Abstract
As RNA virus mutation occurs during replication within host cells, we hypothesized that viral evolution during acute infections in healthy hosts reflects host immune pressure. We therefore investigated the within-host diversification of human respiratory syncytial virus (RSV), a highly prevalent cause of acute respiratory infections. We evaluated healthy adults experimentally infected with an identical inoculum and infants hospitalized with naturally acquired infections. In aggregate, viral diversification in adults peaked at day 3, with overrepresentation of diversity in the matrix protein 2 (M2) and non-structural protein 2 (NS2) genes. In one subject, delayed viral clearance was accompanied by a late peak of diversity at day 10 in known and predicted B and T cell epitopes. In contrast, infant infections showed much less viral diversity. Our findings suggest multiple overlapping mechanisms for early control of acute viral infections, which may differ between age groups and host immune responses.
Keywords: RSV, within-host, diversity, deep sequencing, genomics, viral evolution
INTRODUCTION
RSV is a major cause of respiratory disease in young children (1) and one of the greatest single causes of infant mortality worldwide (2). It is also a major cause of disease in the elderly and those with comorbid conditions (3). In older children and healthy adults, infection with RSV usually results in a milder, acute infection (4). There is no animal reservoir of human RSV. Human immune responses are functionally poor and short-lived, and individuals can be re-infected throughout life (5, 6). Human RSV is comprised of two antigenic subtypes, RSV-A and B, defined by differences in the sequence of the attachment (G) gene, and there are multiple genotypes within each subtype (7). Multiple RSV strains co-circulate, with replacement of the dominant strains in successive seasons, suggesting the impact of population-wide immune pressures (8, 9).
In a previous study, we investigated the within-host diversification of RSV over the course of an individual prolonged infection in an immunocompromised infant suffering from severe combined (T and B cell) immunodeficiency, sampling multiple time points both before and after bone marrow engraftment from his RSV-immune father (10). We showed the existence of within-host viral diversity in the absence of adaptive immunity, an increased rate of diversification in the presence of adaptive immunity, and an overrepresentation of diversity in the G protein. While this study confirmed the dynamic nature of viral diversity and supported the hypothesis that measurable viral diversity increases in the presence of immune pressure, conclusions from this study were limited due to absence of information about the inoculating virus, the fact that this was an analysis of only a single infection, and the abnormally weak, though uniquely informative, host immune function.
Here, we expand these observations to normal human immune responses by analyzing longitudinal within-host data from two cohorts: (a) healthy immune-experienced adults experimentally infected with the same genetically well-characterized ultra-low-passaged RSV-A virus (11) and (b) infants hospitalized with severe manifestations of community-acquired RSV-A infection. These datasets allow us to ask how much diversity emerges over the course of infections in healthy adult hosts; how variable it is across hosts, when controlling for infection with the same virus; as well as how much diversity emerges in infants with severe manifestations of infection. Further, our data sets allow comparison of observed viral diversity in adults, all of whom have prior RSV exposure, and in infants experiencing their first RSV infection. Similar time points within each study subjects’ infection can be analyzed by time from infection and from symptom onset.
MATERIALS AND METHODS
Subjects and RSV sample preparation
Nasal aspirates were obtained prospectively and quantitatively by designated study personnel as described (12–14) and maintained at −80°C. A total of 20 healthy adults were each experimentally infected with a total of 500 μL of Memphis-37b per nares (104 PFU/mL) and monitored as described previously (13, 15). All studies were conducted with Ethics Committee and Institutional Review Board approval and informed consent as appropriate, complying with all relevant guidelines and policies. Viral RNA was extracted from the thawed samples using a QIAsymphony DSP virus/pathogen kit (Qiagen) per the manufacturer’s protocol, including the provided synthetic poly(A). Viral RNA was eluted with 60μL of AVE buffer and stored at −80°C. All samples were treated with Turbo DNase (Life Technologies) using the manufacturer’s rigorous treatment to ensure removal of DNA. Viral loads were determined by a validated two-step reverse transcriptase PCR as previously described (16). By comparing birthdate, age at infection, and local RSV seasonality (defined as Nov 1–April 15), all infants were determined to be experiencing their first RSV-A infection. The time point of infection for the adults was defined as the start of the first sustained detectable viral load. The time point of infection for the infants was defined as the start of the first-occurring respiratory symptoms, as collected at study subject enrollment by a designated team of investigators during care-giver interview on the day of enrollment as described (14); note the infant specimens are distinct from those previously reported from an immunocompromised infant (10).
