Abstract
Aims
To assess the role of oxidative stress in mediating adverse outcomes in metabolic syndrome (MetS) and resultant cardiovascular autonomic neuropathy (CAN), and to evaluate the effects of lifestyle interventions on measures of oxidative stress and CAN in subjects with MetS.
Methods
Pilot study in 25 non-diabetic subjects with MetS (age 49±10 years, 76% females) participating in a 24-week lifestyle intervention (supervised aerobic exercise/Mediterranean diet), and 25 age-matched healthy controls. CAN was assessed by cardiovascular reflex tests, heart rate variability (HRV) and PET imaging with sympathetic analog [11C] meta-hydroxyephedrine ([11C]HED). Specific oxidative fingerprints were measured by liquid-chromatography/mass-spectrometry(LC/MS).
Results
At baseline, MetS subjects had significantly higher oxidative stress markers [3-nitrotyrosine (234±158 vs.54±47 µmol/mol tyrosine), ortho-tyrosine (59±38 vs.18±10 µmol/molphenylalanine, all P<0.0001], and impaired HRV at rest and during deep breathing (P=0.039 and P=0.021 respectively) compared to controls. Twenty-four-week lifestyle intervention significantly reduced all oxidative stress markers(all P < 0.01) but did not change any of the CAN measures.
Conclusions
Subjects with MetS present with signs of CAN and increased oxidative stress in absence of diabetes. The 24-week lifestyle intervention was effective in ameliorating oxidative stress, but did not improve measures of CAN. Larger clinical trials with longer duration are required to confirm these findings.
Keywords: Metabolic Syndrome, Cardiovascular autonomic neuropathy, Oxidative stress, Lifestyle intervention
Introduction
Diabetes mellitus affects approximately 29 million children and adults (9.3% of the population) in the United States (US), and the number is growing rapidly (1; 2). In addition, there are more than 86 million US adults (37% of population), who present with prediabetes defined as either impaired glucose tolerance (IGT) or impaired fasting glucose (IFG), and/or metabolic syndrome (MetS)(1–3). Neuropathies are among the most prevalent and debilitating complications of diabetes mellitus (4). Recent data indicate that diabetic neuropathy may be present in patients with MetS even in the absence of diabetes , and that IGT and/or IFG are independently associated with diabetic neuropathy and its rate of progression (5–9).
Cardiovascular autonomic neuropathy (CAN), documented by changes in cardiovascular reflex tests and/or heart rate variability (HRV), is an independent predictor of cardiovascular disease (CVD) mortality (10–12). Changes in HRV consistent with CAN have been identified at the time of diagnosis in subjects with type 2 diabetes (12; 13), and in patients with impaired glucose tolerance, insulin resistance, and/or the metabolic syndrome (9; 14; 15). These suggest that such impairment may be present either after a relatively brief exposure to sustained hyperglycemia, or develop in conjunction with obesity, insulin resistance and/or intermittent episodes of milder glucose elevations (12; 15). Cross-sectional studies in adults without diabetes provide evidence that indices of CAN are inversely associated with central obesity, insulin resistance, and fasting glucose (9; 14; 15), all components of the MetS. Clustering of major CVD risk factors that includes IFG or IGT, along with low high-density lipoprotein (HDL) cholesterol, elevated triglycerides, elevated blood pressure (BP) and central adiposity are the key features of MetS (16).
Several lines of evidence point towards a central role for oxidative stress and chronic inflammation in the pathogenesis of insulin resistance (17), CVD (18),and the development of diabetic complications (19–21), including CAN (22). Indeed, Houstis and Lander (17), proposed that oxidative stress is causal in all forms of insulin resistance and is central in mediating adverse impact of insulin resistance. However, it has been unclear whether CAN, a major complication of insulin resistant state (both diabetes and MetS) is linked with oxidative stress and will be improved following interventions mitigating oxidative stress.
We hypothesized that components of the MetS in the presence of insulin resistance induce activation of specific oxidative stress and inflammatory pathways, which in turn lead to impaired sympathetic activity, CAN and subsequent increased CVD risk. Furthermore, we hypothesized that lifestyle interventions may improve indices of CAN in subjects with MetS, and that these effects are explained, in part, by lowering oxidation and inflammation biomarkers.
Thus, the goals of the current study were to evaluate the relationship between components of MetS, oxidative stress and CAN, and the effects of an intensive lifestyle intervention on subjects with MetS. We predicted such an intervention would diminish oxidative stress and thereby potentially positively impact CAN.
Subjects, Materials and Methods
Study Design and Patient Population
This investigation combined a cross-sectional case-control pilot study that enrolled 25 subjects with MetS and 25 age-matched healthy controls with a prospective, pilot study of the subjects with MetS before and after participating in the Met Fit Program. The Met Fit Program at the University of Michigan includes a supervised exercise program and evidence-based dietary counseling to improve metabolic health in patients with MetS (23; 24).
Main inclusion criteria for study subjects with MetS were: age18–65 years, impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) (21) and two other components of the MetS as defined by the Adult Treatment Panel (ATP) III (25) among: waist circumference ≥ 102 cm (40 inches) for men and ≥ 88 cm (35 inches) for women; triglycerides ≥150 mg/dl (1.7 mmol/l); HDL cholesterol <40 mg/dl (1.0 mmol/l) in men and <50 mg/dl (1.3 mmol/l) in women; blood pressure (BP) ≥130/≥85 mmHg. Women of childbearing potential were required to use contraception to prevent pregnancy. The control subjects were age-and sex-matched healthy, non-obese (BMI <30) individuals with normal glucose tolerance (26) , normal BP (27), and normal lipid profiles (25).
