Abstract
OBJECTIVE
The retention of patients on antiretroviral therapy (ART) is key to achieving global targets in response to the HIV epidemic. Loss to follow-up (LTFU) can be substantial, with unknown outcomes for patients lost to ART programmes. We examined changes in outcomes of patients LTFU over calendar time, assessed associations with other study and programme characteristics and investigated the relative success of different tracing methods.
METHODS
We performed a systematic review and logistic random-effects meta-regression analysis of studies that traced adults or children who started ART and were LTFU in sub-Saharan African treatment programmes. The primary outcome was mortality, and secondary outcomes were undocumented transfer to another programme, treatment interruption and the success of tracing attempts.
RESULTS
We included 32 eligible studies from 12 countries in sub-Saharan Africa: 20 365 patients LTFU were traced, and 15 708 patients (77.1%) were found. Compared to telephone calls, tracing that included home visits increased the probability of success: the adjusted odds ratio (aOR) was 9.35 (95% confidence interval [CI] 1.85–47.31). The risk of death declined over calendar time (aOR per 1-year increase 0.86, 95% CI 0.78–0.95), whereas undocumented transfers (aOR 1.13, 95% CI 0.96–1.34) and treatment interruptions (aOR 1.31, 95% CI 1.18–1.45) tended to increase. Mortality was lower in urban than in rural areas (aOR 0.59, 95% CI 0.36–0.98), but there was no difference in mortality between adults and children. The CD4 cell count at the start of ART increased over time.
CONCLUSIONS
Mortality among HIV-positive patients who started ART in sub-Saharan Africa, were lost to programmes and were successfully traced has declined substantially during the scale-up of ART, probably driven by less severe immunodeficiency at the start of therapy.
Keywords: HIV, antiretroviral therapy, loss to follow-up, mortality, sub-Saharan Africa
Introduction
Antiretroviral combination therapy (ART) has become more widely available in the low-income and middle-income countries that carry the highest burden of HIV infection and AIDS. By the end of 2015, 17 million people globally received ART, including 12 million persons living with HIV in sub-Saharan Africa [1]. Modelling by UNAIDS and others indicates that there is now a unique opportunity to end the HIV/AIDS epidemic by reaching the 90-90-90 targets by 2020: 90% of HIV infections are diagnosed, 90% of people known to be HIV-positive are on ART and 90% of individuals on ART are virologically suppressed [2, 3].
Retention in care is a key indicator of ART programmes’ effectiveness and key to achieving the 90-90-90 targets. Through active tracing by phone calls and home visits or through data linkage to national population registries, it is possible to determine the true outcome of patients lost to follow-up (LTFU) [4–7]. In the last few years, an increasing number of such tracing studies were conducted to examine mortality, treatment interruption and undocumented transfers to other clinics and to link patients back to care. These studies were first systematically reviewed by Brinkhof et al. in 2009 [8], who found that mortality among patients LTFU was high (around 40%) and was inversely correlated with the rate of LTFU at the level of the programme. This association was subsequently used in a nomogram to estimate overall programme-level mortality [4].
Since then, additional studies have been published, and evidence has emerged that an increasing number of patients LTFU had self-transferred to other clinics, making the prognosis of patients LTFU to the initial programme better than initially estimated [9–11]. Tracing studies in children have also been published. An updated review is now needed that accounts for undocumented transfers and interruption of ART. We systematically reviewed all tracing studies in adults and children LTFU on ART in sub-Saharan Africa. We examined changes in outcomes of patients LTFU over calendar time, assessed associations with other study and programme characteristics and investigated the relative success of different tracing methods.
Methods
This systematic review was part of the research activities of the Measurement and Surveillance of HIV Epidemics (MeSH; see www.mesh.lshtm.ac.uk) and the International epidemiology Databases to Evaluate AIDS (IeDEA; www.iedea.org) consortia. MeSH develops, tests and implements innovative and efficient methods for routine HIV measurement and surveillance among adults and children. IeDEA is a large network of ART programmes in sub-Saharan Africa and other regions [12].
Literature search
We searched three databases (PubMed, EMBASE and LILACS) to identify eligible studies published from 1 January 2009 to 31 December 2015. In PubMed, we used a combination of free text and Medical Subject Headings (MeSH) and then adapted the search to the other data-bases (see Figure S1). The search of LILACS included Spanish, Portuguese and French terms. We also searched conference abstracts to identify studies that we may have missed in the searches of the three literature databases. We covered the Conference on Retroviruses and Opportunistic Infections (CROI, 2014–2015); the Conference on HIV Pathogenesis and Treatment of the International AIDS Society (IAS, 2009–2015) and the International AIDS Conference (AIDS, 2010–2014). We used Google Scholar to identify electronic publications ahead of print of eligible studies presented at CROI, IAS or AIDS. Lastly, we scrutinised the reference lists of previous reviews [8, 9, 13].
Inclusion criteria
We included all full-text articles where patients who started ART for their own health in programmes in sub-Saharan Africa were LTFU and then were actively traced to establish their vital status. A range of definitions of LTFU were used and studies were included in the review regardless of how LTFU was defined. We excluded studies from other regions, case reports, studies related to other interventions, such as prevention of mother-to-child transmission (PMTCT), voluntary counselling and testing (VCT) or post-exposure prophylaxis (PEP), and studies in special patient groups such as patients with tuberculosis, or patients considered stable on ART (based on lack of clinical progression, improved CD4 cell counts, suppressed viral load or good adherence to treatment). If several reports from the same ART programme included overlapping study periods, the article on the most recent periods was included. Two reviewers independently assessed the eligibility of articles (A.M. and O.T.). Discrepancies were resolved in consultation with a third reviewer (M.E.).
Data extraction
Data were extracted in duplicate by A.M. and O.T., or K.Z. and M.C., and subsequently checked by N.A. Standardised data extraction sheets covered characteristics of the ART programme and of eligible patients, including location and setting (urban, mostly urban, mostly rural, rural), whether the programme was public or private, the age of patients in care (children, adults, mostly children, mostly adults), CD4 cell count at ART start and time on ART of patients LTFU. We extracted the definition of LTFU used, the number of patients LTFU, the number of patients traced and their outcome: dead, alive but not on ART (treatment interruption), undocumented transfer and not found. We also extracted the start and end date. We defined the calendar year of a study as the mid-study year, calculated to one decimal place. The methods used to trace patients (telephone calls, home visits or linkage with population registry) were also assessed. Data were double-entered into a database, with discrepancies resolved in discussions with a senior reviewer (M.E. or D.N.).
