Abstract
Introduction
The management of esophageal strictures has evolved from surgical treatment to the endoscopic dilation and, more recently, esophageal stenting.
Clinical case
We describe a case of a two-year-old boy with a double stenosis of the esophagus resulting from accidental ingestion of strong alkaline liquid. After several unsuccessful endoscopic dilations for three years and even topical mitomicin, it was decided to place a dynamic stent developed by the Digestive Surgery and Endoscopic Unit of the Bambino Gesù Hospital, Rome. The stent is a custom silicon device built coaxially on a nasogastric tube that is inserted after stricture dilations, by endoscopic guidance, and then fixed outside the nose. The device was removed after seven weeks with good clinical outcome (no dysphagia more than a year of follow-up).
Conclusion
This case confirms that the dynamic stent is a simple device that may avoid aggressive surgical substitution in cases of refractory strictures.
Keywords: Dilatation; Endoscopy, Gastrointestinal; Esophageal Stenosis
Resumo
Introdução
O tratamento das estenoses esofágicas tem evoluído desde a correção cirúrgica, à dilatação endoscópica e, mais recentemente, à colocação de stents esofágicos.
Caso clínico
Descrevemos o caso de um doente que aos dois anos ingeriu acidentalmente um cáustico e desenvolveu duas estenoses esofágicas recidivantes. Após numerosas dilatações endoscópicas e aplicação tópica de mitomicina, ao fim de três anos, foi decidido colocar um stent dinâmico, desenvolvido pela Unidade de Cirurgia e Endoscopia do Hospital Bambino Gesù, Roma. O dispositivo consiste numa sonda nasogástrica com uma área de maior calibre (stent) que foi posicionada por via endoscópica na zona das estenoses e fixada por via nasal. O dispositivo foi retirado passadas sete semanas com melhoria clínica sustentada (ausência de disfagia mais de um ano após).
Conclusão
Este caso demonstra que o “stent” dinâmico é tecnicamente simples e permite evitar uma solução cirúrgica mutilante em casos de estenose esofágica de difícil controlo.
Palavras-chave: Dilatação, Endoscopia Gastrointestinal, Estenose Esofágica
1. Introduction
Ingestion of corrosive substances remains an important public health problem mostly in developing countries. Children represent 80% of worldwide cases, primarily due to accidental ingestion.1 The caustic substances may cause severe esophageal stenosis. The management of esophageal strictures has evolved and development of endoscopic techniques has led to a more conservative management, rather than more aggressive surgical substitution.
Endoscopic dilation (with balloon or bougienage) is used worldwide, although approximately one-third of patients develop recurrent strictures after dilation (defined as an inability to maintain a satisfactory luminal diameter for four weeks) and others have refractory strictures requiring multiple dilations (defined as inability to achieve a satisfactory luminal diameter over five sessions at two week interval).2, 3 On the other hand, endoscopic dilations have a significant risk of perforation (15–20%) and development of new strictures due to elevated pressure on the esophageal wall.4, 5, 6
In the last decade, esophageal stenting has become popular. Several authors have described their experience with different types of stents, although adapted pediatric devices are still scarce and the majority cause a centrifugal force on the esophageal wall while allowing the passage of food inside the stent. This type of stents may cause stretching and mucosal hypertrophy, with a non-negligible risk of migration, perforation and occlusion.7, 8, 9, 10, 11, 12, 13
Since 1988, the team of the Digestive Surgery and Endoscopy Unit of Bambino Gesù Hospital – Rome have developed a device named “dynamic stent” (DS), which allows food passage between the stent and the esophageal wall, improving esophageal motility and, thus, preventing stricture recurrence.13, 14 The DS is a custom silicon device built coaxially on a nasogastric tube to reach the desirable length and diameter, in order to create a larger area tailored to be placed along the stricture. The published experience of two decades of DS use confirms the safety and efficacy of the device and may even be considered as first option in the treatment of caustic esophageal stenosis.14, 15
We describe a case of a patient with a refractory double caustic stenosis of the esophagus that was treated with a DS in the Unit of Pediatric Gastroenterology, Hospital Center of São João, Porto. Usually the caustic lesions are more severe at the site of functional narrowing of the esophagus (level of crossing the major vessels or just above cardia) and tend to be single with variable length, but on occasions there may occur at more than one site, as in the present case, causing additional therapeutic challenge.
