Abstract
Background
Although there is no consensus on whether pre-operative MRI in women with breast cancer (BC) benefits surgical treatment, MRI continues to be used pre-operatively in practice. This meta-analysis examines the association between pre-operative MRI and surgical outcomes in BC.
Methods
A systematic review was performed to identify studies reporting quantitative data on pre-operative MRI and surgical outcomes (without restriction by type of surgery received or type of BC) and using a controlled design. Random-effects logistic regression calculated the pooled odds ratio (OR) for each surgical outcome (MRI versus no-MRI groups), and estimated ORs stratified by study-level age. Subgroup analysis was performed for invasive lobular cancer (ILC).
Results
Nineteen studies met eligibility criteria: 3 RCTs and 16 comparative studies that included newly diagnosed BC of any type except for 3 studies restricted to invasive lobular cancer (ILC). Primary analysis (85975 subjects) showed that pre-operative MRI was associated with increased odds of receiving mastectomy [OR 1.39 (1.23, 1.57); p<0.001]; similar findings were shown in analyses stratified by study-level median age. Secondary analyses did not find statistical evidence of an effect of MRI on the rates of re-excision, re-operation, or positive margins; however MRI was significantly associated with increased odds of receiving contralateral prophylactic mastectomy [OR 1.91 (1.25, 2.91); p= 0.003]. Subgroup analysis for ILC did not find any association between MRI and the odds of receiving mastectomy [OR 1.00 (0.75, 1.33); p= 0.988] or the odds of re-excision [OR 0.65 (0.35, 1.24); p= 0.192].
Conclusions
Pre-operative MRI is associated with increased odds of receiving ipsilateral mastectomy and contralateral prophylactic mastectomy as surgical treatment in newly diagnosed BC patients.
Keywords: Breast cancer, breast conserving surgery, magnetic resonance imaging, mastectomy, meta-analysis, re-operation
Introduction
The use of pre-operative breast magnetic resonance imaging (MRI) in patients with breast cancer (BC) remains a controversial issue. Despite a decade of evidence suggesting a lack of clinical benefit, counter-balanced by evidence that MRI detects additional disease not seen with conventional imaging in the cancerous breast, there is no consensus on whether it confers benefit or harm1–12. The fact that pre-operative MRI increases the detection of additional disease in the affected breast13 has promulgated the impression that MRI enhances surgical care of newly diagnosed BC patients1;2;4. Conflicting evidence, from both randomized controlled trials (RCTs) and observational studies, regarding whether MRI improves surgical treatment or whether it simply leads to more mastectomies1–3;5–12;14–16, has led to divergent interpretation of its impact and variability in its use as a pre-operative test for BC patients. Previous meta-analyses focusing on preoperative MRI have reported solely on its detection capability13, or have indicated that MRI could increase mastectomy rates but with equivocal findings for some surgical outcomes6. It is not surprising then that guidelines give varying recommendations regarding use of pre-operative MRI in newly diagnosed BC and that there is persistent use of MRI for pre-operative surgical planning in BC10;17–19.
To aid in addressing this controversy, we report a pooled analysis of the association of pre-operative MRI with surgical treatment of women newly diagnosed with invasive BC. We extend our previous meta-analytic work which examined surgical outcomes associated with preoperative MRI, to ensure that estimates of the effect of MRI reflect all current evidence, and to provide precise estimates for patient cohorts including subgroups.
Methods
Literature search and eligibility criteria
A systematic literature search (MEDLINE, Cochrane Database) was performed in December 2016 to identify primary studies that met pre-defined eligibility criteria, adapted from and updated from our earlier systematic review6, as follows: studies of pre-operative MRI that examined surgical outcomes for the ipsilateral breast using a controlled study design (either randomized controlled trial or comparative design); and reporting quantitative data on surgical treatment (initial and final surgery received, or primary surgical treatment) of women with invasive BC; and included cohorts of patients potentially eligible to receive breast conserving surgery (BCS) or mastectomy. Studies reporting on cohorts of BC patients that included those with ductal carcinoma in situ (DCIS) were eligible for inclusion, however, studies restricted to DCIS cases were ineligible. Studies reporting on pre-operative MRI based only on cohorts who had MRI (without a control group that did not receive MRI), or restricted to subjects who had received BCS as their definitive treatment (hence removing the potential to receive mastectomy) were ineligible for inclusion, as these study designs do not allow estimation of the effect of MRI on surgical treatment and cannot quantify the primary endpoint of this meta-analysis.
The literature search process, including search terms and number of citations selected or excluded, is summarized in online-only Appendix 1.
Study endpoints
Primary endpoint for this meta-analysis was receipt of mastectomy as surgical treatment of the affected breast. Additional analyses examined the following secondary endpoints (in studies reporting these outcomes): re-excision after receipt of BCS, positive margins after BCS, any re-operation in the overall study cohorts, and receipt of contralateral prophylactic mastectomy.