Statistical analyses
The primary outcome was the proportion of patients who were found to have died among those successfully traced. Secondary outcomes included the proportion of patients LTFU with undocumented transfer to another clinic, the proportion who interrupted treatment among those successfully traced and the proportion of patients LTFU who were successfully traced. We present proportions with exact binomial 95% confidence intervals (95% CIs) in forest plots and calculated the between-study variance (tau-squared).
We explored associations between outcomes and study characteristics using random intercept logistic meta-regression (binomial-normal) models. These models avoid the biases that arise when normal-normal models (which model the within-study variability via normal approximations) are applied to logit- or arcsine-square-root-transformed proportions [14, 15]. We included the calendar year, the study duration, the study setting (urban or mostly urban versus rural or mostly rural) and the study population (adults or mostly adults versus children or mostly children) as covariates in univariable and multivariable analyses. We also examined the effect of the tracing method on the success of tracing with and without adjusting for the covariates used in the main analysis (calendar year, study duration, setting and population). For the latter analysis, we excluded studies from South Africa where the vital status of patients was ascertained via linkage to the national population registry, using the civil identity document (ID) number [16]. We also examined whether in the treatment programmes the proportion of patients lost to follow-up had increased or decreased over calendar time.
Finally, we revisited the previously reported [4, 8] association between the proportion of patients lost in a programme and their mortality. All analyses were carried out in R version 3.2.3 (R Foundation, Vienna, Austria). Results are presented as odds ratios (ORs), adjusted ORs (aORs) and regression lines with 95% CIs.
Results
Selection and characteristics of studies
The searches identified a total of 1152 publications and 467 abstracts. We excluded 1589 items for the reasons detailed in Figure 1. Thirty articles met inclusion criteria [5, 7, 10, 17–44]. Two studies [27, 35] reported results for children and adults separately. A total of 32 cohorts were therefore included in the analyses. The studies were performed in 12 countries in East Africa, West Africa and Southern Africa (Table 1). The median study duration was 3.75 years (range 4 months to 9 years), and the mid-point of the study period ranged from 2003 to 2011. Most settings were urban or predominantly urban; six were rural studies. Most studies included adults only; two studies [32, 37] included mostly adults (over 90%) and four cohorts children [17, 23, 27, 35]. Definitions of LTFU varied from ‘not seen for a few weeks after a missed appointment’ to ‘not seen for over 6 months’. The most common definition was ‘not seen for at least 3 months’.
Figure 1.
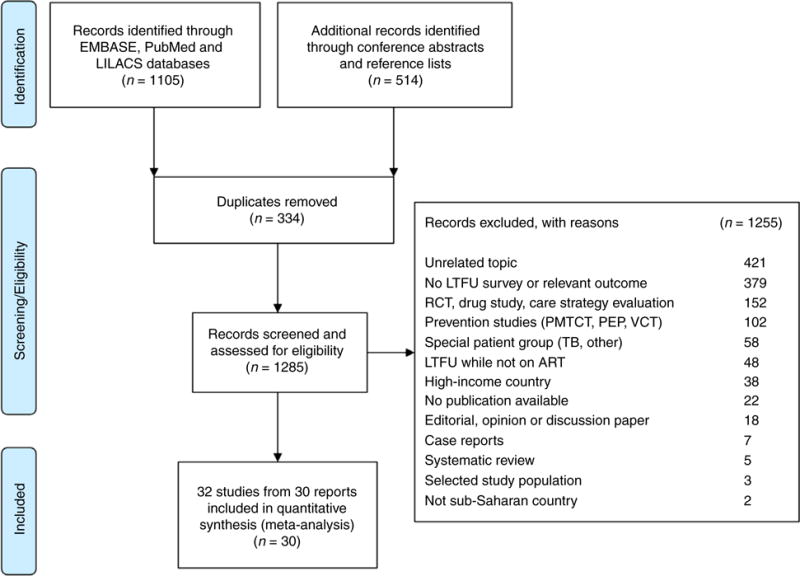
Identification and selection of eligible studies.
Table 1.
Characteristics of ART programmes tracing patients lost to follow-up (LTFU) in sub-Saharan Africa
| Study/Region | Location | Country | Setting | Study population | LTFU definition | Tracing method | Study period |
|---|---|---|---|---|---|---|---|
| East Africa | |||||||
| Geng (2008) [29] | Mbarara | Uganda | Rural | Adults | No visit for >6 months | Home visit | 2004–2007 |
| Alamo (2012) [44, 61] | Kampala | Uganda | Urban | Adults | No visit for >90 days | Home visit, buddy contact | 2001–2010 |
| Makunde (2012) [26] | Tanga City | Tanzania | Urban | Adults | No visit for >3 months | Home visit | 2006–2008 |
| Kiragga (2013) [19] | Kampala | Uganda | Urban | Adults | No visit for >3 months | Telephone, home visit | 2005–2007 |
| Wubshet (2013) [42] | Gondar | Ethiopia | Urban | Adults | Missed appointment >3 months | Home visit | 2005–2010 |
| Geng (2015) [5] | – | Kenya, Uganda, Tanzania | Urban | Adults | Missed appointment >90 days | Home visit | 2009–2012 |
| Mekuria (2015) [39] | Addis Ababa | Ethiopia | Urban | Adults | No visit for >1 month, | Telephone | 2009–2012 |
| Rachlis (2015) [37] | Nyanza | Kenya | Urban | Mostly adults | No visit >3 months | Home visit | 2009–2011 |
| West Africa | |||||||
| Mben (2012) [34] | Yaounde | Cameroon | Urban | Adults | Missed appointment >1 month | Telephone, home visit, relatives | 2006–2007 |
| Onoka (2012) [20] | Emuga State | Nigeria | Mostly urban | Adults | Missed ≥3 appointments | Telephone, home visit | 2007–2007 |
| Saka (2013) [41] | Lome | Togo | Mostly urban | Adults | – | Telephone | 2008–2011 |
| Southern Africa | |||||||