2. Clinical case
A previously healthy two-year-old boy had accidental ingestion of strong alkaline liquid that led to a double stenosis (5 cm apart, at 15 and 20 cm from the mouth) of the esophagus. Each of the stenotic segments was short (less than 2 cm), but caused significant limited visibility and technical difficulty in addressing both for dilation. He was initially treated with endoscopic dilations with (Savary-Gilliard) bougies with recurrence of dysphagia two months later and even after subsequent other bougies dilation. On some occasions, when there was enough visibility of the esophageal lumen through both stenotic segments, TTS balloons of 10 mm were used, but once there was a perforation of the esophageal wall, successfully managed with conservative measures.
Several months after perforation, dysphagia recurred with grade 1 (dysphagia was graded using a previously described scale as follows: grade 0 = able to eat normal diet/no dysphagia; grade 1 = able to swallow some solid foods; grade 2 = able to swallow only semi-solid foods; grade 3 = able to swallow liquids only; grade 4 = total dysphagia),16 that evolved to grade 3 twelve months later. Endoscopic dilations (initially with Savary-Gilliard bougies and after with TTS balloons) were resumed at two-week intervals. Topical mitomicin C was applied on four consecutive sessions initially at 0.1 mg/mL and then raised to 1 mg/mL. However, the stenosis remained refractory and multiple dilation sessions (more than 30, maximum width 12 mm) for 18 months.