Extracted data
Study-specific descriptive information (design, timeframe, aggregate age and tumor size where reported, and histology), and quantitative data on surgical outcomes were extracted by one author (NH). Surgical outcomes were defined as follows: receipt of mastectomy for the affected breast, based on primary surgical treatment or based on data for initial and final surgery (number who had mastectomy, number who had BCS); number with positive margins (or incomplete excision due to positive or non-negative margins) if BCS was initially received; number who had re-excision surgery if BCS was received; number who had any re-operation from total cohort; and receipt of a contralateral prophylactic mastectomy (CPM).
Statistical methods
Random effects meta-analysis using the DerSimonian and Laird method was used to pool odds ratios comparing the number of subjects with the above-defined surgical outcomes (based on number with each surgical outcome, from the total cohort or by receipt of BCS) for cohorts who had MRI versus those who did not have MRI (referent group). Random study effects were included in all models to allow for anticipated heterogeneity between studies beyond what would arise from within study sampling error alone; taking account of both within- and between-study variability provides valid standard errors, confidence intervals (CI), and P-values. Study-specific and pooled data, and the estimated ORs and 95% CI were displayed in forest plots. Statistical significance was set at P <0.05.
Because only some of the studies reporting on CPM provided sufficient data to determine the numerator for this outcome but all reported adjusted ORs, we calculated the pooled OR for CPM using study-specific adjusted OR estimates – hence for this outcome only study-reported ORs and pooled ORs are displayed in forest plots.
To investigate sources of heterogeneity we conducted a subgroup analysis by study-level median (or mean) age, stratifying around the median value. A test for subgroup differences was then conducted and shown in stratified analyses. We also examined our estimates in relation to study timeframe using the median value of the years during which studies were conducted. Analyses were performed for studies that included all BC patients, and separately in subgroup analysis for studies that selected cases with invasive lobular cancer (ILC) or reported subgroup data for ILC. We used Stata version 14.2 (StataCorp 2009; College Station, TX) for meta-analysis.
Results
Our search strategy identified 264 citations (additional details in online Appendix-1); of these 19 studies3;14–16;20–34 met eligibility criteria: 3 RCTs3;15;20 and 16 comparative studies 14;16;21–34; 13 that included newly diagnosed BC of any type and 3 studies32–34 restricted to cases with invasive lobular histology. Of the 19 eligible studies, 3 studies reported outcomes data on all BC types and also provided separately data for invasive lobular cancer (ILC) patients3;14;30. Two of the eligible studies were based on young women15;16and another 2 studies14;26 were based on older women from overlapping datasets (SEER Medicare data in women aged 66 years and older); only one of the 2 overlapping studies was included in each analysis (online Appendix-1 provides additional details). Most studies specified that patients who received neoadjuvant therapy were excluded14;16;21;22;25;27;30–34 (only one study included patients who had neoadjuvant therapy15). Two studies excluded patients with BRCA gene mutations23;31, otherwise this information was not explicitly reported. Because different studies contributed to the various analyses, total number of subjects is shown for each model; the primary endpoint analysis included 85975 subjects of whom 15274 received preoperative MRI.
The characteristics of each study are summarized in online-Appendix 2: studies were heterogeneous in terms of design, and patient and tumor characteristics; extracted variables were inconsistently reported. There was heterogeneity in median or mean age between studies, and within the non-randomized studies patients undergoing MRI generally had younger median or mean age than those who did not have MRI in some studies, as shown in Appendix 2.
Modeled estimates
Table 1, and figures 1–5, show results for surgical outcomes in patients with BC of any histology3;14–16;20–31. Table 1 summarizes the models, including the number of subjects in each model, the overall pooled ORs, and the OR estimates stratified around the median study-level age. Subject numbers varied according to the number of studies reporting data for specific surgical outcomes, and also according to whether the analysis applied to all subjects or only those who received initial BCS. Study-specific data and ORs, and estimated pooled ORs, are displayed in Figures 1–4. Figure 5 shows study-specific adjusted ORs, and estimated pooled ORs for the 3 studies reporting on CPM.
Table 1.