| Maskew (2007) [38] | Johannesburg | South Africa | Urban | Adults | Missed appointment | Telephone | 2006–2007 |
| Yu (2007) [32] | Four facilities | Malawi | Rural | Mostly adults | No visit for >3 months | Home visit | 2004–2006 |
| Bisson (2008) [31] | Gaborone | Botswana | Urban | Adults | Missed appointment >1 month | Telephone, home visit | 2003–2003 |
| Dalai (2008) [24] | Johannesburg | South Africa | Urban | Adults | Missed appointment for ≥6 weeks | Telephone, home visit | 2004–2005 |
| Krebs (2008) [25] | Lusaka | Zambia | Urban | Adults | Missed appointment | Home visit | 2005–2005 |
| Caluwaerts (2009) [36] | Tete | Mozambique | Urban | Adults | No visit for >60 days | Home visit | 2002–2007 |
| Boulle (2010) [7] | Khayelitsha | South Africa | Urban | Adults | No visit for > 6 months | Linkage | 2001–2007 |
| Fox (2010) [18] | Johannesburg | South Africa | Urban | Adults | Missed appointment ≥3 months | Linkage | 2004–2008 |
| McGuire (2010) [27] | Chiradzulu | Malawi | Rural | Adults | Missed appointment >1 month | Home visit | 2004–2007 |
| McGuire (2010) [27] | Chiradzulu | Malawi | Rural | Children | Missed appointment >1 month | Home visit | 2004–2007 |
| Peltzer (2011) [28] | Uthukela | South Africa | Mostly urban | Adults | Missed appointment ≥2 months | Telephone, home visit | 2007–2008 |
| Van Cutsem (2011) [21] | Cape Town | South Africa | Urban | Adults | No visit for >6 months | Linkage | 2001–2008 |
| Weigel (2011) [35] | Lilongwe | Malawi | Urban | Adults | Missed appointment >2 weeks | Telephone, home visit | 2002–2005 |
| Weigel (2011) [35] | Lilongwe | Malawi | Urban | Children | Missed appointment >2 weeks | Telephone, home visit | 2002–2005 |
| Grimwood (2012) [17] | 4 provinces | South Africa | Mostly urban | Children | No visit for ≥3 months | Linkage | 2004–2009 |
| Henriques (2012) [30] | Chiradzulu | Malawi | Rural | Adults | Missed appointment >1 month | Home visit | 2004–2007 |
| Mutevedzi (2013) [22] | Hlabisa | South Africa | Rural | Adults | No visit for 180 days | Linkage | 2004–2011 |
| Tweya (2013) [40] | Lilongwe | Malawi | Urban | Adults | Missed appointment >3 weeks | Telephone, home visit | 2006–2010 |
| Cornell (2014) [10] | 4 provinces | South Africa | Mostly urban | Adults | No visit for ≥6 months | Linkage | 2004–2009 |
| Ardura-Garcia (2015) [23] | Lilongwe | Malawi | Urban | Children | Missed appointment ≥3 weeks | Home visit | 2006–2010 |
| Budgell (2015) [33] | Johannesburg | South Africa | Urban | Adults | Missed appointment ≥3 months | Linkage | 2004–2013 |
–, not reported.
Three studies used phone calls only to trace patients, 22 studies did home visits and seven South African studies used data linkages (Table 1). Among the 25 studies that used home visits and/or telephone calls to trace patients lost to follow-up, nine used clinic staff (health educators, nurses or counsellors), eight employed research assistants for this task, and seven used community health workers or trained outreach workers. Two studies did not report the type of personnel involved [38, 45].
Patients lost and traced
A total of 20 365 patients LTFU were traced (median across studies: 294 patients; range 32–4467). In 30 of the 32 cohorts, the total number of patients LTFU was also given: 31 765 patients (median 588.5, range 68–5780); and in 25 studies, the total number of patients enrolled in the ART programme was reported: 177 890 (median 4674, range 155–34 277). The median percentage of patients LTFU among all patients enrolled was 16.6% (range 2.6–57.4%). Among patients LTFU, the median percentage traced was 81.3% (range 11–100%). The data are summarised in Table 2.
Table 2.
Outcomes of HIV-positive patients lost to follow-up (LTFU) in ART programmes in sub-Saharan Africa
| Number of patients
|
Vital status among successfully traced patients (%)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Enrolled in programme | Lost to programme | Tracing attempted | Successfully traced (100%) | Died | Alive and undocumented transfer | Alive and interruption of ART | Alive total | |
| East Africa | ||||||||
| Geng (2008) [29] | 3628 | 829 | 128 | 111 | 32 (28.8%) | – | 13/67 (19.4%)† | 79 (71.2%) |
| Alamo (2012) [44, 61] | 2713 | 1502 | 164 | 158 | 16 (10.1%) | 86 (54.4%) | 56 (35.4%) | 142 (89.9%) |
| Makunde (2012) [26] | 155 | 89 | 89 | 89 | 14 (15.7%) | 47 (52.8%) | 9 (10.1%) | 75 (84.3%) |
| Kiragga (2013) [19] | 5633 | 806 | 406 | 176 | 107 (60.8%) | 69 (39.2%) | – | 69 (39.2%) |
| Wubshet (2013) [42] | 3012 | 551 | 551 | 486 | 233 (47.9%) | 118 (24.3%) | 135 (27.8%) | 253 (52.1%) |
| Geng (2015) [5] | 34 277 | 5780 | 991 | 860 | 233 (27.1%) | – | – | 627 (72.9%) |
| Mekuria (2015) [39] | 836 | 116 | 116 | 65 | 15 (23.1%) | 19 (29.2%) | – | 50 (76.9%) |
| Rachlis (2015) [37] | – | 1071 | 851 | 569 | 193 (33.9%) | 154 (27.1%) | 177 (31.1%) | 376 (66.1%) |