Three years after the initial accident a DS was considered. Despite significant dysphagia (grade 3), the boy had adequate growth and weight gain mostly due to adjustment of food consistency to match his tolerance. Ethical clearance was obtained and parents provided informed consent. The exact location and extent of the stenosis were previously assessed radiologically with contrast (Fig. 1) and radio-opaque skin markers were applied. The two stenosis were then dilated until an adequate caliber was obtained (with Savary-Gilliard bougies of 7 and 9 mm followed by balloon of 12 mm) that allowed the placement of the stent (Fig. 2). The DS was then inserted through the mouth and the correct position was radiologically confirmed with the largest diameter at the level of the strictures using the skin markers as reference. Fig. 3 shows the actual device and scheme of the location of the device after insertion. The nasogastric tube of the stent was then passed with a backward movement through the nasopharynx and nose, and fixed externally with adhesive tape to avoid distal displacement (Fig. 4). On the following day, the patient started being fed orally with soft diet that gradually progressed to normal food. Treatment with proton pump inhibitor (omeprazole 20 mg id) and corticosteroid (prednisone 1 mg/kg/day once daily) was maintained during three days, according to the published protocol.14, 15
Figure 1.
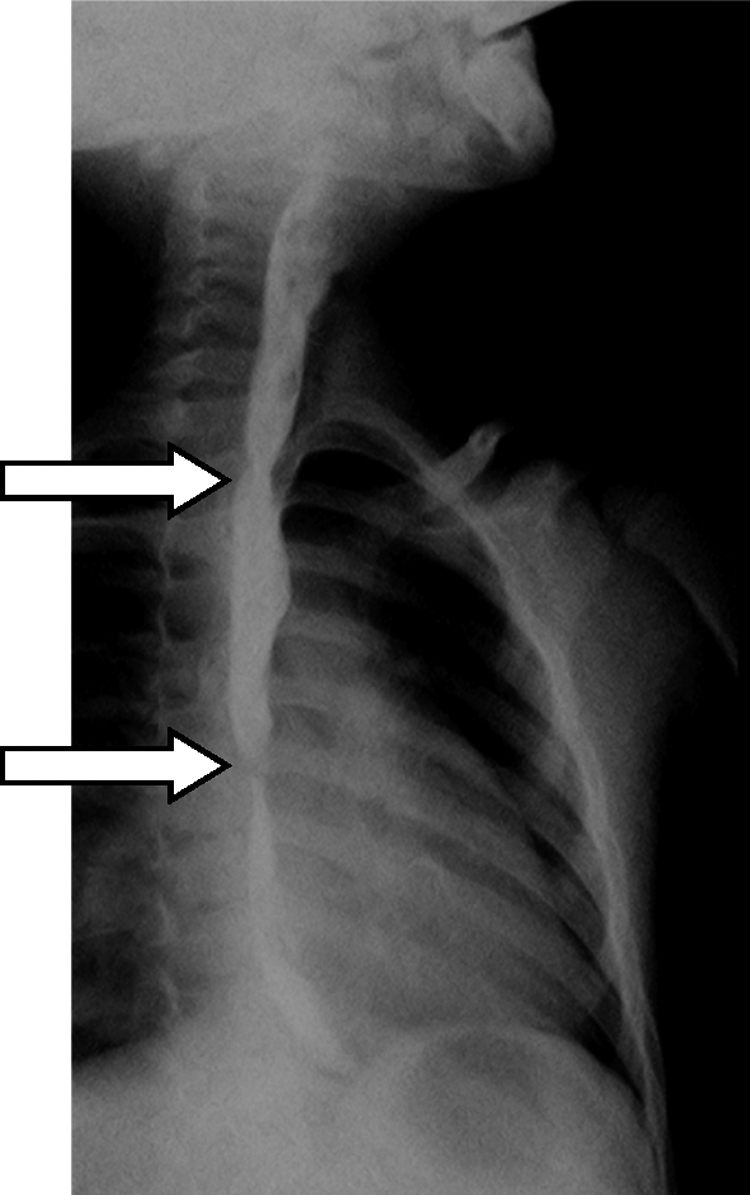
Esophagogram showing the exact location and extent of the stenosis (arrows).
Figure 2.
Balloon dilation of the stenosis before stent placement.
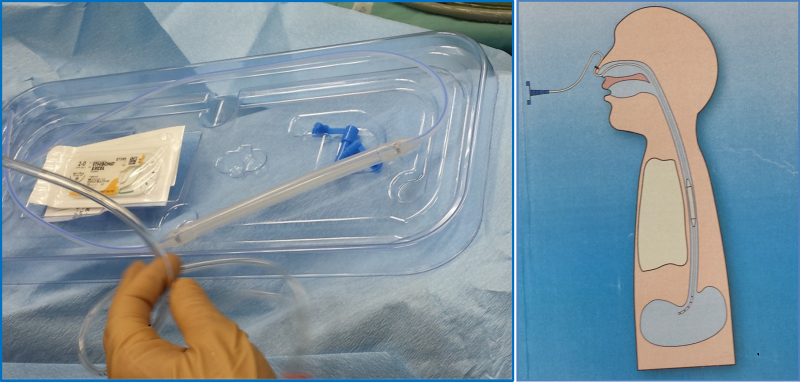
Figure 3.
Dynamic stent – preview of the commercial version to be available in the market after full approval of the regulatory agency (left); schematic illustration of the intra-esophageal placement of the device (right).
Figure 4.
