (80%, normal: 50–70), elevation of inflammatory parameters (C-reactive protein: 2.46 mg/dl (normal 0–0.3) and erythrocyte sedimentation rate 33 mm (normal 0–15) and mild elevation of alkaline phosphatase 170:U/L (normal: 40–129). The remaining analytical assessment was normal, including tumor markers and human immunodeficiency virus antibody test. Infection by Mycobacterium tuberculosis was excluded. Upper gastrointestinal endoscopy revealed pyloric substenosis, yet allowing the passage of the endoscope to the duodenum, probably resulting from previous duodenal ulcer. Biopsies were performed in normal mucosa, which revealed chronic gastritis due to Helicobacter pylori. Gastroduodenal transit was normal. An abdominal ultrasound identified in segment IV of the liver a pseudonodular lesion, hypoechoic, heterogeneous with partially undefined limits with 45 × 33 mm (Fig. 1). A contrast-enhanced computed tomography (CT) scan showed the same lesion, presenting low enhanced in the arterial phase, with progressive enhanced in the late phase (Fig. 2). To further characterize the lesion a magnetic resonance imaging (MRI) was performed revealing the same nodular lesion with lobulated and undefined limits of 4 cm in its greatest diameter, hypointense signal on T1 and hyperintense signal on T2 (Fig. 3). These images were suggestive of cholangiocarcinoma but secondary deposit cannot be excluded. A CT-guided liver biopsy revealed an inflammatory pseudotumor/inflammatory myofibroblast tumor (Fig. 4). The patient was then referred to referral Hospital in hepatobiliary surgery where he underwent left hepatectomy extended to segments V and VIII. The histological examination of surgical tissue demonstrated abscesses due to Actinomyces (Fig. 5). The patient was treated with doxycycline for three months. Eighteen months after the surgery, he is completely asymptomatic.
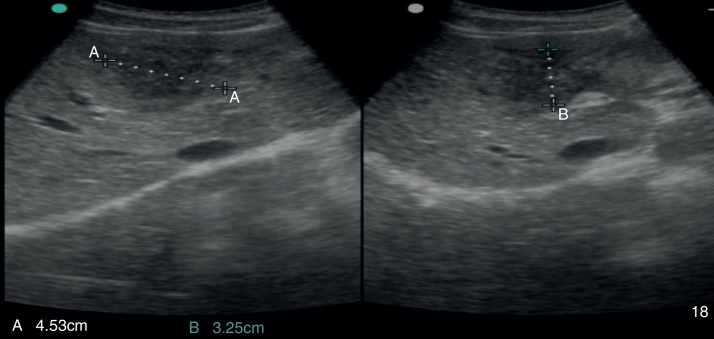
Figure 1.
Abdominal ultrasound: pseudonodular lesion, hypoechoic, heterogeneous and partially undefined in segment IV of the liver.
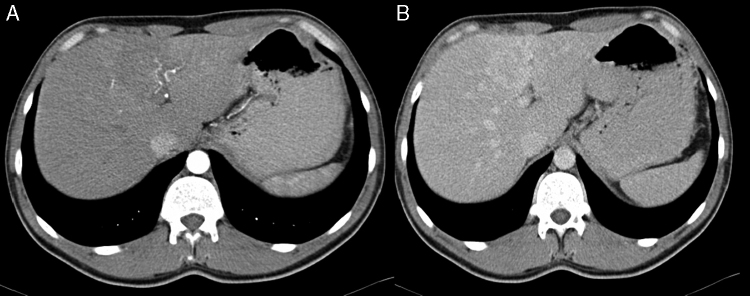
Figure 2.
Contrast-enhanced computed tomography scan: low enhanced lesion in the arterial phase (A), with progressive enhanced in the late phase (B).