| Western Africa | ||||||||
| Mben (2012) [34] | – | 238 | 238 | 238 | 98 (41.2%) | 22 (9.2%) | – | 140 (58.8%) |
| Onoka (2012) [20] | 1034 | 219 | 150 | 100 | 51 (51.0%) | 15 (15.0%) | 18 (18.0%) | 49 (49.0%) |
| Saka (2013) [41] | 16 617 | 1216 | 1004 | 202 | 88 (43.6%) | – | – | 114 (56.4%) |
| Southern Africa | ||||||||
| Maskew (2007) [38] | 5849 | 154 | 154 | 70 | 19 (27.1%) | 6 (8.6%) | – | 51 (72.9%) |
| Yu (2007) [32] | 5009 | 253 | 253 | 185 | 127 (68.6%) | 20 (10.8%) | 37 (20.0%) | 58 (31.4%) |
| Bisson (2008) [31] | 410 | 68 | 68 | 46 | 40 (87.0%) | – | – | 6 (13.0%) |
| Dalai (2008) [24] | 1631 | 267 | 267 | 173 | 83 (48.0%) | 30 (17.3%) | – | 90 (52.0%) |
| Krebs (2008) [25] | – | – | 654 | 417 | 192 (46.0%) | – | – | 225 (54.0%) |
| Caluwaerts (2009) [36] | 2818 | 594 | 594 | 214 | 118 (55.1%) | 43 (20.1%) | 46 (21.5%) | 96 (44.9%) |
| Boulle (2010) [7] | 6402 | 628 | 293 | 293 | 96 (32.8%) | – | – | 197 (67.2%) |
| Fox (2010) [18] | 11 694 | 2435 | 1037 | 1037 | 333 (32.1%) | – | – | 704 (67.9%) |
| McGuire (2010) [27]* | – | 75 | 32 | 19 | 11 (57.9%) | – | – | 8 (42.1%) |
| McGuire (2010) [27] | – | 1186 | 624 | 344 | 233 (67.7%) | 63 (18.3%) | 34/152 (22.3%)† | 111 (32.3%) |
| Peltzer (2011) [28] | 727 | 169 | 169 | 147 | 82 (55.8%) | 58 (39.5%) | – | 65 (44.2%) |
| Van Cutsem (2011) [21] | 6411 | 627 | 295 | 295 | 109 (36.9%) | – | – | 186 (63.1%) |
| Weigel (2011) [35] | – | 1840 | 659 | 486 | 201 (41.4%) | 127 (26.1%) | 23 (4.7%) | 285 (58.6%) |
| Weigel (2011) [35]* | – | – | 65 | 48 | 16 (33.3%) | 1 (2.1%) | 9 (18.8%) | 32 (66.7%) |
| Grimwood (2012) [17]* | 3563 | 211 | 93 | 93 | 36 (38.7%) | – | – | 57 (61.3%) |
| Henriques (2012) [30] | 6727 | 583 | 305 | 170 | 114 (56.4%) | – | – | 88 (43.6%) |
| Mutevedzi (2013) [22] | 4674 | 558 | 558 | 394 | 115 (29.2%) | – | – | 279 (70.8%) |
| Tweya (2013) [40] | 21 382 | 4560 | 4467 | 3176 | 952 (30.0%) | 744 (23.4%) | 957 (30.1%) | 2224 (70.0%) |
| Cornell (2014) [10] | 19 481 | 2624 | 2624 | 2624 | 972 (37.0%) | – | – | 1652 (63.0%) |
| Ardura-Garcia (2015) [23]* | 985 | 251 | 201 | 158 | 17 (10.8%) | 41 (25.9%) | 41 (25.9%) | 141 (89.2%) |
| Budgell (2015) [33] | 12 222 | 2265 | 2265 | 2265 | 434 (19.2%) | – | – | 1831 (80.8%) |
| Overall | 177 890 | 31 765 | 20 365 | 15 708 | 5380 (34.1%) | 1663 | 1555 | 10360 (65.9%) |
–, not reported.
Study or study subgroup of children.
Only a subsample of patients found to be alive was interviewed.
Seventeen studies reported the time from the start of ART to the last patient contact: the median was 5.3 months (range 1.3–14.4). Median CD4 cell counts at the start of ART among patients LTFU were available from 11 studies in adults. The median CD4 cell count increased from 71 cells/μl in the earliest study to 138 cells/μl in the most recent study [5]. Time on ART before LTFU was correlated with study duration, and median CD4 cell count correlated with mid-study calendar year (Figure S1).
Outcomes
The total number of patients successfully traced, that is the number of patients whose outcome could be ascertained, was 15 708. The median percentage of successfully traced patients was 73.4% across the 32 cohorts (range 20.2–100%). A total of 5380 (34.2% of patients successfully traced) had died (Table 2). Furthermore, 18 studies reported 1663 undocumented transfers of patients to another clinic. The median percentage of patients transferred among those successfully traced was 23.9% (range 2.1–54.4%). Thirteen studies reported on patients who had interrupted ART.
Meta-regression analyses
The tracing method used in the different studies was strongly associated with the probability of successful tracing of patients: compared to telephone calls only, tracing that included home visits substantially increased the probability of success. The univariable regression model predicted an increase in the proportion successfully traced from about 20% to 60% if home visits were included in the tracing strategy. The OR of successful tracing, comparing home visits with telephone calls, was 5.71 (95% CI 1.23–26.42), and the aOR was 9.35 (1.85–47.31).
For mortality, the forest plot indicated that deaths among patients successfully traced declined over calendar time (Figure 2). Meta-regression analyses confirmed this association: in univariable analysis, predicted mortality declined from around 56% in 2003 to 24% in 2011 (Figure 3, panel a). In multivariable analysis, the adjusted odds ratio (aOR) of death per 1-year increase in calendar year was 0.86 (95% CI 0.78–0.95) (Table 3). Mortality also decreased with longer study duration (aOR per 1-year increase 0.84, 95% CI 0.77–0.92) and was lower in urban than in rural areas (aOR 0.59, 95% CI 0.36–0.98). There was no evidence that mortality differed between adults and children (Table 3).
Figure 2.