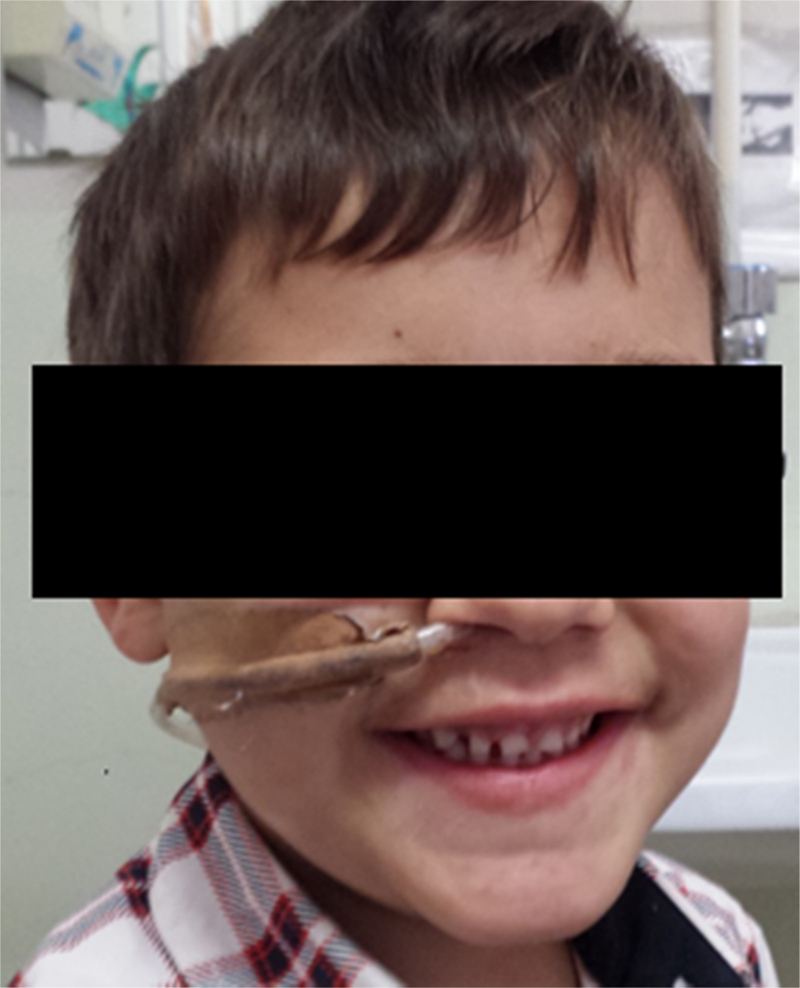
Patient with the dynamic stent illustrating the exterior fixation.
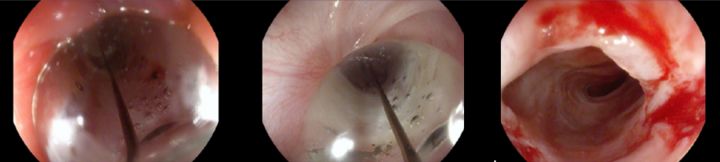
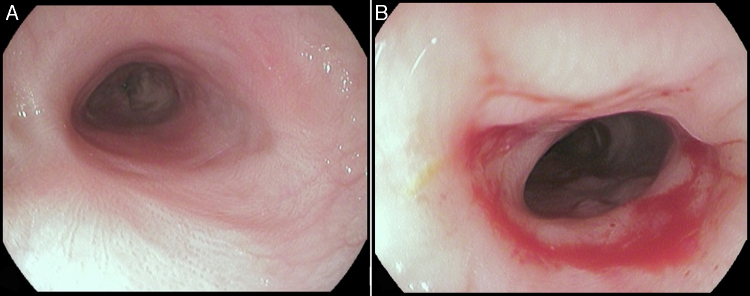
The stent was removed after seven weeks under general anesthesia with endoscopic control of the esophageal wall. At that time, the patient was eating solids without dysphagia (grade 0). The upper endoscopy performed after the stent removal showed an adequate esophageal lumen without any ulceration (Fig. 5). The whole process and device were well tolerated without adverse events.
Figure 5.
Endoscopic aspect of the esophageal mucosa after stent removal (A – distal stenosis; B – proximal stenosis).
More than a year later, the patient remains asymptomatic on normal oral feeding (no dysphagia – grade 0).
3. Discussion
The stenting use for esophageal strictures has evolved rapidly over the past 10 years and it is widely performed in adults, especially for treatment of malignant strictures.17 More recently, temporary stent placement has increasingly been used for refractory benign esophageal strictures.2
Self-expandable metal stents (SEMS) were the first devices used,18 but they were associated with high complication rate, mostly due to hyperplastic tissue ingrowth (as high as 80%) and migration rate of 26.4%.17, 19 To overcome the problem, fully covered SEMS seem preferable, but currently data on their use in refractory benign esophageal strictures is still limited.20, 21, 22, 23
Self-expandable plastic stents (SEPS) have been proposed as an alternative to SEMS. Two recent reviews, found a clinical success rate of only 45–52% with SEPS use for the treatment of benign esophageal strictures in 172 and 130 patients, respectively, with a significant stent migration rate of 24–31%.24, 25 Although effective, SEPS design needs further improvement to reduce the risk of migration.