Models comparing breast cancer surgical outcomes in subjects who had pre-operative MRI versus those who did not have MRI
| Model comparing surgical outcomes in those who had MRI versus those who did not have MRI [Number of studies in model] | Number of subjects† included in analysis | Pooled Odds Ratio [Referent: No MRI] | Pooled OR stratified by study-level aggregate age§ [Referent: No MRI] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median or mean age < 55 years§ | Median or mean age ≥ 55 years§ | ||||||||
| MRI | No MRI | OR | 95% CI | P value* | OR (95% CI) | P* | OR (95% CI) | P* | |
| Surgical treatment: receipt of mastectomy [15 studies] | 15274 | 70701 | 1.39 | 1.23, 1.57 | < 0.001 | 1.33 (1.09, 1.62) | 0.005 | 1.43 (1.21, 1.68) | < 0.001 |
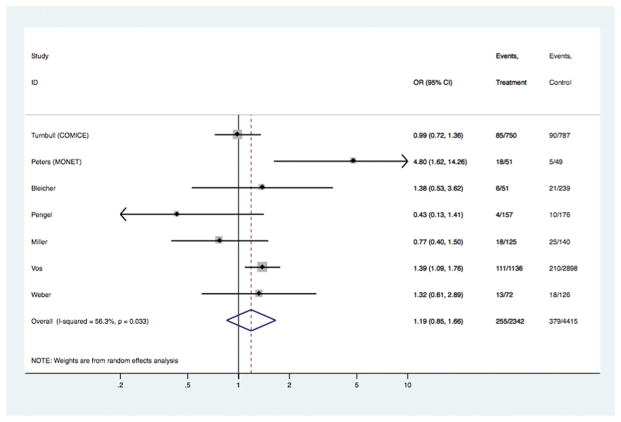
| Re-excision or further local excision, where received BCS [7 studies] | 2342 | 4415 | 1.19 | 0.85, 1.66 | 0.316 | 1.48 (0.74, 3.00) | 0.268 | 1.08 (0.73, 1.60) | 0.712 |
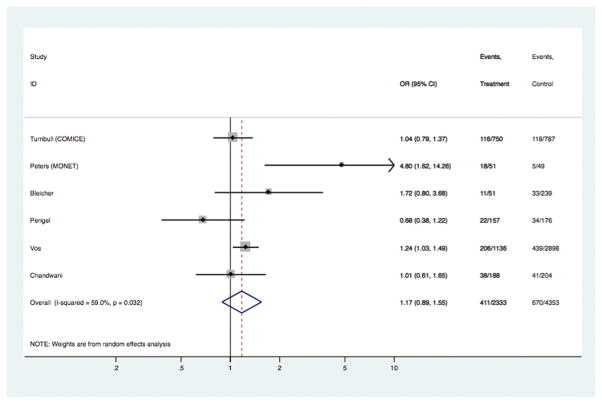
| Positive margins or incomplete excision, where received initial BCS [ 6 studies] | 2333 | 4353 | 1.17 | 0.89, 1.55 | 0.26 | 2.66 (0.98, 7.25) | 0.056 | 1.08 (0.88, 1.31) | 0.467 |
| Any reoperation [10 studies] | 6351 | 23927 | 0.89 | 0.72, 1.10 | 0.272 | 0.88 (0.31, 2.56) | 0.818 | 0.94 (0.78, 1.12) | 0.473 |
| Receipt of contralateral prophylactic mastectomy [3 studies] | 15461 | 110624 | 1.91‡ | 1.25, 2.91 | 0.003 | NA** | - | NA** | - |
Subject numbers varied according to the number of studies contributing outcomes data to each model
P value for model comparing estimates of each outcome in those who had MRI relative to those who did not have MRI (referent group)
Estimates stratified around the median value [55.1 years] for study-level median or mean age
Pooled OR in model calculated directly from adjusted study-specific ORs
NA: not applicable (analysis based on three studies that did not report study-level median or mean age)
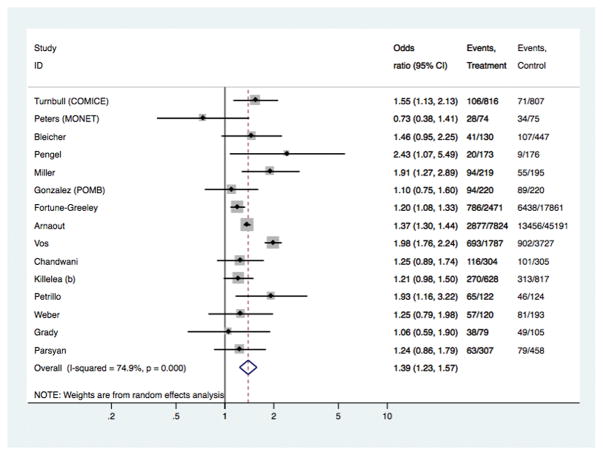
Fig 1.
Models comparing surgical outcomes in breast cancer patients who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for receipt of mastectomy as surgical treatment.
[Study-specific OR for Fortune-Greeley et al was based on the adjusted OR reported in that study]
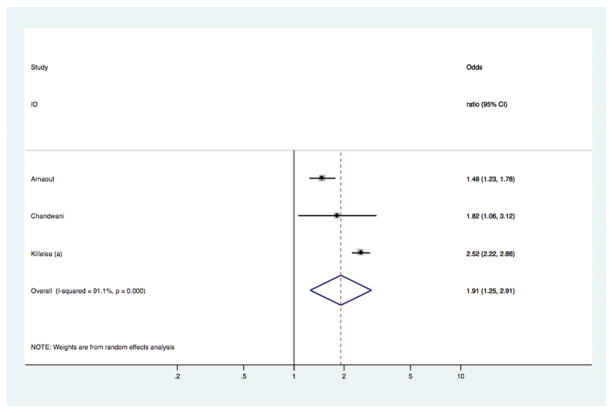
Fig 5.
Models comparing surgical outcomes in breast cancer patients who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for receipt of contralateral prophylactic mastectomy.
[Study-specific adjusted ORs were used in this analysis – see Statistical methods]
Fig 4.