Main criteria for exclusion were: nursing mothers or pregnant women and subjects with pre-existing cardiovascular disease, severe systemic disease with recognized complication neuropathy (e.g., chronic alcohol abuse, vitamin B12 deficiency, hypothyroidism, drug-induced neuropathy) and neurologic disease (e.g., Parkinson’s disease, epilepsy, recent stroke). Subjects taking drugs which interfere with the uptake or metabolism of catecholamines, subjects with known history of chronic kidney disease and subjects that took systemic investigational drugs within six months were also not enrolled in the study.
All subjects were evaluated with comprehensive physical examination, anthropometric parameters and standardized BP measurements at baseline and follow-up, and fasting blood samples were obtained for glucose and insulin levels, lipid profile and biomarkers of oxidative stress. Detailed evaluations for CAN and oxidative biomarkers were obtained at baseline in all subjects, and also at 12 and 24 weeks in MetS subjects as described below. The control group did not undergo PET testing due to a previously established normative database of [11C]HED retention in healthy controls (24).
Intervention
Subjects with MetS completed a 24-week intervention designed to provide strategies to reduce the risks associated with the MetS, following a modified protocol from the MetFit program (23; 24) . Exercise was individually tailored based on fitness that was assessed at baseline by entry treadmill that estimated peak METs(metabolic equivalent) in all subjects with MetS. Subjects had free access to the exercise facility and participated in a weekly group exercise session with the exercise physiologist and at least three other individual sessions at their convenience with the goal of a minimum of 180 minutes/week at 60–85% of maximum heart rate. Subjects completed weekly exercise logs that listed cardiovascular exercise, physical activity minutes and strength training each week; these were reviewed and analyzed by exercise physiologists. The nutrition component was taught by a registered dietitian and derived from the American Dietetic Association Evidence Based Nutrition Practice Guidelines (http://adaevidencelibrary.com/topic.cfm?cat=2651). The intervention was a calorie-controlled, cardio-protective Mediterranean style eating plan (28) and consisted of four 45-minute interactive group nutrition education sessions. Nutrition intake was monitored by phone dietary calls and weekly collected diet logs that were analyzed using NDS-R (Nutrition Data System for Research, University of Minnesota). Adherence to the Mediterranean diet was assessed using a previously validated Mediterranean Diet Score (MDS). The MDS is 10-point scale that ranges from 0–9 and incorporates the prominent characteristics of the diet. A higher MDS indicated better adherence to the diet (29). Throughout the intervention, weekly diet and exercise logs were collected. The weekly dietary scores were collected and change in MDS from baseline to the end of the study, were monitored for adherence to the diet throughout the study. Vital signs and urinary ketones were collected weekly. In addition, in a subset of the population, VO2 measurements were recorded at baseline and post-intervention.
All subjects signed a written informed consent document and the University of Michigan Institutional Review Board approved the study.
Detailed phenotype characterization of subjects with MetS
A detailed phenotypic characterization of subjects with MetS, including fasting glucose and insulin levels, lipid profiles, and oral glucose tolerance test was done at baseline and end of study after a 12-hour fast. Insulin sensitivity was evaluated with the homeostasis model assessment (HOMA-IR) and with the insulin sensitivity index (ISI) from the OGTT as previously described (30; 31). Separated plasma was frozen to −80°C for further measurement of oxidative stress and inflammation biomarkers.
Quantization of highly sensitive and specific stable products of oxidation using liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) All reagents were obtained from Sigma Chemical (St. Louis, MO) or Aldrich Chemical (Milwaukee, WI) unless otherwise specified. Cambridge Isotope Laboratories (Andover, MA) supplied 13C-labeled amino acids. Protein-bound oxidized phenylalanine and tyrosine moieties (3-nitrotyrosine o, o’-dityrosine and ortho-tyrosine) in plasma were quantified by isotope dilution LC/MS as described previously(32). Briefly, plasma proteins were precipitated with ice-cold trichloroacetic acid (10% vol/vol) and then delipidated with water/methanol/water-washed diethyl ether (1:3:7; vol/vol/vol). Known amounts of 13C6 isotopically labeled internal standards were added. The precipitated plasma proteins were hydrolyzed at 110°C for 24 hours in 4M methanesulfonic acid solution saturated with 1% benzoic acid. After solid-phase extraction, the oxidized amino acids were quantified by LC-ESI-MS/MS with multiple reaction monitoring by integrating peak areas of the labeled standards and the analytes. The levels of the oxidized amino acids were then normalized to the precursor amino acid tyrosine (for 3-nitrotrosine and o,o’-dityrosine) and phenylalanaine (for ortho-tyrosine) content. The levels of oxidized products are expressed as the ratio of the oxidized product over the total tyrosine or phenylalanine. Intraassay coefficients of variation for ortho-tyrosine, o,o’-dityrosine, and 3-nitrotyrosine were 2.92%, 3.81%, and 3.7%, respectively.
Systematic evaluation of CAN using HRV tests
Standardized CAN evaluations were performed on all subjects after an overnight fast. Subjects were asked to avoid caffeine and tobacco products for 8 h prior to testing. The electrocardiogram recordings were obtained in the supine position using a physiologic monitor (Nightingale PPM2, Zoe Medical Inc.), and data were collected during a resting study (5 min) and during several standardized cardiovascular autonomic reflex tests (CARTs) obtained under paced breathing (R-R response to deep breathing, Valsalva maneuver, and postural changes) as previously described (33). Each clinical challenge was separated by a standard period of rest to allow the subject’s HR to return to baseline. HRV studies were analyzed according to current guidelines (12; 34; 35) using the continuous wavelet transform methods with the ANX 3.1 (ANSAR Inc. Philadelphia, PA). This method incorporates respiratory activity in the formula and is reported to be superior for the analysis of nonstationary signals compared with Fourier transform. The following measures of CAN were predefined as outcomes of interests: resting low frequency power (LF), resting high frequency power (HF), resting LF/HF ratio, deep breathing LF, deep breathing HF, deep breathing LF: HF ratio, Valsalva LF, Valsalva HF, Valsalva LF: HF ratio, Standing LF, Standing HF and Standing ratio
PET Imaging [13N] ammonia and [11C]meta-hydroxyephedrine ([11C]HED)
All PET studies were performed on a Siemens/ECAT Exact HR+ PET scanner. Dynamic PET scans with [13N]ammonia (20 min acquisition, 20 image frames) and [11C]HED (60 min acquisition, 23 image frames) were acquired using previously published method (36). Following image reconstruction, software was used to reorient and reslice the raw transaxial PET data into cardiac short-axis view data sets.