An alternative treatment option that has more recently been introduced is the biodegradable stent. Only case reports and small number of cohort studies on the use of biodegrable stents have been published and the majority included few patients.26, 27, 28 The largest studies with 20–30 patients with refractory benign esophageal strictures showed a recurrence rate of dysphagia of 50–75% at six months follow-up with need of repeated stenting procedure.29, 30, 31 That recurrence rate might be explained by the relatively low radial force and degradable nature of these stents. In 2011, a nonrandomized, head-to-head comparison between biodegradable stents and SEPS found similar long term relief of dysphagia (33% and 31%, respectively) in patients with refractory benign esophageal strictures.32
The first largest study of stenting use in pediatric population was published in 1996, included 69 children with corrosive esophageal strictures and showed a significant difference between stent use and traditional therapy (68% versus 83% healing rate). The stents were left in place for one year with no serious complications.33
Despite this promising results, until now the number of studies published about stenting use in pediatric population is still scarce, the majority also include adults and the specific use in corrosive strictures are limited to cohort series that include benign esophageal strictures of other etiologies. Manfredi et al. treated 24 children with anastomotic esophageal stenosis after esophageal atresia repair and the clinical success was only 39% at 30 days and 26% at 90 days, with a high stricture recurrence after stent removal. The results were independent on the type of stent used (they included Polyflex airway stents, AERO fully covered tracheobronchial stents and ALIMAXX-ES fully covered esophageal stents).34 Recently (2015), Lange et al. published their experience with fully covered SEMS use in children with benign esophageal strictures of different etiologies (but no one with corrosive strictures). Between 2006 and 2014, 11 children were treated with a SEMS (biliary, bronchial and colonic commercialized stents). At follow-up 55% were successfully treated without further intervention, but 36% needed restenting at a maximum of four times and 27% did not improved, requiring surgery. They reported only two stent migration.35 Zhang et al. used a newly designed fully covered SEMS with intermittent connectors in order to reduce stent migration and they were custom-made for individual patients. Although the results were disappointing: among the five patients with postoperative esophageal restenosis, ulcerative stricture was observed in two patients and stent migration occurred in three of them.36 The first report of the use of a biodegradable stent in a child was made by Vandenplas et al. in 2009, but after four months symptom free, the patient developed a severe distal esophageal stenosis.37 More recently (2013), a retrospective case series of seven patients with caustic esophageal strictures included two children (5 and 14 years) that were treated with a biodegradable stent. Both of them required multiple dilations post-stent placement (10–12 procedures) due to recurrent dysphagia.28
As esophageal stenting results are not optimal and there is no specially pediatric designed esophageal stents commercially available, other type of custom-made devices has been used. Woynarowski et al. developed a specific double lumen, varying diameter, perforated, esophageal closure protection tube built coaxially over a thin naso-gastric tube. This device was used in two patients with refractory esophageal stenosis after caustic injury. However the good clinical outcome, a long-term use of a nasogastric tube (5–9 months) was required and the results after removing the device in one case were not reported.38, 39
Altogether the reported use of physical force stenting in pediatric patients is still not optimal. A different approach of inserting a non-pressing device through the stenosis and intermittent “dilation” being performed by the swallowed food seems an attractive idea that reduces the sheer continuous pressure of the device and the risk of migration. In this regard, the DS appears to be the pediatric device that included larger number of patients. The published experience in 2011 included 80 children aged between 3 months and 10 years, the majority (55) with caustic esophageal stenosis. The strictures were successfully treated in 88.6% of cases and 50% required only one stenting procedure.14 The complications described were essentially related to partial displacement of the stent in 14.7% and the stent migration into the stomach in only two cases that were easily recovered endoscopically.14 These complication rates are largely inferior to the described for conventional stents with a migration rate of 24–31% with need of surgery for stent retrieval in a non-negligible number of cases.19, 24, 25, 34, 40 The median time of nasogastric tube placement was shorter (39 days with a maximum of 65 days) compared with other devices and, even if the nasogastric tube has a strong psychological impact, it was well tolerated with good compliance by children and parents.14 In 2013, this group published their experience with DS use in postoperative stenosis in 26 children with esophageal atresia and the success rate was 80.7% with a mean follow-up of 5.4 years.15
In conclusion, at present the agreement for treatment of esophageal stenosis in pediatric patients is low and the reasons are varied. These include small series of cases that have been analyzed and the scarcity of dedicated pediatric devices.1, 14, 34, 35, 36 In the future, prospective, multicenter studies will be required to optimize indications and protocols for esophageal stent placement in pediatric patients after ingestion of corrosive substances.