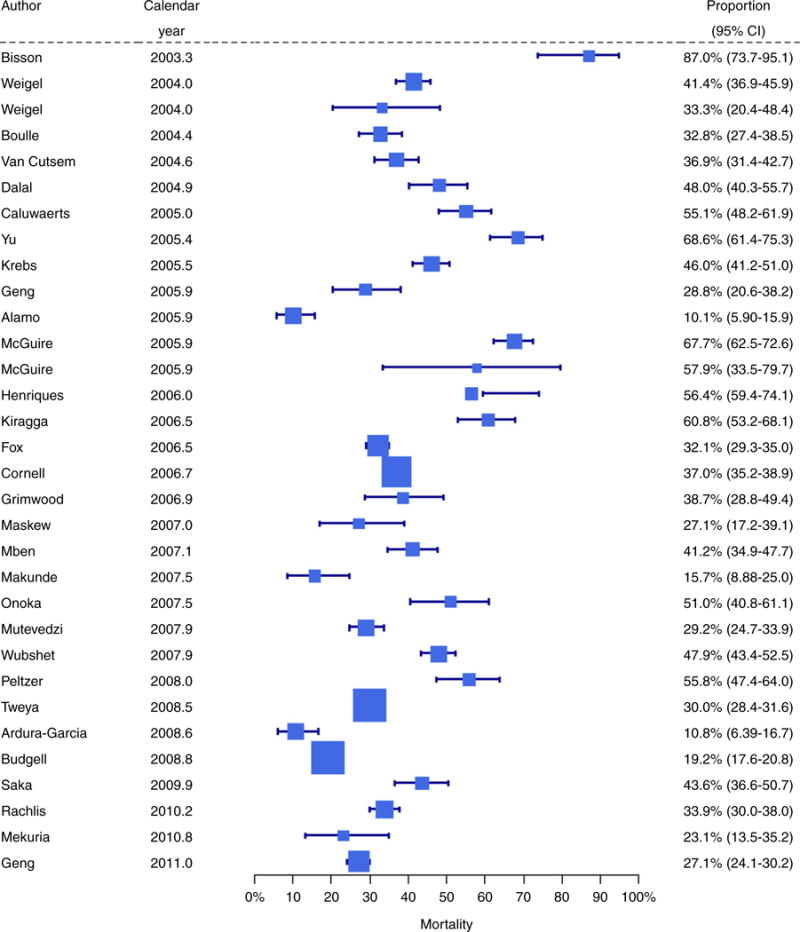
Forest plot of mortality among patients successfully traced in 32 cohorts of patients LTFU in ART programmes in sub-Saharan Africa. Study-specific mortality estimates are shown with exact binomial 95% confidence intervals. The studies are ordered by the mid-point of the study periods. Mortality among LTFU patients that were successfully traced decreased over time. The size of each square is inversely proportional to the variance of the estimate for that study.
Figure 3.
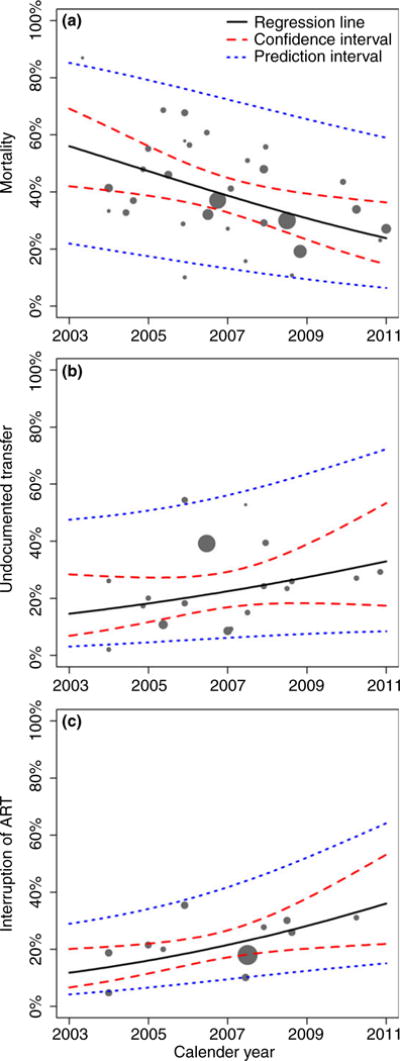
Estimated change in mortality (a), undocumented transfers (b) and interruption of ART (c) over calendar time among patients LTFU and successfully traced in studies from sub-Saharan Africa. The area of each circle is inversely proportional to the variance of the estimate for that study. Results from univariable random-effects meta-regression analyses.
Table 3.
Meta-regression analyses for mortality, undocumented transfer and ART interruption
| Outcome | Variable | Crude OR (95% CI) |
Adjusted* aOR (95% CI) |
|
|---|---|---|---|---|
| Mortality | Calendar year | Per 1-year increase | 0.84 (0.74–0.96) | 0.86 (0.78–0.95) |
| Study duration | Per 1-year increase | 0.82 (0.74–0.92) | 0.84 (0.77–0.92) | |
| Setting | Rural | 1 | 1 | |
| Urban | 0.51 (0.25–1.01) | 0.59 (0.36–0.98) | ||
| Population | Children | 1 | 1 | |
| Adults | 1.51 (0.63–3.62) | 1.47 (0.78–2.78) | ||
| Undocumented transfer | Calendar year | Per 1-year increase | 1.14 (0.94–1.38) | 1.13 (0.96–1.34) |
| Study duration | Per 1-year increase | 1.13 (0.96–1.34) | 1.17 (1.01–1.36) | |
| Setting | Rural | 1 | 1 | |
| Urban | 1.89 (0.63–5.73) | 1.57 (0.59–4.24) | ||
| Population | Children | 1 | 1 | |
| Adults | 2.38 (0.67–8.39) | 2.78 (0.93–8.30) | ||
| Interruption of ART | Calendar year | Per 1-year increase | 1.20 (1.03–1.39) | 1.31 (1.18–1.45) |
| Study duration | Per 1-year increase | 1.13 (0.97–1.33) | 1.22 (1.11–1.34) | |
| Setting | Rural | 1 | 1 | |
| Urban | 1.02 (0.45–2.28) | 0.51 (0.31–0.84) | ||
| Population | Children | 1 | 1 | |
| Adults | 0.89 (0.34–2.34) | 0.90 (0.51–1.58) |
Adjusted for all variables listed.
The proportion of undocumented transfers among those successfully traced increased with calendar time (Figure 3, panel b), but the association failed to reach statistical significance (aOR 1.13, 95% CI 0.96–1.34). Undocumented transfers were associated with longer study duration and tended to be more common in adults than in children. The proportion of patients who had interrupted ART increased both with calendar time (Figure 3, panel c) and with longer study duration.
Interruptions of ART were also more common in rural settings than in urban settings (Table 3). The variables included in the multivariable meta-regression models explained substantial proportions of the between-study heterogeneity. The model for mortality reduced tau-squared from 0.6279 to 0.2765 (a reduction by 56%). For undocumented transfer, the corresponding reductions were from 0.5852 to 0.3706 (a 37% reduction), and for interruption of ART from 0.3409 to 0.0597 (an 82% reduction).
There was no evidence of a change in the proportion lost to follow-up over calendar years (P = 0.76). Finally, as in the previous analysis [4,8], the proportion of patients LTFU in a programme was negatively associated with their mortality, with the regression line shifted towards lower mortality (Figure S4).