Myocardial blood flow
Eight short-axis slices covering the left ventricle (LV) from apex to base were used for quantitative analyses. Each of the 8 slices were divided circumferentially into 12 arc-shaped myocardial regions (‘sectors’), to give 96 independent LV regions. Time-activity data for each sector was extracted from the PET data and stored for kinetic analysis. Quantitative estimates of regional myocardial perfusion were obtained for each sector using the validated kinetic modeling methods of Hutchins et al (37).
[11C]HED retention analysis
The 8 short axis slices from the [11C]HED study were divided into 60 sectors to generate 480 independent LV regions. The measured [11C]HED radioactivity concentration in each sector in the final image frame (40–60 min) was normalized to the calculated integral of the total radioactivity in the blood pool throughout the PET study to obtain a [11C]HED “retention index” (RI, units: mL blood/min/mL tissue) for each LV sector, as previously described (38). Polar maps of regional [11C]HED retention were generated and saved for visual inspection of [11C]HED retention deficits. A quantitative measure of the degree of cardiac denervation in each subject was generated by statistically comparing the measured [11C]HED RI value of each sector in the subject’s [11C]HED polar map to the mean and standard deviation of the RI data for that particular sector contained in our database of healthy nondiabetic subjects. Using this standard ‘z-score analysis’ (39) sectors in the subject’s [11C]HED polar map with RI values more than 2.5 standard deviations (SD) compared with the age-matched healthy control data mean values were considered to be ‘abnormal’. The fraction of LV sectors that was abnormal was used as a measure of the ‘extent’ of [11C]HED retention deficits.
Outcome Measures
The primary outcomes were the change in [11C]HED RI and the change in oxidative stress markers after the 24-week intervention with Met Fit program for subjects with MetS. Secondary outcomes included changes in: HRV, waist circumference, BMI, glucose tolerance, insulin sensitivity, lipids, pre- and post- exercise HR, BP and VO2.
Statistical Analysis
The differences in the characteristics of the subjects with MetS and healthy controls were assessed using the t-test for normally distributed continuous variables and chi square test for categorical variables. The change in outcome measures from baseline to 24 weeks was evaluated using paired t-test. All statistical analysis was performed using the statistical software SAS version 9.4 (SAS Inc, NC).
Results
Phenotypic Differences between subjects with MetS and Healthy controls
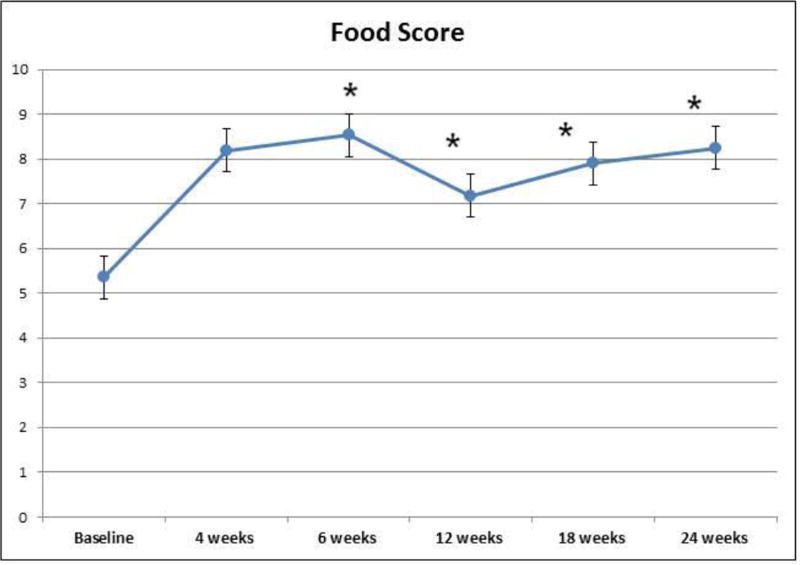
Data is presented for 25 subjects with MetS who met the inclusion criteria for the study and 25 age-and-sex-matched healthy controls. Clinical characteristics at baseline for these subjects are shown in Table 1. As anticipated, subjects with MetS had significantly higher weight, BMI, waist circumference, mean fasting plasma glucose, fasting insulin, systolic blood pressure, diastolic blood pressure and triglycerides levels, and significantly lower high-density lipoprotein (HDLc) levels compared with healthy controls (p<0.001). At baseline all subjects were consuming a Western style diet as documented by a low MDS (Figure 1).
Table 1.