We wish to report the DS use as a valid option in the treatment of recurrent esophageal stenosis as a mean to avoid surgery, undoubtedly much more aggressive. This device represents a new concept of functional dilation and deserves consideration in all cases of recurrent esophageal stenosis after the usual initial dilating procedures. Despite having been described for some years, the publications of use of this type of device is still limited to their developers, derived from the fact that they have been in-house custom made, but a commercial version will be available soon in the market.
Ethical disclosures
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data
The authors declare that no patient data appear in this article.
Right to privacy and informed consent
The authors declare that no patient data appear in this article.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgement
To Dall’Oglio Luigi of the Digestive Surgery and Endoscopy Unit of Gesù Hospital – Rome, for the authorization and manufacture of the device used in our patient.
References
- 1.Contini S., Scarpignato C. Caustic injury of the upper gastrointestinal tract: a comprehensive review. World J Gastroenterol. 2013;19:3918–3930. doi: 10.3748/wjg.v19.i25.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Boeckel P.G., Siersema P.D. Refractory esophageal strictures: what to do when dilation fails. Curr Treat Options Gastroenterol. 2015;13:47–58. doi: 10.1007/s11938-014-0043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochman M.L., McClave S.A., Boyce H.W. The refractory and the recurrent esophageal stricture: a definition. Gastrointest Endosc. 2005;62:474–475. doi: 10.1016/j.gie.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 4.Tiryaki T., Livanelioglu Z., Atayurt H. Early bouginage for relief of stricture formation following caustic esophageal burns. Pediatr Surg Int. 2005;21:78–80. doi: 10.1007/s00383-004-1331-3. [DOI] [PubMed] [Google Scholar]
- 5.De Peppo F., Zaccara A., Dall’Oglio L., Abriola G.F., Ponticelli A., Marchetti P. Stenting for caustic strictures: esophageal replacement replaced. J Pediatr Surg. 1998;33:54–57. doi: 10.1016/s0022-3468(98)90361-x. [DOI] [PubMed] [Google Scholar]
- 6.Baskin D., Urganci N., Abbasoglu L., Alkim C., Yalcin M., Karadag C. A standardized protocol for the acute management of corrosive ingestion in children. Pediatr Surg Int. 2004;20:824–828. doi: 10.1007/s00383-004-1294-4. [DOI] [PubMed] [Google Scholar]
- 7.Broto J., Asensio M., Vernet J.M. Results of a new technique in the treatment of severe esophageal stenosis in children: poliflex stents. J Pediatr Gastroenterol Nutr. 2003;37:203–206. doi: 10.1097/00005176-200308000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Berkovits R.N., Bos C.E., Wijburg F.A., Holzki J. Caustic injury of the oesophagus. Sixteen years experience, and introduction of a new model oesophageal stent. J Laryngol Otol. 1996;110:1041–1045. doi: 10.1017/s0022215100135716. [DOI] [PubMed] [Google Scholar]
- 9.Broto J., Asensio M., Marhuenda C., Vernet J.M., Acosta D., BoixOchoa J. Intraesophageal stent in the prevention of stenosis caused by caustic ingestion. Cir Pediatr. 1999;12:107–109. [PubMed] [Google Scholar]
- 10.Best C., Sudel B., Foker J.E., Krosch T.C., Dietz C., Khan K.M. Esophageal stenting in children: indications, application, effectiveness and complications. Gastrointest Endosc. 2009;70:1248–1538. doi: 10.1016/j.gie.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Atabek C., Surer I., Demirbag S., Caliskan B., Ozturk H., Cetinkursun S. Increasing tendency in caustic esophageal burns and long-term polytetrafluorethylene stenting in severe cases: 10 years experience. J Pediatr Surg. 2007;42:636–640. doi: 10.1016/j.jpedsurg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Vanderplas Y., Hauser B., Devreker T., Urbain D., Reynaert H. A biodegradable esophageal stent in the treatment of corrosive esophageal stenosis in a child. J Pediatr Gastroenterol Nutr. 2009;49:254–257. doi: 10.1097/MPG.0b013e31819de871. [DOI] [PubMed] [Google Scholar]