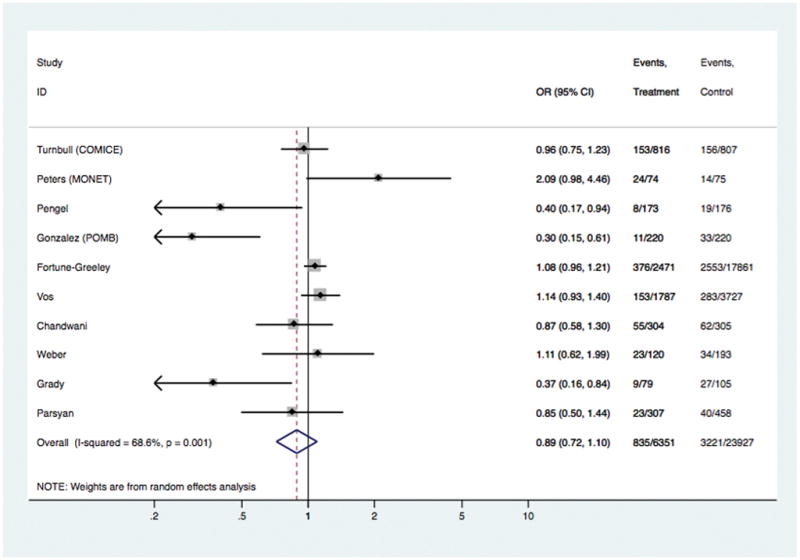
Models comparing surgical outcomes in breast cancer patients who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for ‘any reoperation’.
In the overall analyses (Table 1), there was consistent evidence that MRI was significantly associated with increased odds of receiving mastectomy as treatment for BC [OR 1.39 (95%CI 1.23, 1.57); p<0.001], with similar findings shown in analyses stratified by study-level median age. The association between MRI and receipt of mastectomy was also evident in analyses stratified by timeframe of study recruitment (estimates not shown). There was no statistical evidence that MRI had an effect on the odds of re-excision, or the odds of positive margins in those who received BCS – the point estimate for the latter varied in subgroup analysis (Table 1). Pooled OR for ‘any re-operation’ also showed no effect from pre-operative MRI [OR 0.89 (95%CI 0.72, 1.10); p= 0.272]. Pre-operative MRI significantly increased the odds of receiving CPM [OR 1.91 (95%CI 1.25, 2.91); p= 0.003]
Modeled estimates – subgroup analysis for ILC
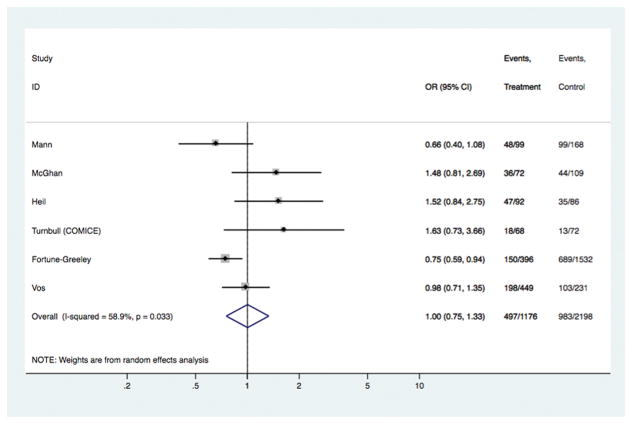
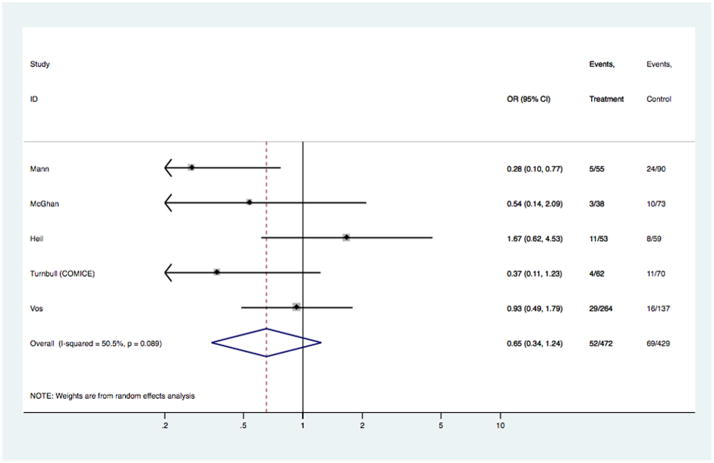
Table 2, and figures 6–7, show results for surgical outcomes in the subgroup of patients with invasive lobular histology based on data from six studies3;14;30;32–34. Table 2 summarizes models for rates of re-excision and mastectomy reported in these studies, including the number of subjects in each model and the pooled ORs; age-stratified analyses were not performed because study-level aggregate age was >54 years across studies. Study-specific data and ORs, and estimated pooled ORs, are displayed in Figures 6–7. There was no evidence of an association between pre-operative MRI and the odds of receiving mastectomy [OR 1.00 (95%CI 0.75, 1.33); p= 0.988] or the odds of re-excision surgery [OR 0.65 (95%CI 0.35, 1.24); p= 0.192]. These results were unchanged in analyses stratified by timeframe of study recruitment. Surgical outcomes for the ILC subgroup that were reported by only one study were not examined in pooled analysis.