Clinical Characteristics at Baseline in Subjects with MetS and Controls
| Variable | MetS (N=25) |
Healthy Controls (N=25) |
P value |
|---|---|---|---|
| Age, years | 49±10 | 44±9 | 0.092 |
| Male: Female | 6:19 | 6:19 | 1.00 |
| Weight, mean ±SD, kg | 103±16 | 70±13 | <0.0001 |
| Height, cm | 169±12 | 169±11 | 0.95 |
| Body Mass Index, kg/m2 | 36±5 | 24±3 | <0.0001 |
| Waist circumference, cm | 108±11 | 81±10 | <0.0001 |
| Fasting glucose, mg/dl | 101±9 | 87±6 | <0.0001 |
| Fasting insulin, units | 31±15 | 12±3 | <0.0001 |
| Systolic BP, mmHg | 132±14 | 115±13 | <0.0001 |
| Diastolic BP, mmHg | 77±10 | 68±8 | 0.0016 |
| Triglyceride, mg/dl | 198±82 | 72±32 | <0.0001 |
| HDL cholesterol, mg/dl | 41±10 | 63±13 | <0.0001 |
| LDL cholesterol, mg/dl | 110±37 | 105±23 | 0.56 |
| HOMA-IR | 7±3 | 2±0.8 | <0.0001 |
| 3-nitrotyrosine, µmol/mol | 234.3±158.2 | 53.7±46.8 | <0.0001 |
| -ortho-tyrosine, µmol/mol | 59.2± 38.2 | 18.1±10.0 | <0.0001 |
| o, o’-dityrosine, µmol/mol | 14.2±13.7 | 8.5±10.3 | 0.11 |
| Resting LF, msec | 3.8±6.4 | 2.8±1.7 | 0.46 |
| Resting HF, msec | 1.9±3.2 | 1.7±1.1 | 0.82 |
| Resting LF/HF ratio | 6.5±6.7 | 3.5±2.1 | 0.039 |
| Deep Breathing LF, msec | 0.7±0.5 | 0.5±0.4 | 0.32 |
| Deep Breathing HF, msec | 13.5±16.1 | 18.2±19.4 | 0.35 |
| Deep Breathing LF/HF ratio | 0.3±0.9 | 0.05±0.04 | 0.021 |
| Standing LF, msec | 2.8±3.7 | 3.1±2.8 | 0.77 |
| Standing HF, msec | 1.8±4.1 | 1.3±2.0 | 0.58 |
| Standing ratio | 6.7±4.0 | 9.3±5.6 | 0.069 |
| Valsalva LF, msec | 5.3±4.6 | 4.1±2.4 | 0.24 |
| Valsalva HF, msec | 2.1±3.1 | 1.5±1.1 | 0.37 |
| Valsalva Ratio | 4.2±2.3 | 3.3±1.3 | 0.38 |
| E/I ratio | 1.18±0.18 | 1.21±0.11 | 0.61 |
All data are presented as mean ±SD, BP : blood pressure, LF: low frequency power, HF: high frequency power, E/I ratio: expiration/inspiration ratio
Figure 1.
The changes in food score from baseline to 24 weeks in subjects with MetS
Protein-bound 3-nitrotyrosine and ortho-tyrosine levels were markedly elevated at baseline in subjects with MetS compared to healthy controls (234.3±158.2 vs. 53.7±46.8 µmol /mol tyrosine and 59.2±38.2 vs. 18.1±10.0 µmol /mol phenylalanine, respectively; (p<0.0001) In contrast, o,o’-dityrosine levels were not significantly different (Table 1).
Subjects with MetS had a higher LF/HF ratio (6.5±6.7 vs. 3.5±2.1, P =0.039) and deep breathing LF/HF ratio (0.3±0.9 vs. 0.05±0.04, P=0.021) compared to healthy controls (Table 1), but there were no differences in the standardized CARTs ratios or other CAN parameters between the 2 groups. These data strongly support elevated oxidative stress and impaired autonomic function suggesting evidence of CAN in MetS subjects at baseline.
We next performed a correlation analysis as a first step to explore any potential association between oxidative stress biomarkers and CAN parameters and found non-significant associations between these measures (Supplemental Data Table).
Effects of Lifestyle Intervention on Components of the Metabolic Syndrome and Cardio-Respiratory Fitness
The adherence to the Mediterranean diet was excellent with the MDS food score increasing to more than 8 at 4 and 6 weeks (Figure 1) and continued to be maintained at or near 8 up to 24 weeks. In addition, all subjects increased their exercise time to an average of 210 min/week.
After 24 weeks of the adherence to the Met Fit Program, subjects with MetS showed a significant reduction in weight, BMI, waist circumference, systolic blood pressure, diastolic blood pressure and triglycerides levels (all p<0.01), fasting insulin (p=0.030) and a significant increase in HDLc levels (p=0.011) (Table 2). The change in the exercise-related variables is presented in Table 2. As compared to baseline, at the end of the study, subjects with MetS had a significant change in pre-exercise HR (85 vs 72 beat/min, p=0.0019), post-exercise HR (157 vs. 109 beats/min, p<0.0001), and post-exercise systolic BP (169 vs. 129 mm Hg, p<0.0001). These data strongly support the effectiveness of the lifestyle intervention in improving measures of MetS and insulin sensitivity.
Table 2.
Effect of lifestyle intervention in subjects with Metabolic Syndrome from baseline to 24 weeks
| Variable | MetS Baseline (N=25) |
MetS 24 weeks (N=23) |
Difference (24 weeks – Baseline) |
P value |
|---|---|---|---|---|
| Weight, kg | 102±16 | 99±15 | −3±4 | 0.0025 |
| Body Mass Index, kg/m2 | 36±4 | 34±5 | −1±1 | 0.0015 |
| Waist circumference, cm | 108±11 | 105±9 | −3±4 | 0.0022 |
| Fasting glucose, mg/dl | 101±9 | 99±7 | −1±8 | 0.34 |
| Fasting insulin, units | 31±15 | 24±13 | −7±14 | 0.030 |
| Systolic BP, mmHg | 132±13 | 126±13 | −6±10 | 0.0045 |
| Diastolic BP, mmHg | 77±10 | 72±10 | −6±8 | 0.0016 |
| Triglyceride, mg/dl | 198±82 | 132±52 | −68±70 | 0.00012 |
| HDL cholesterol, mg/dl | 41±10 | 48±13 | 6±10 | 0.011 |
| LDL cholesterol, mg/dl | 110±37 | 102± 25 | −7±32 | 0.34 |
| Pre-exercise HR, beats/min | 72±14 | 85±13 | 15±19 | <0.0001 |
| Pre-exercise SBP, mmHg | 114±21 | 119±15 | 3±23 | 0.59 |
| Pre-exercise DBP, mmHg | 75±15 | 72±6 | −0.4±11 | 0.90 |
| Post-exercise HR (BPM) | 157±17 | 109±17 | −47±31 | <0.0001 |
| Post-exercise SBP, mmHg | 169±19 | 129±16 | −40.5±22 | <0.0001 |
| Post-exercise DBP, mmHg | 73±9 | 66±9 | −6±12 | 0.08 |
| Resting VO2 | 266±52 | 252±37 | −17±57 | 0.30 |
| REE (Kcal/day) | 1874±375 | 1739±300 | −185±299 | 0.082 |
| Exercise Time (min/week) | 0 | 210±122 | 210±122 | <0.0001 |
All data are presented as mean±SD, HDL: high density lipoproteins, LDL: low density lipoproteins, HR: heart rate, SBP: systolic blood pressure, DBP: diastolic blood pressure, REE: resting energy expenditure.