Deep sequencing and variant calling
Samples with viral loads above approximately 105 PFUe/mL for adults and all samples for infants were submitted for sequencing. For all infant and adult samples as well as the inoculum strain used for adult experimental infections (Memphis-37b), RNA amplification and sequencing via the Illumina platform were performed as described previously (10, 17). The reads were assembled using the VICUNA assembly program (18) and variant calling was performed using V-Phaser 2 (19) to compare reads to consensus sequences. Sensitivity of the variant calling process was improved by reducing the effects of sequencing error at the tail ends of reads; V-Phaser 2 was run with the parameter of -i 15. All other parameters were set to software default. For the adults, reads were compared to a consensus sequence generated from an aggregate consensus of all the Memphis-37b infected adult samples. Because each infant dataset represented a separate natural infection, a viral consensus sequence was constructed for each individual infant (see GenBank BioProject number PRJNA227457). Each consensus sequence was assembled using reads from the earliest sample in the time course with the highest genome coverage.
Diversity analysis
Calculation of diversity
For each time point within an individual infant or adult dataset, the amino acid frequencies at each residue position in the viral genome were determined by V-Phaser 2; only amino acid sites with high quality coverage of at least 100x were considered. Diversity of the viral population was calculated at a position at all time points in an individual study subject dataset if the frequency of the dominant amino acid was below 95% at any time point in that dataset. Diversity at variant positions was calculated as the Shannon entropy index, Si = −Σapa,ilog2pa,i where pa,i is the frequency of amino acid a at position i and Si is in units of bits. The overall diversity for each time point was calculated by summing Si over all variant positions that met criteria.
Estimation of transmission bottleneck size
For the adult datasets that shared the amino acid variant present in the inoculum, the effective viral population size during the transmission bottleneck was calculated with the sample from the first time point, using previously described methods (20).
Identification of known and predicted T and B cell epitopes
Predicted CD4 and CD8 T cell epitopes were previously identified by Kim et al. (11). Predicted B cell epitopes were identified and compiled from the literature (21–24). The variants observed in study subject G13 and G14 were represented using Circos (25).
Bootstrapping method for calculation of significance of overrepresentation
To determine if the set of variants across all infant samples and the set of variants across all adult samples were overrepresented in specific genes or in epitope regions, bootstrap simulations were run. In each of the total 10,000 simulations, the same number of variants as the observed set was randomly selected from all possible genome codon positions without replacement. P-values were calculated from the distribution of sites from the simulations. Statistical analysis were performed in Python and the R computing environment (26).
Comparison of adult and infant diversity
To compare the viral diversity in the infant and adult populations over time, we first matched the samples by inferring time from infection. Since symptom onset in adults generally occurs at the time that RSV is first detectable (15) and since the infant samples analyzed for diversity are from at least day 3 after the reported onset of symptoms (other than a single time point), we compared the adult and infant diversity starting on day 3 for both datasets. Further, for the purposes of this analysis, we made the simplifying assumption that the adult and infant data points could be treated as comparable. The PM samples for adults were assigned to midpoints between days (e.g., Day 2 PM = 2.5). The combined data were fit to a regression model with an indicator variable for adult vs infant samples (Y = β0 + β1X1 + β2X2 + β3X1X2).