Table 2.
Models comparing breast cancer surgical outcomes§ in subjects with invasive lobular cancer who had pre-operative MRI versus those who did not have MRI
| Model comparing surgical outcomes in those who had MRI versus those who did not have MRI [Number of studies in model] | Number of subjects† included in analysis | Pooled Odds Ratio [Referent: No MRI] | |||
|---|---|---|---|---|---|
| MRI | No MRI | OR | 95% CI | P value* | |
| Surgical treatment: receipt of mastectomy [6 studies] | 1176 | 2198 | 1.00 | 0.75, 1.33 | 0.988 |
| Re-excision or further local excision, where received BCS [5 studies] | 472 | 429 | 0.65 | 0.35, 1.24 | 0. 192 |
Surgical outcomes reported by only one study (positive margins; any reoperation) were not examined in pooled analysis
Subject numbers varied according to the number of studies contributing data to each model
P value for model comparing estimates of each outcome in those who had MRI relative to those who did not have MRI (referent group)
Fig 6.
Models comparing surgical outcomes in patients with invasive lobular cancer who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for receipt of mastectomy as surgical treatment.
Fig 7.
Models comparing surgical outcomes in patients with invasive lobular cancer who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for re-excision surgery.
Discussion
Various reviews have discussed the clinical application of pre-operative MRI in newly diagnosed BC patients, including screening of the contralateral breast and monitoring response to neoadjuvant therapy1;4;11;35–37, or have examined its impact on long-term outcomes38. In this work we systematically evaluate and synthesize the evidence on pre-operative MRI and surgical treatment of BC patients to provide updated estimates of the association between receipt of MRI and surgical outcomes. We have focused on studies of invasive BC because a systematic review has recently reported on pre-operative MRI in patients with DCIS35. Our study-level pooled analysis, based on 19 studies that had a control group in the design3;14–16;20–34, did not find any evidence that pre-operative MRI was associated improved surgical outcomes. The primary analysis included 85975 subjects and showed that MRI was associated with increased odds of receiving mastectomy [OR 1.39 (95%CI 1.23, 1.57); p<0.001] for BC treatment. That estimate was essentially unchanged in analyses stratified by median study-level aggregate age, and the association persisted in analyses stratified by study timeframe, indicating that the increased mastectomy rate was not a function of older studies done when MRI–guided biopsy was not widely available.
The increased odds of receiving mastectomy as BC treatment attributed to MRI can only benefit patients if it translates into a decrease in local recurrence rates. However, the evidence clearly shows that pre-operative MRI does not reduce the risk of BC recurrence38. Individual patient data meta-analysis has shown that 8-year local recurrence-free survival did not significantly differ between patients who had MRI and those who did not have MRI (P=0.87), and the associated multivariable models found no effect from MRI on local recurrence-free survival: hazard ratio for MRI (versus no MRI) was 0.88 (P=0.65), whereas age, margin status, and tumor grade were significantly associated with local recurrence-free survival in that meta-analysis38. A recent study with long follow-up similarly found no significant effect from pre-operative MRI: 15-year local failure rates were reported as 8% for patients with and 8% for patients without MRI (P=0.59)39
The secondary analyses we report considered surgical outcomes examined in only some of the studies, including re-excision rates, rates of positive margins or incomplete excision, and re-operation rates; we did not find statistical evidence of a beneficial effect from MRI on any of these end-points in the overall or the stratified analyses (Table 1). The point estimate for positive margins or incomplete excision [OR 2.66 (95%CI 0.98, 7.25), P= 0.056] in studies with younger study-level median age in the stratified analyses may be interpreted as potential harm, however this estimate was short of statistical significance and had wide confidence intervals (Table 1). The lack of a benefit in reducing the need for additional surgery is particularly noteworthy given the increased rate of mastectomy associated with MRI use. If patients with more extensive disease are being appropriately identified pre-operatively with MRI and undergo initial mastectomy, the remainder with relatively limited disease on MRI should be more likely to require only one surgery for successful breast conservation, but this was not the case based on meta-analysis. For the secondary endpoint, CPM, there was evidence that pre-operative MRI was significantly associated with increased odds of receiving contralateral prophylactic mastectomy [OR 1.91 (1.25, 2.91)].
In spite of the continuing lack of evidence that MRI is beneficial in the newly diagnosed BC patient, its use has increased since 200340;41. A recent study of a population- based sample of 377 surgeons (77% response rate) treating BC patients between 2013 and 2015 found that 26% would obtain an MRI for an uncomplicated, screen-detected clinical stage 1 BC, 60% in a BC patient 45 years of age or younger, and 54% for a triple negative BC patient42. Of responding surgeons, 29% incorrectly indicated that MRI decreases the need for re-excision in patients undergoing breast conserving surgery and 41% did not believe that the likelihood of mastectomy was increased by pre-operative MRI42. These findings show that the evidence regarding the lack of benefit of MRI on peri-operative outcomes remains unknown to a substantial proportion of surgeons, and underscores the need for further educational outreach to the surgical community, underpinned by comprehensive and up-to-date synthesis of the literature as provided in our meta-analysis. This work can also be used to support the development of clinical guidelines on this controversial aspect of breast cancer treatment.