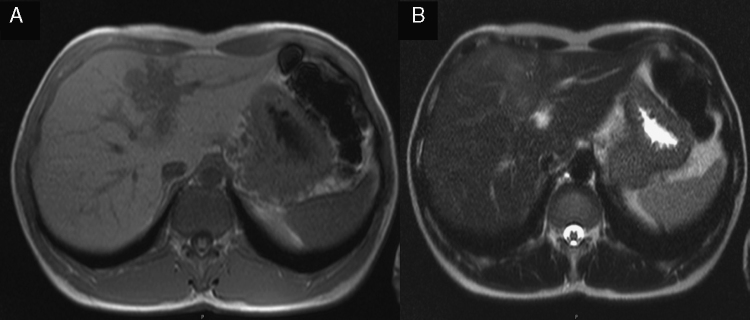
Figure 3.
MRI: nodular lesion with lobulated and undefined limits, hypointense signal on T1 (A) and hyperintense signal on T2 (B).
Figure 4.
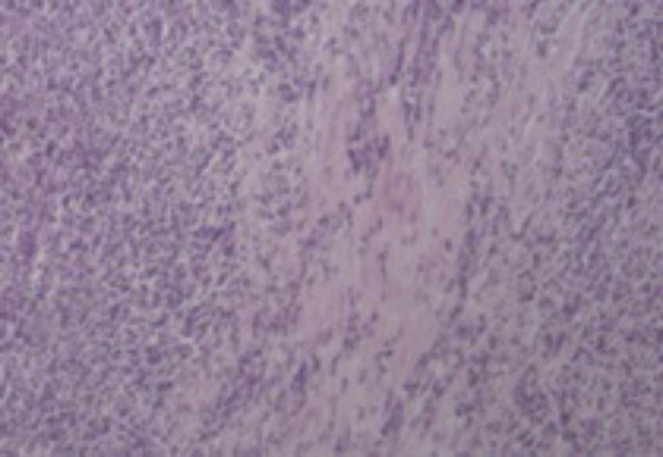
Histology of liver fragment: inflammatory pseudotumor (hematoxylin and eosin 100×).
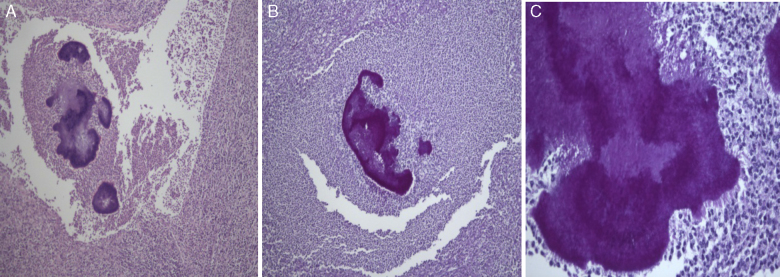
Figure 5.
Histological examination allowed the observation of abcesses in several areas. A central zone with neutrophil granulocytes and necrotic tissue is surrounded by a band of fibrous connective tissue that constitutes the abscess capsule, showing the plugs of Actinomyces (A – hematoxylin and eosin, magnification 5×). At higher magnification, filamentous structures corresponding to aggregates of Actinomyces were identified (B and C – PAS 5× and 40× magnification respectively).
3. Discussion
Actinomycosis is a rare cause of intra-abdominal infection. The main risk factors associated with this condition are loss of integrity of the gastrointestinal mucosa, previous abdominal surgery, intra-abdominal infection, gastrointestinal foreign body and imunossupression.5, 6 Liver involvement occurs by hematogenous spread via the portal vein from either a mucosal injury or infection.7 Sharma et al. conducted a study which found that about 17.5% of cases of abdominal actinomycosis were associated with previous abdominal or pelvic procedures performed within the last year.8 In this case, the time between diagnosis and the initiation of duodenal ulcer was one year. Despite the known major risk factors, hepatic actinomycosis is cryptogenic in 80% of cases and4, 6 polymicrobial in 33%.9 As in our case, there is male predominance (70–74%) in immunocompetent adults, aged between 30 and 50 years.4, 5, 8 Due to an insidious course, it takes several weeks to months9, 10 of symptoms to make the diagnosis with a range of 4 days to 18 months until presentation.10 In addition, both clinical and laboratory manifestations are nonspecific. The most frequent symptoms are fever (83.3%), abdominal pain (74.5%) and weight loss (50.9%).11, 12 Analytically presents with leukocytosis (75%), elevated alkaline phosphatase (83.3%) and erythrocyte sedimentation rate,6, 8, 13 as in this case. Some studies have demonstrated an association with elevated levels of CA 19.9, but with lower values than those described for malignant conditions.14
In imaging studies, particularly in ultrasound, CT and MRI, hepatic actinomycosis presents with a single hypodense lesion in 66% of cases. In the remaining 33% it may present as multiple hepatic abscesses.2, 5, 10, 15, 16 Thus, it is necessary to distinguish it from both malignant lesions (primary or secondary tumor deposits) and benign lesions (cystic lesions, pyogenic abscesses, amebiasis or echinococcosis),5 taking into account the characteristics of the lesion. In about 44.7% of the cases mimics a malignant lesion5, 9 and the right lobe is the most affected (56.5%).2, 5, 16 MRI is characterized by hypointense signal on T1-and hyperintense signal on T2.17 In our case, the lesion presents features of malignancy, including heterogeneous content and undefined limits which associated with MRI study requires differential diagnosis with cholangiocarcinoma, despite the young age of the patient. The diagnosis of hepatic abscess was also considered because the patient had fever for a long period, however the imagiologic studies did not support this hypothesis.