Effects of Lifestyle Intervention on Oxidative Stress Biomarkers
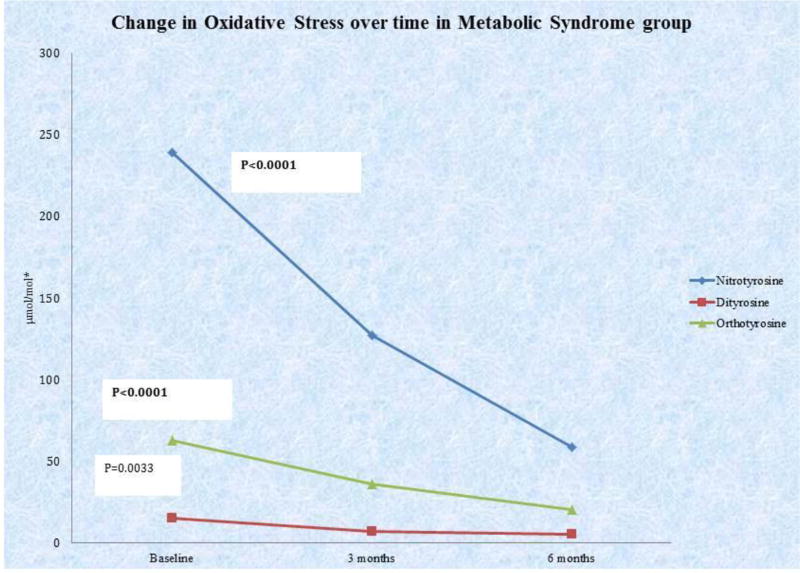
After implementation of the MetFit exercise program and Mediterranean diet at both the 3-month and 6-month time periods, patients showed significant reduction in oxidative stress levels (Figure 2). All markers of oxidative stress measured in this study (3-nitrotyrosine, o’o’-dityrosine, and ortho-tyrosine) significantly decreased from baseline to 3 months, and from baseline to 6 months (p<0.005 for all values). 3-nitrotyrosine and ortho-tyrosine levels also decreased significantly between 3 and 6 months, p=0.0005 and p=0.0001, respectively. All three markers of oxidative stress (3-nitrotyrosine, o’o’-dityrosine, and ortho-tyrosine) were significantly reduced in subjects with MetS at 24 weeks compared to baseline (58.9±55.0 vs. 234.3±158.2 µmol/mol tyrosine (p<0.0001), 5.2±9.5 vs. 14.2±13.8 µmol/mol tyrosine (p=0.0033), and 20.4±10.9 vs. 59.2±38.2 µmol/mol phenylalanine (p<0.0001), respectively). Thus, improvement in parameters of MetS and insulin sensitivity are accompanied by significant amelioration of oxidative stress.
Figure 2. Summary of changes in oxidative stress over treatment period.
*3-Nitrotyrosine measured as µmol 3-nitrotyrosine/mol tyrosine. o,o’-dityrosine measured as µmol ortho-tyrosine /mol tyrosine. ortho-tyrosine measured as µmol ortho-tyrosine /mol phenylalanine.
Effects of Lifestyle Intervention on Measures of CAN
After the 24-week lifestyle intervention, subjects with MetS did not demonstrate any significant changes in any of the CAN measures including CARTS, HRV indices, or LV sympathetic innervation as assessed by the [11C]HED RI (Table 3). None of the indices of HRV including resting and deep-breathing LF/HF ratio improved during the follow-up. The [11C]HED RI remained unchanged (0.066 mL blood/min/mL tissue at baseline and 0.067 mL blood/min/mL tissue at 24 weeks). Measures of CAN thus did not exhibit salutary changes in response to the intervention.
Table 3.