Although we had relatively fewer data in the ILC models (3374 ILC cases in the primary analysis, Table 2), the pooled estimates represent the largest comparative analysis in this subgroup of patients in the existing literature, to the best of our knowledge. These analyses did not find any evidence of association between pre-operative MRI and the odds of receiving mastectomy or the odds of re-excision surgery in BC patients with ILC. The present results differ from our earlier systematic review on surgical outcomes from MRI, which had shown that preoperative MRI increased mastectomy rates in ILC – however the updated analysis included more studies. A substantial proportion of surgeons (72%) in the survey study discussed above favour the use of MRI for patients with ILC42. While this is understandable given the tendency of mammography to underestimate the extent of lobular carcinoma43, our meta-analysis does not indicate an improvement in surgical outcomes in women with ILC undergoing the test, suggesting that it is an unnecessary cost44 when used routinely based on a diagnosis of ILC. Some of the confusion regarding pre-operative indications for MRI stems from guidelines that were predominantly based on expert opinion10, promulgating its use for pre-operative evaluation of the ipsilateral and contralateral breast, even while radiologists acknowledge that the level of consensus on its use in patients with invasive ductal cancer undergoing primary surgery is low45. Now that a large body of evidence from comparative studies exists and has been synthesized in this meta-analysis, indicating that pre-operative MRI is not associated with improved surgical treatment, and given the broader health context of appropriate use of medical services that also recognises drivers of ineffective practice46;47, it is time to question the routine use of pre-operative MRI for BC patients.
Limitations of this meta-analysis include the heterogeneity between groups and across studies, and that most studies used a comparator group rather than randomization, with only 3 RCTs contributing data into pooled estimates – hence bias and confounding cannot be eliminated, and interpretation of our findings should factor in the heterogeneity between studies. Furthermore, inconsistent reporting of study-level data for variables such as age and tumour size (Appendix 2) limited the scope for statistical adjustments. However, our main analyses (studies of all BC histological types) used stratification around the median study-level aggregate age to allow for the observed imbalance in median or mean age amongst some of the non-randomized studies. Our estimates therefore represent the most comprehensive evidence summary on preoperative MRI and surgical outcomes, despite the above-mentioned limitations inherent in study-level meta-analysis. Use of individual person data meta-analysis would allow some of these issues to be better addressed38, however this was not feasible to undertake for this pooled study.
This meta-analysis of studies comparing surgical outcomes in BC patients who received pre-operative MRI and those who did not receive MRI shows that the use of pre-operative MRI is not associated with improved surgical treatment. The outcome associated with pre-operative MRI is an increased likelihood of receipt of mastectomy for the cancerous breast, and increased likelihood of contralateral prophylactic mastectomy, as surgical treatment in newly diagnosed BC patients. Our findings can be used to guide breast surgical practice, to inform transparent discussion with BC patients on the consequences of having pre-operative MRI, and to assist in the development of evidence-based clinical guidelines on pre-operative testing in newly affected women.
Supplementary Material
Fig 2.
Models comparing surgical outcomes in breast cancer patients who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for re-excision surgery in those who had breast conservation.
Fig 3.
Models comparing surgical outcomes in breast cancer patients who had pre-operative MRI versus those who did not have MRI: study-specific and pooled odds ratios for positive margins in those who had breast conservation.
Acknowledgments
NH receives funding via a National Breast Cancer Foundation (Australia) Breast Cancer Research Leadership Fellowship.
Footnotes
Disclosures/COI: None to declare.