Definitive diagnosis is based on blood cultures,6, 7, 9 microscopy (percutaneous biopsy, laparoscopic or surgical tissue specimens) and macroscopic examination, which reveal basophilic filament aggregates and yellow “sulfur granules”.4, 6, 9 In our patient histological diagnosis of inflammatory pseudotumor probably corresponds to a fragment of the capsule of abscess, which is constituted by fibrous connective tissue with focal inflammatory infiltrate.
The rate of preoperative diagnosis is less than 10%,4, 18 despite advanced imaging techniques. In addition, blood cultures are positive in only 15.4% of the cases4, 8 and percutaneous biopsy diagnosis is not always conclusive.
Therapeutic options available are antibiotics, surgical resection and percutaneous drainage either alone or in combination.6, 9 There are no current guidelines available, particularly in what pertains the indication for surgery. In the literature, are not described guidelines for therapy, particularly for surgery indication.9 Early initiation of antibiotics is usually effective.8 Surgery should be reserved for the cases in which percutaneous drainage is not possible and for larger lesions in which there is a greater amount of necrotic tissue and therefore lower penetrance of antibiotic.10
The recommended antibiotics are a penicillin derivative, tetracycline or clindamycin.6 Duration of treatment is variable with courses lasting from 3 to 6 months.5, 10 Despite the usual delay in making the diagnosis and consequently in initiating therapy, the outcome is excellent, with a mortality rate of 7.6%.2, 5, 9, 16, 19
In conclusion, primary hepatic actinomycosis is a rare disease and difficult to diagnosis, since its clinical and analytical presentation is nonspecific. The differential diagnosis is extensive encompassing both benign and malignant conditions. However, it has a good prognosis after appropriate treatment.
Ethical disclosures
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent
The authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Brook I. actinomycosis: diagnosis and management. South Med J. 2008;101:1019–1023. doi: 10.1097/SMJ.0b013e3181864c1f. [DOI] [PubMed] [Google Scholar]
- 2.Wong J.J., Kinney T.B., Miller F.J., Rivera S.G. Hepatica actinomycotic abscesses: diagnosis and management. Am J Roentgenol. 2006;186:174–176. doi: 10.2214/AJR.04.1691. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi Y., Isowa N., Nakazaki H., Takeda K., Tokuyasu H., Saitoh Y. Acute cardiac tamponade caused by extension of multiple hepatic actinomycotic abscesses. Intern Med. 2012;51:305–309. doi: 10.2169/internalmedicine.51.6422. [DOI] [PubMed] [Google Scholar]
- 4.Lall T., Shehab T.M., Valenstein P. Isolated heoatic actinomycosis: a case report. J Med Case Rep. 2010;4:45. doi: 10.1186/1752-1947-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L.W., Chang L.C., Shie S.S., Chien R.N. Solitary actinomycotic abscesses of liver: report of two cases. Int J Clin Pract. 2006;60:104–107. doi: 10.1111/j.1368-5031.2005.00691.x. [DOI] [PubMed] [Google Scholar]