Change in Heart Rate Variability and Retention Index from baseline to 24 weeks in Subjects with MetS
| Variable | MetS Baseline (N=25) |
MetS 24 weeks (N=23) |
Difference |
P value |
|---|---|---|---|---|
| Resting LF, msec | 3.8±6.4 | 2.2±1.8 | −1.7±6.4 | 0.22 |
| Resting HF, msec | 1.9±3.2 | 1.2±1.1 | −0.7±2.8 | 0.23 |
| Resting LF/HF ratio | 6.5±6.7 | 5.9±4.1 | −0.9±5.0 | 0.37 |
| Deep Breathing LF, msec | 0.7±0.5 | 0.7±0.6 | 0.1±0.5 | 0.28 |
| Deep Breathing HF, msec | 13.5±16.1 | 11.8±10.6 | 0.2±6.0 | 0.86 |
| Deep Breathing LF/HF ratio | 0.30 ±0.9 | 0.10±0.1 | −0.17±0.91 | 0.38 |
| Valsalva LF, msec | 5.3±4.6 | 4.9±3.7 | −0.1±3.6 | 0.94 |
| Valsalva HF, msec | 2.1±3.1 | 1.8±2.8 | 0.2±3.2 | 0.80 |
| Valsalva ratio | 4.2±2.3 | 6.3±3.4 | 2.8±15.8 | 0.39 |
| Standing LF, msec | 2.8±3.7 | 3.8±5.8 | 1.0±6.4 | 0.48 |
| Standing HF, msec | 1.8±4.1 | 1.7±3.5 | −0.2±5.5 | 0.87 |
| Standing ratio | 6.7±4.0 | 7.7±4.5 | 0.74±5.3 | 0.51 |
| E/I ratio | 1.18±0.18 | 1.15±0.09 | 0.03±0.18 | 0.48 |
| Retention Index, mL blood/min/mL tissue | 0.068 ±0.016 | 0.068±0.014 | 0.003± 0.007 | 0.14 |
All data are presented as mean±SD, BP: blood pressure, LF: low frequency power, HF: high frequency power, E/I ratio: expiration/inspiration ratio
Discussion
The major goal of this study was to understand the link between MetS, oxidative stress and CAN. First, we found that compared with healthy controls, subjects with MetS present with evidence of CAN as documented by changes in HRV and in the LV sympathetic innervation as assessed by 11C–HED PET, and elevated markers of oxidative stress. We then tested the hypothesis whether a therapeutic life style intervention will have salutary effect on both oxidative stress and CAN. A 24-week lifestyle intervention comprised of (180 min/week) aerobic exercise and adherence to a Mediterranean-like diet induced a significant improvement in all the components of the MetS, suggesting its effectiveness and adherence by the study subjects. There was a marked improvement in all oxidative stress markers (3-nitrotyrosine, o’o’-dityrosine, and ortho-tyrosine) in the MetS subjects with the lifestyle intervention suggesting a significant improvement in the pro-inflammatory milieu. Surprisingly, the measures of CAN did not change during this time calling into question prevailing view that improvement of oxidative stress would mitigate complications resulting from MetS
Several large observational epidemiologic studies and randomized trials have demonstrated beneficial effects of the Mediterranean diet on CVD outcomes (40–42). Mechanisms postulated to mediate these benefits include: reduction in low-grade inflammation and oxidative stress (43), improvement in endothelial function, increased adiponectin concentrations, decreased blood coagulation, and improved apolipoprotein profiles with lower concentrations of oxidized LDL cholesterol (40–42). However, the metabolic pathways through which the Mediterranean diet influences CVD risk remain largely unknown. Oxidative stress in response to disturbed glycemic milieu, as observed in diabetes, has been implicated in the pathophysiology of various microvascular complications of diabetes including neuropathy (20–22; 44). Several pathways can promote oxidative stress in the prediabetic state and contribute to tissue damage in MetS including the glycoxidation pathway, myeloperoxidase (MPO) pathway and reactive nitrogen species (RNS) pathway. The products characteristic of these pathways include- ortho-tyrosine for glycoxidation pathway o,o’-dityrosine for MPO mediated oxidation and 3-nitrotyrosine for RNS pathway (45). These covalent protein modifications can be used not only as primary biomarkers of oxidative stress but also as predictors of the improved response to the intervention. At baseline, MetS individuals exhibited higher levels of oxidative stress involving RNS and glycoxidation pathways (increase in 3-nitrotyrosine and ortho-tyrosine). The implications of increased tyrosine and phenylalanine oxidation are broad. Importantly, several clinical and animal studies have shown that both oxidized tyrosine and phenylalanine moieties are associated with a proinflammatory state, such as atherosclerosis, (21; 46), diabetes (45; 47; 48) , lupus (49), interstitial lung disease (50), and rheumatoid arthritis (51). Although some proteins and tyrosine residues are known to be preferentially nitrated (52), much less is known about the other modifications. Further research is warranted to address specific protein targets of MetS; the present study only provides an overall measure of protein oxidation.
All three oxidative modifications ortho-tyrosine, 3-nitrotyrosine, and o,o′-dityrosine, improved with the intervention in MetS patients, suggesting that the intervention is effective in lowering oxidative stress broadly across all pathways. This could partly be attributed to the effect of the components of the Mediterranean diet which includes plant-based foods (vegetables, fruits, nuts, legumes, and unprocessed cereals), low consumption of meat and meat products and low consumption of dairy products. These data thus support the notion that subjects with MetS can alter the course of their pre-diabetes and improve some of the risk factors for complications by lifestyle changes. The significant reduction in these markers in response to 24 weeks of lifestyle intervention could pave the way for future therapeutic strategies for reducing the inflammatory response resulting in the damage of the nerves as seen in diabetic neuropathy.
Exercise and lifestyle interventions have been shown to prevent the onset of type 2 diabetes in patients with impaired glucose tolerance. Several clinical and population-based studies reported associations among fitness, physical activity, and autonomic or peripheral nerve function. For instance, the Diabetes Prevention Program (DPP) conclusively provided evidence that lifestyle intervention was superior to placebo or metformin in improving measures of CAN in 2,980 DPP participants with IGT (14) . Furthermore, decreases in heart rate and QT indexes and increases in HRV over time were associated with a lower risk of developing diabetes (14). Recent data from other clinical trials also support the notion that increased physical activity improves peripheral nerve function in subjects with MetS or painful diabetic neuropathy, yet all these studies were longer in duration (53–55). However, in this study the reduction in markers of oxidative stress after the lifestyle intervention did not translate into improvement in markers of CAN which could be due to the shorter duration of intervention and smaller sample size. Alternatively, CAN once developed may be uncoupled from oxidative stress and may not be readily reversible in the short-term and may need sustained intervention.
The strengths of our study are the state-of-the-art techniques used to assess markers of oxidative stress and validated measures CAN, the comprehensive data collection performed meticulously under standardized conditions, and access to a supervised and rigorously implemented lifestyle intervention program. Limitations of this pilot study include the relatively short duration of the intervention, and the sample size that likely contributed to a reduced ability to demonstrate an improvement in indices of CAN, in spite of the improvement in indices of oxidative stress. No differences in measures of CAN were observed between subjects who adhered to the lifestyle intervention and those who did not (data not shown). Although subjects with MetS had elevated levels of oxidative stress biomarkers, we could not demonstrate any significant correlations between these markers and measures of CAN, which could be due to the small sample size and no clear evidence of cardiovascular autonomic dysfunction in the MetS cohort compared with the healthy controls cohort.