Discussion
We systematically reviewed studies of adult and paediatric patients who started ART in clinics and treatment programmes in sub-Saharan Africa, were LTFU and were subsequently traced. We analysed trends over calendar years in mortality, rates of undocumented transfers to other clinics and rates of ART interruption. We found that mortality in these patients decreased substantially over time, with a corresponding increase in undocumented transfers to other clinics and in the rate of interrupting therapy. We also examined the determinants of successful tracing of patients LTFU and found that home visits were more effective than tracing through phone calls alone. Finally, we updated a previous analysis [4,8] and confirmed the negative association between the proportion of patients LTFU in a treatment programme and their mortality.
Strengths of our study include the comprehensive literature search, which identified many studies from sub-Saharan Africa and the inclusion of studies both in adults and in children. In our study and previous reviews [8, 9], the results from individual studies were highly heterogeneous. We therefore focussed on identifying possible sources of heterogeneity, using state-of-the-art metaregression models, rather than on calculating potentially misleading combined estimates [45]. We excluded studies of selected patients on stable ART [46–48]: mortality in these patients will by definition be lower than in other patients LTFU.
Calendar year, study duration and study setting explained some of the heterogeneity in mortality across studies. Unfortunately, the CD4 cell count at the start of ART was not reported consistently and could therefore not be included in the analyses. Nevertheless, the decline in mortality over time may be explained by the increase in CD4 cell counts and reduction in severe immunodeficiency observed over time in adults and children starting ART [49, 50], which in turn is likely attributable to changes in HIV treatment guidelines [51], and earlier HIV diagnosis and enrolment into care associated with wider HIV testing [52, 53]. Calendar year may thus be seen as a proxy variable that captured the changes at the individual and health system levels associated with the scaling up of ART. We acknowledge that all outcomes in our study occurred conditional on LTFU, and changes in the determinants of LTFU could therefore also explain the trends observed. Of note, although the proportion of patients LTFU in a treatment programme was associated with mortality, the proportion lost remained fairly constant over time.
We reviewed the abstracts of major conferences to identify studies that we may have missed in our literature searches but did not include abstracts because the data from abstracts were generally incomplete. Missing data were also a problem in the published reports: only 18 of 32 cohorts reported on transfers to other clinics, 13 studies reported on the proportion of patients who interrupted ART and 11 studies gave the CD4 cell count of adults at the start of ART. Studies in children and in rural settings were rare and the statistical power of comparisons therefore limited. Another limitation is the definition of LTFU that was not standardised across studies. Although our search was comprehensive, we may have missed studies that remained unpublished, or studies that were published in reports or in journals not indexed in the databases searched, thus potentially introducing publication bias [54]. Few African journals are indexed in major databases: a survey of 158 African medical journals found that only 18 were indexed on Medline and 10 in EMBASE [55].
Both of the previous reviews [8, 9] reported a mortality rate of around 40% among patients LTFU and successfully traced. Similar to the present study, Wilkinson et al. [9] found a decline in mortality from 50% to 30% when comparing study periods before and after December 2007. The percentage of patients who transferred to another clinic overall was 19% in their review and the percentage stopping ART 29% [9]. Our study extends their analysis, showing that the decline in mortality was accompanied by an increase in CD4 cell counts, transfers to other clinics and in treatment interruptions. Recent studies have shown that an important proportion of patients who are lost at one clinic remain on ART and in care at another clinic. For example, a study in Lilongwe, Malawi, found that among patients LTFU and found to be alive on tracing, a majority (56%) were still taking ART, sourced from another clinic [40]. Similarly, a study of adults starting ART in Uganda, Tanzania and Kenya found that 59% of patients interviewed had reconnected to care at a different clinic [11]. Recently, an analysis of all public-sector CD4 cell count and viral load test results in South Africa found that systemwide retention in the healthcare system was much higher than retention at the initiating clinic [56].
A study of South African treatment programmes, where the vital status of patients known to have transferred out could be ascertained by linkage to the national population register, reported that mortality in transferred patients was higher than in patients retained in the clinic in the 3 months after transfer, but comparable to or lower than mortality in patients retained in the clinic after 3 months [10]. The higher early mortality probably reflects transfers of patients with advanced disease to better equipped facilities, whereas mortality later on reflects the transfer and self-transfer of stable patients [10].
Estimations of mortality and life expectancy in HIV-infected populations rely on the complete ascertainment of deaths; however, the proportion of patients lost to follow-up in HIV care programmes is high, especially in sub-Saharan Africa [57]. As patients who are LTFU experience higher mortality than those remaining in care [4, 10, 11], failing to account for deaths of these patients leads to the underestimation of overall programme-level mortality, that is the mortality of all patients who started ART. In the absence of data from tracing studies on mortality of patients LTFU, investigators need to make assumptions on their likely mortality. For example, a study from Uganda [58] assumed that 30% of patients LTFU had died, whereas in Rwanda, investigators assumed that about 50% of patients LTFU had died [59]. Our regression analyses can inform such assumptions, taking into account the proportion of patients LTFU in the treatment programme and calendar year. A nomogram [4] or more sophisticated methods can then be used to obtain estimates of overall mortality that are corrected for LTFU [19].
Only one of the included studies assessed the cost-effectiveness of tracing patients lost to follow-up [47]. In this study, a social worker contacted patients by phone who had initiated antiretroviral therapy (ART) in the Themba Lethu Clinic in Johannesburg and were late for a scheduled visit. The worker could determine the status of 260 of 493 (53%) patients lost to follow-up. Twenty patients returned to care as a result of the intervention, at a cost of $432 per patient returned [47]. Kessler et al. simulated the cost-effectiveness of different strategies to retain patients in care in East Africa from a payer’s perspective, assessing both interventions that aim to decrease the risk of loss to follow-up and interventions tracing patients lost to follow-up [60]. They found that tracing of patients alone was associated with both minimal costs and minimal benefits, whereas a risk reduction strategy had a greater impact on the quality-adjusted life-years (QALYs) gained. The risk reduction strategy was, however, a more expensive intervention [60].
Our study has important implications. First, patients who started therapy and were lost to follow-up in recent years will likely have better outcomes than patients enrolling in earlier years of the scale-up of ART. Mortality was also lower in urban than in rural areas. Our results thus caution against the simplistic imputation of mortality rates based on older studies, or studies from other settings. Second, although mortality has declined, it is still much higher than mortality among patients retained in care, and efforts to retain patients in care and to bring patients back to care must continue, both to improve outcomes in individuals and to prevent HIV transmissions at the population level. Third, HIV care programmes should collect time-updated contact information on all patients to allow the effective tracing of patients LTFU through home visits, and a system of unique IDs should be introduced at the national level to link patients transferring between clinics.