Reference List
- 1.Morrow M, Waters J, Morris E. MRI for breast cancer screening, diagnosis, and treatment. Lancet. 2011;378:1804–1811. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 2.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA: a Cancer Journal for Clinicians. 2009;59:290–302. doi: 10.3322/caac.20028. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 4.Pilewskie M, King TA. Magnetic resonance imaging in patients with newly diagnosed breast cancer: a review of the literature. Cancer. 2014;120:2080–2089. doi: 10.1002/cncr.28700. [DOI] [PubMed] [Google Scholar]
- 5.Jatoi I, Benson JR. The case against routine preoperative breast MRI. [Review] Future Oncology. 2013;9:347–353. doi: 10.2217/fon.12.186. [DOI] [PubMed] [Google Scholar]
- 6.Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Annals of Surgery. 2013;257:249–255. doi: 10.1097/SLA.0b013e31827a8d17. [DOI] [PubMed] [Google Scholar]
- 7.Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. Journal of Clinical Oncology. 2008;26:386–391. doi: 10.1200/JCO.2006.09.5448. [DOI] [PubMed] [Google Scholar]
- 8.Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007;16:Suppl-44. doi: 10.1016/j.breast.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Morrow M. Magnetic resonance imaging in the breast cancer patient: curb your enthusiasm. Journal of Clinical Oncology. 2008;26:352–353. doi: 10.1200/JCO.2007.14.7314. [DOI] [PubMed] [Google Scholar]
- 10.Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. European Journal of Cancer. 2010;46:1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Brennan ME, Houssami N, Lord S, et al. Magnetic resonance imaging screening of the contralateral breast in women with newly diagnosed breast cancer: systematic review and meta-analysis of incremental cancer detection and impact on surgical management. Journal of Clinical Oncology. 2009;27:5640–5649. doi: 10.1200/JCO.2008.21.5756. [DOI] [PubMed] [Google Scholar]
- 12.Ciatto S. Pre-operative breast MRI: what do women want? Breast. 2010;19:435–436. doi: 10.1016/j.breast.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. Journal of Clinical Oncology. 2008;26:3248–3258. doi: 10.1200/JCO.2007.15.2108. [DOI] [PubMed] [Google Scholar]
- 14.Fortune-Greeley AK, Wheeler SB, Meyer AM, et al. Preoperative breast MRI and surgical outcomes in elderly women with invasive ductal and lobular carcinoma: a population-based study. Breast Cancer Research & Treatment. 2014;143:203–212. doi: 10.1007/s10549-013-2787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez V, Sandelin K, Karlsson A, et al. Preoperative MRI of the breast (POMB) influences primary treatment in breast cancer: a prospective, randomized, multicenter study. World Journal of Surgery. 2014;38:1685–1693. doi: 10.1007/s00268-014-2605-0. [DOI] [PubMed] [Google Scholar]
- 16.Petrillo A, Porto A, Fusco R, et al. Surgical impact of preoperative breast MRI in women below 40 years of age. Breast Cancer Research & Treatment. 2013;140:527–533. doi: 10.1007/s10549-013-2651-6. [DOI] [PubMed] [Google Scholar]
- 17.Landercasper J, Bailey L, Berry TS, et al. Measures of Appropriateness and Value for Breast Surgeons and Their Patients: The American Society of Breast Surgeons Choosing Wisely Initiative. Annals of Surgical Oncology. 2016;23:3112–3118. doi: 10.1245/s10434-016-5327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gradishar WJ, Anderson BO, Balassanian R, et al. Invasive Breast Cancer Version 1. 2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2016;14:324–354. doi: 10.6004/jnccn.2016.0037. [DOI] [PubMed] [Google Scholar]
- 19.American College of Radiology (ACR) ACR practice guideline for the performance of contrast-enhanced magnetic resonance imaging (MRI) of the breast. 2008 doi: 10.1016/j.jacr.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Peters NH, van ES, van den Bosch MA, et al. Preoperative MRI and surgical management in patients with nonpalpable breast cancer: the MONET - randomised controlled trial. European Journal of Cancer. 2011;47:879–886. doi: 10.1016/j.ejca.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. Journal of the American College of Surgeons. 209:180–187. doi: 10.1016/j.jamcollsurg.2009.04.010. 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pengel KE, Loo CE, Teertstra HJ, et al. The impact of preoperative MRI on breast-conserving surgery of invasive cancer: a comparative cohort study. Breast Cancer Research & Treatment. 2009;116:161–169. doi: 10.1007/s10549-008-0182-3. [DOI] [PubMed] [Google Scholar]
- 23.Miller B, Abbott A, Tuttle T. The Influence of Preoperative MRI on Breast Cancer Treatment. Annals of Surgical Oncology. 2012;19:536–540. doi: 10.1245/s10434-011-1932-8. [DOI] [PubMed] [Google Scholar]
- 24.Grady I, Gorsuch-Rafferty H, Hadley P. Preoperative staging with magnetic resonance imaging, with confirmatory biopsy, improves surgical outcomes in women with breast cancer without increasing rates of mastectomy. Breast Journal. 2012;18:214–218. doi: 10.1111/j.1524-4741.2012.01227.x. [DOI] [PubMed] [Google Scholar]
- 25.Weber JJ, Bellin LS, Milbourn DE, Verbanac KM, Wong JH. Selective preoperative magnetic resonance imaging in women with breast cancer: no reduction in the reoperation rate. Archives of Surgery. 2012;147:834–839. doi: 10.1001/archsurg.2012.1660. [DOI] [PubMed] [Google Scholar]