Therefore, this study illustrates the need for larger studies focusing on the relationship between oxidative stress and autonomic health.
In conclusion, in this pilot trial, 24 weeks of lifestyle intervention combined with a Mediterranean diet successfully improved markers of oxidative stress and components of metabolic syndrome in subjects with MetS, but failed to improve CAN. Larger, long term prospective studies are needed to assess the effect of these interventions on CAN.
Supplementary Material
Acknowledgments
This work was supported in part by the following grants: AMERICAN DIABETES ASSOCIATION-1-08-CR-48 (RPB), R01HL102334-01 (RPB), DK089503, DK097153 DK081943 and DK DK082841 (SP). This study was also supported by Grant Number P30DK020572 (MDRC) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:None of the authors reported any conflict of interest.
Author contribution
RPB, SP, DR designed the study. RPB drafted the manuscript. SP, MJ, DR, LA MR, and EW provided critical input to the manuscript.
References
- 1.Group IDFDA: Update of mortality attributable to diabetes for the IDF Diabetes Atlas. Estimates for the year 2013. Diabetes Res Clin Pract. 2015;109:461–465. doi: 10.1016/j.diabres.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Professional Practice Committee for the Standards of Medical Care in Diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S107–108. doi: 10.2337/dc16-S018. [DOI] [PubMed] [Google Scholar]
- 4.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneap Minn) 2012;18:60–84. doi: 10.1212/01.CON.0000411568.34085.3e. [DOI] [PubMed] [Google Scholar]
- 6.Asghar O, Petropoulos IN, Alam U, Jones W, Jeziorska M, Marshall A, Ponirakis G, Fadavi H, Boulton AJ, Tavakoli M, Malik RA. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37:2643–2646. doi: 10.2337/dc14-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bongaerts BW, Rathmann W, Heier M, Kowall B, Herder C, Stockl D, Meisinger C, Ziegler D. Older subjects with diabetes prediabetes are frequently unaware of having distal sensorimotor polyneuropathy: the KORA F4 study. Diabetes Care. 2013;36:1141–1146. doi: 10.2337/dc12-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im S, Kim SR, Park JH, Kim YS, Park GY. Assessment of the medial dorsal cutaneous, dorsal sural, and medial plantar nerves in impaired glucose tolerance and diabetic patients with normal sural and superficial peroneal nerve responses. Diabetes Care. 2012;35:834–839. doi: 10.2337/dc11-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31:464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 10.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 11.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, Genuth S, Grimm RH, Corson MA, Prineas R. Effects of cardiac autonomic dysfunction on mortality risk in the action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27:639–653. doi: 10.1002/dmrr.1239. [DOI] [PubMed] [Google Scholar]
- 13.Singh JP, Larson MG, O’Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study) Am J Cardiol. 2000;86:309–312. doi: 10.1016/s0002-9149(00)00920-6. [DOI] [PubMed] [Google Scholar]
- 14.Carnethon MR, Prineas RJ, Temprosa M, Zhang ZM, Uwaifo G, Molitch ME. The association among autonomic nervous system function, incident diabetes, and intervention arm in the diabetes prevention program. Diabetes Care. 2006;29:914–919. doi: 10.2337/diacare.29.04.06.dc05-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler D, Voss A, Rathmann W, Strom A, Perz S, Roden M, Peters A, Meisinger C, Group KS. Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population the KORA S4 survey. Diabetologia. 2015;58:1118–1128. doi: 10.1007/s00125-015-3534-7. [DOI] [PubMed] [Google Scholar]
- 16.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 18.Heinecke JW. Oxidative stress: new approaches to diagnosis and prognosis in atherosclerosis. Am J Cardiol. 2003;91:12A–16A. doi: 10.1016/s0002-9149(02)03145-4. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 20.Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 21.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 22.Pop-Busui R, Kirkwood I, Schmid H, Marinescu V, Schroeder J, Larkin D, Yamada E, Raffel DM, Stevens MJ. Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol. 2004;44:2368–2374. doi: 10.1016/j.jacc.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Rubenfire M, Mollo L, Krishnan S, Finkel S, Weintraub M, Gracik T, Kohn D, Oral EA. Themetabolic fitness program lifestyle modification for the metabolic syndrome using the resources of cardiac rehabilitation. Journal of cardiopulmonary rehabilitation and prevention. 2011;31:282–289. doi: 10.1097/HCR.0b013e318220a7eb. [DOI] [PubMed] [Google Scholar]
- 24.Walden P, Jiang Q, Jackson EA, Oral EA, Weintraub MS, Rubenfire M. Assessing the incremental benefit of an extended duration lifestyle intervention for the components of the metabolic syndrome. Diabetes Metab Syndr Obes. 2016;9:177–184. doi: 10.2147/DMSO.S94772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 26.Standards of medical care in diabetes--2009. Diabetes Care. 2009;32(Suppl 1):S13–61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention Detection Evaluation Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 28.de Lorgeril M, Salen P. The Mediterranean-style diet for the prevention of cardiovascular diseases. Public Health Nutr. 2006;9:118–123. doi: 10.1079/phn2005933. [DOI] [PubMed] [Google Scholar]