Supplementary Material
Figure S1. Scatter plots of median time on ART against study duration (upper panel) and median CD4 cell count (on log scale) against mid study calendar year (lower panel).
Figure S2. Forest plot of proportion who had undocumented transfers to other clinics among patients successfully traced in 18 cohorts of patients LTFU in ART programmes in sub-Saharan Africa.
Figure S3. Forest plot of proportion who interrupted treatment among patients successfully traced in 16 cohorts of patients LTFU in ART programmes in sub-Saharan Africa. Study-specific proportions estimates are shown with exact binomial 95% confidence intervals.
Figure S4. Estimated change in mortality among patients LTFU with proportion of patients LTFU in programme.
Acknowledgments
We are grateful to Doris Kopp and Beatrice Minder for their expert help with literature searches. Funding was provided by the Bill and Melinda Gates Foundation and the National Institutes of Health.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.UNAIDS. Global AIDS Update 2016. Geneva. 2016 (Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf) [ 31 May 2016]
- 2.UNAIDS. ‘15 by 15’ – a global target achieved. 2015 (Available from: http://www.unaids.org/en/resources/documents/2015/15_by_15_a_global_target_achieved) [1 May 2016]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90. An ambitious treatment target to help end the AIDS epidemic. 2014 (Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf) [5 April 2015]
- 4.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8:e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of Mortality among HIV-infected people on antiretroviral therapy treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2:e107–e116. doi: 10.1016/S2352-3018(15)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yiannoutsos CT, Johnson LF, Boulle A, et al. Estimated mortality of adult HIV-infected patients starting treatment with combination antiretroviral therapy. Sex Transm Infect. 2012;88(Suppl 2):i33–i43. doi: 10.1136/sextrans-2012-050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 8.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson LS, Skordis-Worrall J, Ajose O, et al. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Heal. 2015;20:365–379. doi: 10.1111/tmi.12434. [DOI] [PubMed] [Google Scholar]
- 10.Cornell M, Lessells R, Fox MP, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicenter cohort study. J Acquir Immune Defic Syndr. 2014;67:e67–e75. doi: 10.1097/QAI.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng EH, Odeny TA, Lyamuya R, et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis. 2016;62:935–944. doi: 10.1093/cid/civ1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger M, Ekouevi DK, Williams C, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–1264. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon JH, Elliott JH, Hong SY, Bertagnolio S, Jordan MR. Effects of physical tracing on estimates of loss to follow-up, mortality and retention in low and middle income country antiretroviral therapy programs: a systematic review. PLoS ONE. 2013;8:e56047. doi: 10.1371/journal.pone.0056047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stijnen T, Hamza TH, Ozdemir P. Random effects metaanalysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 15.Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol. 2008;61:41–51. doi: 10.1016/j.jclinepi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LF, Dorrington RE, Laubscher R, et al. A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. J Int AIDS Soc. 2015;18:20628. doi: 10.7448/IAS.18.1.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimwood A, Fatti G, Mothibi E, Malahlela M, Shea J, Eley B. Community adherence support improves programme retention in children on antiretroviral treatment: a multicentre cohort study in South Africa. J Int AIDS Soc. 2012;15:17381. doi: 10.7448/IAS.15.2.17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Heal. 2010;15:405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiragga AN, Castelnuovo B, Musomba R, et al. Comparison of methods for correction of mortality estimates for loss to follow-up after ART initiation: a case of the Infectious Diseases Institute, Uganda. PLoS ONE. 2013;8:e83524. doi: 10.1371/journal.pone.0083524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onoka CA, Uzochukwu BS, Onwujekwe OE, et al. Retention and loss to follow-up in antiretroviral treatment programmes in southeast Nigeria. Pathog Glob Heal. 2012;106:46–54. doi: 10.1179/2047773211Y.0000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Cutsem G, Ford N, Hildebrand K, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS ONE. 2011;6:e14684. doi: 10.1371/journal.pone.0014684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutevedzi PC, Lessells RJ, Newell ML. Disengagement from care in a decentralised primary health care antiretroviral treatment programme: cohort study in rural South Africa. Trop Med Int Heal. 2013;18:934–941. doi: 10.1111/tmi.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ardura-Garcia C, Feldacker C, Tweya H, et al. Implementation and operational research: early tracing of children lost to follow-up from antiretroviral treatment: true outcomes and future risks. J Acquir Immune Defic Syndr. 2015;70:e160–e167. doi: 10.1097/QAI.0000000000000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalal RP, MacPhail C, Mqhayi M, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 25.Krebs DW, Chi BH, Mulenga Y, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–317. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 26.Makunde WH, Francis F, Mmbando BP, et al. Lost to follow up and clinical outcomes of HIV adult patients on antiretroviral therapy in care and treatment centres in Tanga city, NorthEastern Tanzania, Tanzan. J Health Res. 2012;14:250–6. [PubMed] [Google Scholar]
- 27.McGuire M, Munyenyembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 28.Peltzer K, Ramlagan S, Khan MS, Gaede B. The social and clinical characteristics of patients on antiretroviral therapy who are ‘lost to follow-up’ in KwaZulu-Natal, South Africa: a prospective study. SAHARA J. 2011;8:179–186. doi: 10.1080/17290376.2011.9725002. [DOI] [PubMed] [Google Scholar]
- 29.Geng EH, Emenyonu N, Bwana MB, Glidden DV, Martin JN. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300:506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henriques J, Pujades-Rodriguez M, McGuire M, et al. Comparison of methods to correct survival estimates and survival regression analysis on a large HIV African cohort. PLoS ONE. 2012;7:e31706. doi: 10.1371/journal.pone.0031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: Implications for global scale-up efforts. PLoS ONE. 2008;3:1–6. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JK-L, Chen SC-C, Wang K-Y, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budgell EP, Maskew M, Long L, Sanne I, Fox MP. Does most mortality in patients on ART occur in care or after lost to follow-up? Evidence from the Themba Lethu Clinic, South Africa. J Acquir Immune Defic Syndr. 2015;70:323–328. doi: 10.1097/QAI.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mben JM, Kouanfack C, Essomba CN, et al. Operational research and HIV policy and guidelines: lessons from a study of patients lost to follow-up from a public antiretroviral treatment program in Cameroon. J Public Heal Policy. 2012;33:462–477. doi: 10.1057/jphp.2012.31. [DOI] [PubMed] [Google Scholar]