- 13.Kramer R.E., Quiros J.A. Esophageal stents for severe strictures in young children: experience, benefits, and risk. Curr Gastroenterol Rep. 2010;12:203–210. doi: 10.1007/s11894-010-0105-4. [DOI] [PubMed] [Google Scholar]
- 14.Foschia F., De Angelis P., Torroni F., Romeo E., Caldaro T., Abriola G.F. Custom dynamic stent for esophageal strictures in children. J Pediatr Surg. 2011;46:848–853. doi: 10.1016/j.jpedsurg.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Caldaro T., Torroni F., De Angelis P., Abriola G.F., Foschia F., Rea F. Dynamic esophageal stents. Dis Esophagus. 2013;26:388–391. doi: 10.1111/dote.12048. [DOI] [PubMed] [Google Scholar]
- 16.Knyrim K., Wagner H., Bethge N., Keymling M., Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329:1302–1307. doi: 10.1056/NEJM199310283291803. [DOI] [PubMed] [Google Scholar]
- 17.Hirdes M.M., Vleggaar F.P., Siersema P.D. Stent placement for esophageal strictures: an update. Expert Rev Med Devices. 2011;8:733–755. doi: 10.1586/erd.11.44. [DOI] [PubMed] [Google Scholar]
- 18.Siersema P.D. Stenting for benign esophageal strictures. Endoscopy. 2009;41:363–373. doi: 10.1055/s-0029-1214532. [DOI] [PubMed] [Google Scholar]
- 19.Thomas T., Abrams K.R., Subramanian V., Mannath J., Ragunath K. Esophageal stents for benign refractory strictures: a meta-analysis. Endoscopy. 2011;43:386–393. doi: 10.1055/s-0030-1256331. [DOI] [PubMed] [Google Scholar]
- 20.Eloubeidi M.A., Lopes T.L. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol. 2009;104:1374–1381. doi: 10.1038/ajg.2009.133. [DOI] [PubMed] [Google Scholar]
- 21.Bakken J.C., Wong Kee Song L.M., de Groen P.C., Baron T.H. Use of a fully covered self-expandable metal stent for the treatment of benign esophageal diseases. Gastrointest Endosc. 2010;72:712–720. doi: 10.1016/j.gie.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Eloubeidi M.A., Talreja J.P., Lopes T.L., Al-Awabdy B.S., Shami V.M., Kahaleh M. Success and complications associated with placement of fully covered removable self-expandable metal stents for benign esophageal diseases (with videos) Gastrointest Endosc. 2011;73:673–681. doi: 10.1016/j.gie.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Hirdes M.M., Siersema P.D., Vleggaar F.P. A new fully covered metal stent for the treatment of benign and malignant dysphagia: a prospective follow-up study. Gastrointest Endosc. 2012;75:712–718. doi: 10.1016/j.gie.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 24.Repici A., Hassan C., Sharma P., Conio M., Siersema P. Systematic review: the role of self-expanding plastic stents for benign oesophageal strictures. Aliment Pharmacol Ther. 2010;31:1268–1275. doi: 10.1111/j.1365-2036.2010.04301.x. [DOI] [PubMed] [Google Scholar]
- 25.Ham Y.H., Kim G.H. Plastic and biodegradable stents for complex and refractory benign esophageal strictures. Clin Endosc. 2014;47:295–300. doi: 10.5946/ce.2014.47.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canhoto M., Barbeiro S., Silva F., Arroja B., Gonçalves C., Cotrim I. Benign refractory esophageal stenosis treated with biodegradable stent: 18 months follow-up. GE J Port Gastrenterol. 2013;20:229–231. [Google Scholar]