- 29.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 32.Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino Acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. doi: 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and 28. Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC) Circulation. 2009;119:2886–2893. doi: 10.1161/CIRCULATIONAHA.108.837369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 35.Bernardi L, Spallone V, Stevens M, Hilsted J, Frontoni S, Pop-Busui R, Ziegler D, Kempler P, Freeman R, Low P, Tesfaye S, Valensi P. Investigation methods for cardiac autonomic function in human research studies. Diabetes Metab Res Rev. 2011;27:639–653. doi: 10.1002/dmrr.1224. [DOI] [PubMed] [Google Scholar]
- 36.Stevens MJ, Dayanikli F, Raffel DM, Allman KC, Sandford T, Feldman EL, Wieland DM, Corbett J, Schwaiger M. Scintigraphic assessment of regionalized defects in myocardial sympathetic innervation and blood flow regulation in diabetic patients with autonomic neuropathy. J Am Coll Cardiol. 1998;31:1575–1584. doi: 10.1016/s0735-1097(98)00128-4. [DOI] [PubMed] [Google Scholar]
- 37.Hutchins GD. Quantitative evaluation of myocardial blood flow with [13N]ammonia. Cardiology. 1997;88:106–115. doi: 10.1159/000177316. [DOI] [PubMed] [Google Scholar]
- 38.Raffel DM, Koeppe RA, Little R, Wang CN, Liu S, Junck L, Heumann M, Gilman S. PET measurement of cardiac and nigrostriatal denervation in Parkinsonian syndromes. J Nucl Med. 2006;47:1769–1777. [PubMed] [Google Scholar]
- 39.Stevens MJ, Raffel DM, Allman KC, Schwaiger M, Wieland DM. Regression and progression of cardiac sympathetic dysinnervation complicating diabetes: an assessment by C-11 hydroxyephedrine and positron emission tomography. Metabolism. 1999;48:92–101. doi: 10.1016/s0026-0495(99)90016-1. [DOI] [PubMed] [Google Scholar]
- 40.Mitjavila MT, Fandos M, Salas-Salvado J, Covas MI, Borrego S, Estruch R, Lamuela-Raventos R, Corella D, Martinez-Gonzalez MA, Sanchez JM, Bullo M, Fito M, Tormos C, Cerda C, Casillas R, Moreno JJ, Iradi A, Zaragoza C, Chaves J, Saez GT. The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clinical nutrition. 2013;32:172–178. doi: 10.1016/j.clnu.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Razquin C, Martinez JA, Martinez-Gonzalez MA, Mitjavila MT, Estruch R, Marti A. A 3 years follow-up of a Mediterranean diet rich in virgin olive oil is associated with high plasma antioxidant capacity and reduced body weight gain. Eur J Clin Nutr. 2009;63:1387–1393. doi: 10.1038/ejcn.2009.106. [DOI] [PubMed] [Google Scholar]
- 42.Zamora-Ros R, Serafini M, Estruch R, Lamuela-Raventos RM, Martinez-Gonzalez MA, Salas-Salvado J, Fiol M, Lapetra J, Aros F, Covas MI, Andres-Lacueva C, Investigators PS. Mediterranean diet non enzymatic antioxidant capacity in the PREDIMED study: evidence for a mechanism of antioxidant tuning. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2013;23:1167–1174. doi: 10.1016/j.numecd.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a Therapeutic Target for Diabetic Neuropathies. Curr Diab Rep. 2016;16:29. doi: 10.1007/s11892-016-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep. 2007;7:257–264. doi: 10.1007/s11892-007-0041-3. [DOI] [PubMed] [Google Scholar]
- 45.Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem. 2005;280:22706–22714. doi: 10.1074/jbc.M500839200. [DOI] [PubMed] [Google Scholar]
- 46.Shao B, Pennathur S, Heinecke JW. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J Biol Chem. 2012;287:6375–6386. doi: 10.1074/jbc.M111.337345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pop-Busui R, Oral E, Raffel D, Byun J, Bajirovic V, Vivekanandan-Giri A, Kellogg A, Pennathur S, Stevens MJ. Impact of rosiglitazone and glyburide on nitrosative stress and myocardial blood flow regulation in type 2 diabetes mellitus. Metabolism. 2009;58:989–994. doi: 10.1016/j.metabol.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Vivekanadan-Giri A, Wang JH, Byun J, Pennathur S. Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage. Reviews in endocrine & metabolic disorders. 2008;9:275–287. doi: 10.1007/s11154-008-9093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, Gillespie BW, Carmona-Rivera C, Liu X, Subramanian V, Hasni S, Thompson PR, Heinecke JW, Saran R, Pennathur S, Kaplan MJ. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis & rheumatology. 2014;66:2532–2544. doi: 10.1002/art.38703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pennathur S, Vivekanandan-Giri A, Locy ML, Kulkarni T, Zhi D, Zeng L, Byun J, de Andrade JA, Thannickal VJ. Oxidative Modifications of Protein Tyrosyl Residues Are Increased in Plasma of Human Subjects with Interstitial Lung Disease. Am J Respir Crit Care Med. 2016;193:861–868. doi: 10.1164/rccm.201505-0992OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vivekanandan-Giri A, Slocum JL, Byun J, Tang C, Sands RL, Gillespie BW, Heinecke JW, Saran R, Kaplan MJ, Pennathur S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Annals of the rheumatic diseases. 2013;72:1725–1731. doi: 10.1136/annrheumdis-2012-202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanski J, Schoneich C. Protein nitration in biological aging: proteomic and tandem mass spectrometric characterization of nitrated sites. Methods Enzymol. 2005;396:160–171. doi: 10.1016/S0076-6879(05)96016-3. [DOI] [PubMed] [Google Scholar]
- 53.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, Fallucca F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20:216–223. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Smith AG, Russell J, Feldman EL, Goldstein J, Peltier A, Smith S, Hamwi J, Pollari D, Bixby B, Howard J, Singleton JR. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. doi: 10.2337/dc06-0224. [DOI] [PubMed] [Google Scholar]
- 55.Singleton JR, Marcus RL, Jackson JE, M KL, Graham TE, Smith AG. Exercise increases cutaneous nerve density in diabetic patients without neuropathy. Annals of clinical and translational neurology. 2014;1:844–849. doi: 10.1002/acn3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.