- 35.Weigel R, Hochgesang M, Brinkhof MW, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caluwaerts C, Maendaenda R, Maldonado F, Biot M, Ford N, Chu K. Risk factors and true outcomes for lost to follow-up individuals in an antiretroviral treatment programme in Tete, Mozambique. Int Health. 2009;1:97–101. doi: 10.1016/j.inhe.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Rachlis B, Ochieng D, Geng E, et al. Implementation and operational research: evaluating outcomes of patients lost to follow-up in a large comprehensive care treatment program in western Kenya. J Acquir Immune Defic Syndr. 2015;68:e46–e55. doi: 10.1097/QAI.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–857. [PubMed] [Google Scholar]
- 39.Mekuria LA, Prins JM, Yalew AW, Sprangers MAG, Nieuwkerk PT. Retention in HIV care and predictors of attrition from care among HIV-infected adults receiving combination anti-retroviral therapy in Addis Ababa. PLoS ONE. 2015;10:e0130649. doi: 10.1371/journal.pone.0130649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tweya H, Feldacker C, Estill J, et al. Are they really lost? ‘True’ status and reasons for treatment discontinuation among HIV infected patients on antiretroviral therapy considered lost to follow up in Urban Malawi. PLoS ONE. 2013;8:e75761. doi: 10.1371/journal.pone.0075761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saka B, Landoh DE, Patassi A, et al. Loss of HIV-infected patients on potent antiretroviral therapy programs in Togo: risk factors and the fate of these patients. Pan Afr Med J. 2013;15:35. doi: 10.11604/pamj.2013.15.35.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wubshet M, Berhane Y, Worku A, Kebede Y. Death and seeking alternative therapy largely accounted for lost to follow-up of patients on ART in northwest Ethiopia: a community tracking survey. PLoS ONE. 2013;8:e59197. doi: 10.1371/journal.pone.0059197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alamo S, Wabwire-Mangen F, Kenneth E, Sunday P, Laga M, Colebunders RL. Task-shifting to community health workers: evaluation of the performance of a peer-led model in an antiretroviral program in Uganda. AIDS Patient Care STDS. 2012;26:101–107. doi: 10.1089/apc.2011.0279. [DOI] [PubMed] [Google Scholar]
- 44.Talisuna-Alamo S, Colebunders R, Ouma J, et al. Socioeconomic support reduces nonretention in a comprehensive, community-based antiretroviral therapy program in Uganda. J Acquir Immune Defic Syndr. 2012;59:e52–e59. doi: 10.1097/QAI.0b013e318246e2aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller CM, Ketlhapile M, Rybasack-Smith H, Rosen S. Why are antiretroviral treatment patients lost to follow-up? A qualitative study from South Africa. Trop Med Int Heal. 2010;15(Suppl 1):48–54. doi: 10.1111/j.1365-3156.2010.02514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen S, Ketlhapile M. Cost of using a patient tracer to reduce loss to follow-up and ascertain patient status in a large antiretroviral therapy program in Johannesburg, South Africa. Trop Med Int Heal. 2010;15(Suppl 1):98–104. doi: 10.1111/j.1365-3156.2010.02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Connor C, Osih R, Jaffer A. Loss to follow-up of stable antiretroviral therapy patients in a decentralized down-referral model of care in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2011;58:429–432. doi: 10.1097/QAI.0b013e318230d507. [DOI] [PubMed] [Google Scholar]
- 49.IeDea, Collaborations ARTC. Avila D, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65:e8–e16. doi: 10.1097/QAI.0b013e3182a39979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koller M, Patel K, Chi BH, et al. Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2015;68:62–72. doi: 10.1097/QAI.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutimura E, Addison D, Anastos K, et al. Trends in and correlates of CD4+ cell count at antiretroviral therapy initiation after changes in national ART guidelines in Rwanda. AIDS. 2015;29:67–76. doi: 10.1097/QAD.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. International Center for AIDS Care and Treatment Programs. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011;25:1523–1533. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman S, Wu Y, Lahuerta M, et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS. 2014;28:2429–2438. doi: 10.1097/QAD.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegfried N, Busgeeth K, Certain E. Scope and geographical distribution of African medical journals active in 2005. S Afr Med J. 2006;96:533–8. [PubMed] [Google Scholar]
- 56.Fox M, Bor J, MacLeod W, et al. Is retention on ART underestimated due to patient transfers? Estimating systemwide retention using a national labs database in South Africa; 21st International AIDS Conference; Durban, South Africa. TUAB0205. (Available from: http://www.aids2016.org/Portals/0/File/AIDS2016_Abstracts_LOW.pdf?ver=2016-08-10-154247-087) [Google Scholar]
- 57.Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low- and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69:98–108. doi: 10.1097/QAI.0000000000000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 59.Nsanzimana S, Remera E, Kanters S, et al. Life expectancy among HIV-positive patients in Rwanda: A retrospective observational cohort study. Lancet Glob Heal. 2015;3:e169–e177. doi: 10.1016/S2214-109X(14)70364-X. [DOI] [PubMed] [Google Scholar]
- 60.Kessler J, Nucifora K, Li L, Uhler L, Braithwaite S. Impact and cost-effectiveness of hypothetical strategies to enhance retention in care within HIV treatment programs in East Africa. Value Health. 2015;18:946–955. doi: 10.1016/j.jval.2015.09.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alamo ST, Colebunders R, Ouma J, et al. Return to normal life after AIDS as a reason for lost to follow-up in a community-based antiretroviral treatment program. J Acquir Immune Defic Syndr. 2012;60:e36–e45. doi: 10.1097/FTD.0b013e3182526e6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatter plots of median time on ART against study duration (upper panel) and median CD4 cell count (on log scale) against mid study calendar year (lower panel).
Figure S2. Forest plot of proportion who had undocumented transfers to other clinics among patients successfully traced in 18 cohorts of patients LTFU in ART programmes in sub-Saharan Africa.
Figure S3. Forest plot of proportion who interrupted treatment among patients successfully traced in 16 cohorts of patients LTFU in ART programmes in sub-Saharan Africa. Study-specific proportions estimates are shown with exact binomial 95% confidence intervals.
Figure S4. Estimated change in mortality among patients LTFU with proportion of patients LTFU in programme.