- 6.Christodoulou N., Papadokis I., Velegrakis M. Actinomycotic liver abscesses. Case report and review of the literature. Chir Ital. 2004;56:141–146. [PubMed] [Google Scholar]
- 7.Wayne M.G., Naran R., Chauhdry A., Steele J. Hepatic actinomycosis mimicking an isolated tumor recurrence. World J Surg Oncol. 2011;9:70. doi: 10.1186/1477-7819-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma M., Briski L.E., Khatib R. Hepatic actinomycosis: an overview of salient features and outcome of therapy. Scand J Infect Dis. 2002;34:386–391. doi: 10.1080/00365540110080304. [DOI] [PubMed] [Google Scholar]
- 9.Kanellopoulou T., Alexopoulou A., Tanouli M.I., Tiniakos D., Giannopoulos D., Koskinas J. Primary hepatic actinomycosis. Am J Med Sci. 2010;339:362–365. doi: 10.1097/MAJ.0b013e3181cbf47c. [DOI] [PubMed] [Google Scholar]
- 10.Lai A.T., Lam C.M., Ng K.K., Yeung C., Ho W.L., Poon L.T. Hepatic actinomycosis presenting as a liver tumour: case report and a literature review. Asian J Surg. 2004;27:345–347. doi: 10.1016/S1015-9584(09)60066-X. [DOI] [PubMed] [Google Scholar]
- 11.Islam T., Athar M.N., Athar M.K., Usman M.H., Misbah B. Hepatic actinomycosis with infiltration of the diaphragm and right lung: a case report. Can Respir J. 2005;12:336–337. doi: 10.1155/2005/804093. [DOI] [PubMed] [Google Scholar]
- 12.Kanellopoulou T., Alexopoulou A., Tiniakos D., Koskinas J., Archimandritis A.J. Primary hepatic actinomycosis mimicking metastatic liver tumour. J Clin Gastroenterol. 2010;44:458–459. doi: 10.1097/MCG.0b013e3181d2ef30. [DOI] [PubMed] [Google Scholar]
- 13.Min K.W., Paik S.S., Han H.H., Jang K.S. Hepatobiliary and pancreatic: hepatic actinomycosis. J Gastroenterol Hepatol. 2012;27:844. doi: 10.1111/j.1440-1746.2012.07087.x. [DOI] [PubMed] [Google Scholar]
- 14.Soardo G., Bason L., Intini S., Avellini C., Sechi L.A. Elevated serum CA 19-9 in hepatic actinomycosis. Scand J Gastroenterol. 2005;40:1372–1373. doi: 10.1080/00365520510024232. [DOI] [PubMed] [Google Scholar]
- 15.Ha K.K., Lee H.J., Kim H., Ro H.J., Park Y.H., Cha S.J. Abdominal actinomycosis: CT findings in 10 patients. AJR. 1993;161:791–794. doi: 10.2214/ajr.161.4.8372760. [DOI] [PubMed] [Google Scholar]
- 16.Felekouras E., Menenakos C., Griniatsos J., Deladetsima I., Kalaxanisi N., Nikiteas N. Liver ressection in cases of isolated hepatic actinomycosis: case report and review of the literatura. Scand J Infect Dis. 2004;36:535–538. doi: 10.1080/00365540410020866-1. [DOI] [PubMed] [Google Scholar]
- 17.KimHS, Park N.H., Park K.A., Kang S.B. A case of pelvic actinomycosis with hepatic actinomycotic pseudotumour. Gynecol Obstet Invest. 2007;64:95–99. doi: 10.1159/000100058. [DOI] [PubMed] [Google Scholar]
- 18.Brow J.R. human actinomycosis: CT features. J Comput Assist Tomogr. 1986;10:335–337. doi: 10.1097/00004728-198603000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Lakshmana Kumar Y.C., Javherani R., Malini A., Prasad S.R. Primary hepatic actinomycosis. Trans R Sos Trop Med Hyg. 2005;99:868–870. doi: 10.1016/j.trstmh.2005.06.004. [DOI] [PubMed] [Google Scholar]