- 27.Basha J., Appasani S., Vaiphei K., Gupta V., Singh K., Kochhar R. Biodegradable stents: truly biodegradable with good tissue harmony. Endoscopy. 2013;45:E116–E117. doi: 10.1055/s-0032-1326111. [DOI] [PubMed] [Google Scholar]
- 28.Karakan T., Utku O.G., Dorukoz O., Sen I., Colak B., Erdal H. Biodegradable stents for caustic esophageal strictures: a new therapeutic approach. Dis Esophagus. 2013;26:319–322. doi: 10.1111/j.1442-2050.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 29.Repici A., Vleggaar F.P., Hassan C., van Boeckel P.G., Romeo F., Pagano N. Efficacy and safety of biodegradable stents for refractory benign esophageal strictures: the BEST (Biodegradable Esophageal Stent) study. Gastrointest Endosc. 2010;72:927–934. doi: 10.1016/j.gie.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Ibrahim M., Vandermeeren A., van Mael V., Deprez P. Belgian multicenter study experience with biodegradable ella-stent in benign strictures of the digestive tract. Endoscopy. 2010 [Google Scholar]
- 31.Hirdes M.M., Siersema P.D., van Boeckel P.G., Vleggaar F.P. Single and sequential biodegradable stent placement for refractory benign esophageal strictures: a prospective follow-up study. Endoscopy. 2012;44:649–654. doi: 10.1055/s-0032-1309818. [DOI] [PubMed] [Google Scholar]
- 32.van Boeckel P.G., Vleggaar F.P., Siersema P.D. A comparison of temporary self-expanding plastic and biodegradable stents for refractory benign esophageal strictures. Clin Gastroenterol Hepatol. 2011;9:653–659. doi: 10.1016/j.cgh.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Mutaf O. Treatment of corrosive oesophageal strictures by long-term stenting. J Pediatr Surg. 1996;31:681–685. doi: 10.1016/s0022-3468(96)90674-0. [DOI] [PubMed] [Google Scholar]
- 34.Manfredi M.A., Jennings R.W., Anjum M.W., Hamilton T.E., Smithers C.J., Lightdale J.R. Externally removable stents in the treatment of benign recalcitrant strictures and esophageal perforations in pediatric patients with esophageal atresia. Gastrointest Endosc. 2014;80:246–252. doi: 10.1016/j.gie.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Lange B., Kubiak R., Wessel L.M., Kähler G. Use of fully covered self-expandable metal stents for benign esophageal disorders in children. J Laparoendosc Adv Surg Tech. 2015;25:335–341. doi: 10.1089/lap.2014.0203. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Ren L., Huoa J., Zhua Z., Liu D. The use of retrievable fully covered self-expanding metal stent in refractory postoperative restenosis of benign esophageal stricture in children. J Pediatr Surg. 2013;48:2235–2240. doi: 10.1016/j.jpedsurg.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Vandenplas Y., Hauser B., Devreker T., Urbain D., Reynaert H. A biodegradable esophageal stent in the treatment of a corrosive esophageal stenosis in a child. J Pediatr Gastroenterol Nutr. 2009;49:254–257. doi: 10.1097/MPG.0b013e31819de871. [DOI] [PubMed] [Google Scholar]
- 38.Woynarowski M., Dądalski M., Wojno V., Teisseyre M., Szymczak M., Chyżyńska A. Nasogastric tube as protection for recurrent oesophageal stricture: a case report. World J Gastroenterol. 2014;20:4806–4810. doi: 10.3748/wjg.v20.i16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woynarowski M., Dądalski M., Wojno V., Teisseyre M., Hurkała L., Płowiecki E. Novel, double-lumen removable stent to treat caustic esophageal stenosis. Endoscopy. 2014;46:E378–E379. doi: 10.1055/s-0034-1377373. [DOI] [PubMed] [Google Scholar]
- 40.Khara H.S., Diehl D.L., Gross S.A. Esophageal stent fracture: case report and review of the literature. World J Gastroenterol. 2014;20:2715–2720. doi: 10.3748/wjg.v20.i10.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]