- 26.Killelea BK, Long JB, Chagpar AB, et al. Trends and clinical implications of preoperative breast MRI in Medicare beneficiaries with breast cancer. Breast Cancer Research & Treatment. 2013;141:155–163. doi: 10.1007/s10549-013-2656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killelea BK, Grube BJ, Rishi M, Philpotts L, Tran EJ, Lannin DR. Is the use of preoperative breast MRI predictive of mastectomy? World Journal of Surgical Oncology. 2013;11:154. doi: 10.1186/1477-7819-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandwani S, George PA, Azu M, et al. Role of preoperative magnetic resonance imaging in the surgical management of early-stage breast cancer. Annals of Surgical Oncology. 2014;21:3473–3480. doi: 10.1245/s10434-014-3748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnaout A, Catley C, Booth CM, et al. Use of Preoperative Magnetic Resonance Imaging for Breast Cancer: A Canadian Population-Based Study. JAMA Oncology. 2015;1:1238–1250. doi: 10.1001/jamaoncol.2015.3018. [DOI] [PubMed] [Google Scholar]
- 30.Vos EL, Voogd AC, Verhoef C, Siesling S, Obdeijn IM, Koppert LB. Benefits of preoperative MRI in breast cancer surgery studied in a large population-based cancer registry. British Journal of Surgery. 2015;102:1649–1657. doi: 10.1002/bjs.9947. [DOI] [PubMed] [Google Scholar]
- 31.Parsyan A, Moldoveanu D, Balram B, et al. Influence of preoperative magnetic resonance imaging on the surgical management of breast cancer patients. American Journal of Surgery. 2016;211:1089–1094. doi: 10.1016/j.amjsurg.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Mann RM, Loo CE, Wobbes T, et al. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Research & Treatment. 2010;119:415–422. doi: 10.1007/s10549-009-0616-6. [DOI] [PubMed] [Google Scholar]
- 33.McGhan LJ, Wasif N, Gray RJ, et al. Use of preoperative magnetic resonance imaging for invasive lobular cancer: good, better, but maybe not the best? Annals of Surgical Oncology. 2010;17:Suppl-62. doi: 10.1245/s10434-010-1266-y. [DOI] [PubMed] [Google Scholar]
- 34.Heil J, Buhler A, Golatta M, et al. Does a supplementary preoperative breast MRI in patients with invasive lobular breast cancer change primary and secondary surgical interventions? Annals of Surgical Oncology. 2011;18:2143–2149. doi: 10.1245/s10434-011-1565-y. [DOI] [PubMed] [Google Scholar]
- 35.Fancellu A, Turner RM, Dixon JM, Pinna A, Cottu P, Houssami N. Meta-analysis of the effect of preoperative breast MRI on the surgical management of ductal carcinoma in situ. British Journal of Surgery. 2015;102:883–893. doi: 10.1002/bjs.9797. [DOI] [PubMed] [Google Scholar]
- 36.Marinovich ML, Houssami N, Macaskill P, et al. Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. Journal of the National Cancer Institute. 2013;105:321–333. doi: 10.1093/jnci/djs528. [DOI] [PubMed] [Google Scholar]
- 37.Marinovich ML, Macaskill P, Irwig L, et al. Meta-analysis of agreement between MRI and pathologic breast tumour size after neoadjuvant chemotherapy. British Journal of Cancer. 2013;109:1528–1536. doi: 10.1038/bjc.2013.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houssami N, Turner R, Macaskill P, et al. An individual person data meta-analysis of preoperative magnetic resonance imaging and breast cancer recurrence. Journal of Clinical Oncology. 2014;32:392–401. doi: 10.1200/JCO.2013.52.7515. [DOI] [PubMed] [Google Scholar]
- 39.Vapiwala N, Hwang WT, Kushner CJ, Schnall MD, Freedman GM, Solin LJ. No impact of breast magnetic resonance imaging on 15-year outcomes in patients with ductal carcinoma in situ or early-stage invasive breast cancer managed with breast conservation therapy. Cancer. 2017;123:1324–1332. doi: 10.1002/cncr.30479. [DOI] [PubMed] [Google Scholar]
- 40.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Internal Medicine. 2014;174:114–121. doi: 10.1001/jamainternmed.2013.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wernli KJ, DeMartini WB, Ichikawa L, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Internal Medicine. 2014;174:125–132. doi: 10.1001/jamainternmed.2013.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrow, Abrahamse P, Hofer TP, Ward KC, et al. Trends in reoperation after initial lumpectomy for breast cancer: Addressing overtreatment in surgical management. Annals of Surgical Oncology. 2017 doi: 10.1001/jamaoncol.2017.0774. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan ME, McKessar M, Snook K, Burgess I, Spillane AJ. Impact of selective use of breast MRI on surgical decision-making in women with newly diagnosed operable breast cancer. Breast. 2017;32:135–143. doi: 10.1016/j.breast.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Onega T, Tosteson AN, Weiss J, et al. Costs of diagnostic and preoperative workup with and without breast MRI in older women with a breast cancer diagnosis. BMC Health Services Research. 2016;16:76. doi: 10.1186/s12913-016-1317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knuttel FM, Menezes GL, van den Bosch MA, Gilhuijs KG, Peters NH. Current clinical indications for magnetic resonance imaging of the breast. Journal of Surgical Oncology. 2014;110:26–31. doi: 10.1002/jso.23655. [DOI] [PubMed] [Google Scholar]
- 46.Saini V, Garcia-Armesto S, Klemperer D, et al. Drivers of poor medical care. Lancet. 2017 Jan 06;2017 doi: 10.1016/S0140-6736(16)30947-3. [DOI] [PubMed] [Google Scholar]
- 47.Brownlee S, Chalkidou K, Doust J, et al. Evidence for overuse of medical services around the world. Lancet. 2017 Jan 06;2017 doi: 10.1016/S0140-6736(16)32585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.