Phylogenetic analysis of RSV consensus sequences
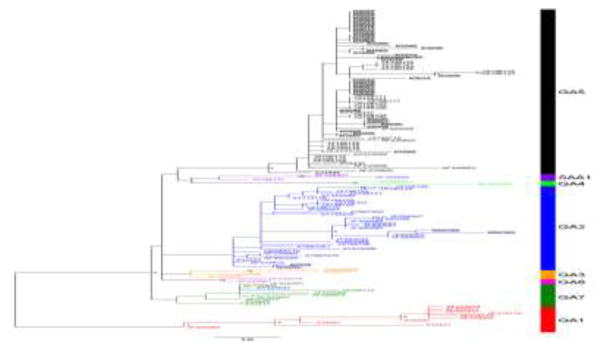
Genotypes of the 43 infant consensus sequences and the Memphis-37b consensus sequence were identified by comparing the second hypervariable region (last 270 nucleotides) of the G gene. Previously characterized RSV-A clinical isolates retrieved from GenBank for comparison included the 63 isolates from literature references and 31 original isolates described in Tapia et al. (7) MUSCLE multiple sequence alignment and phylogenetic tree construction were performed in MEGA6 (27). The maximum likelihood tree was constructed using the Hasegawa-Kishino-Yano substitution model and the combined gamma distribution and invariant sites model for rate variation, and tested with 1,000 bootstrap replicates. The tree was rooted using the ATCC VR-26 Long strain as the outgroup and visualized with FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
RESULTS
We deep sequenced the virus preparation (Memphis-37b) administered to the healthy adult subjects to define the extent of diversity within the inoculum, achieving an average depth of >7000x. Only a single amino acid residue (L protein position 176) was noted to have a minor population greater than 5% (82% N, 18% H). The observation of diversity at this site is consistent with previously published diversity described in a sister virus (Memphis-37c) sequenced by conventional Sanger methods (11) (Supplemental Figure 1).
We experimentally inoculated 20 healthy adults with a total of 500 μL of Memphis-37b per nares (104 PFU/mL) and monitored them with twice daily nasal wash collections. We successfully obtained deep sequence data from samples from 19/20 (95%) subjects (previously described placebo recipients) (13), comprising 155/166 (93%) of the samples sequenced (selected on the basis of viral load >105 PFUe/mL). We found diversity at site L-176 in 11/19 (58%) study subjects, allowing us to estimate the effective viral population size for the transmission bottleneck. The lower bound for the effective bottleneck size had a mean of 25 viral particles and a standard deviation of 35 viral particles (Supplemental Figure 2).
Over the first few days of infection, diversity in the viral population was high compared to the inoculum (Figure 1). Diversity subsequently decreased, with a late rise at day 10 attributable mostly to a single study subject (G13). We did not observe a strong correlation between diversity and viral load, indicating that our detection of diversity was not biased toward samples with higher viral titers (Supplemental Figure 3). Overall in these data sets, variants in the non-structural protein 2 (NS2; p=0.015) and matrix protein 2 (M2; p=0.01) were overrepresented (Supplemental Figure 4).
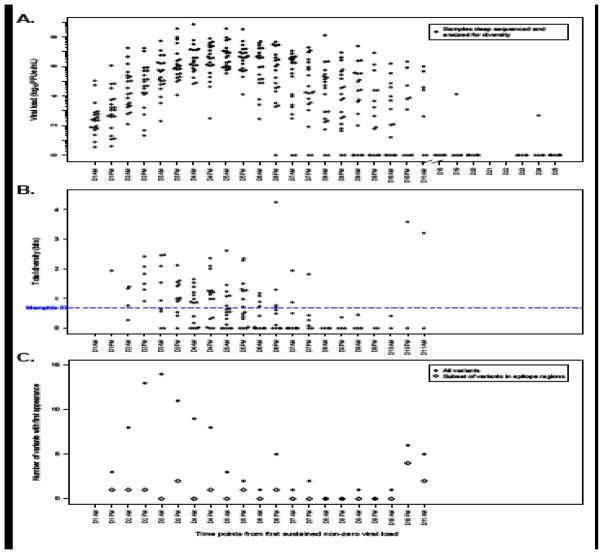
Figure 1.
Results from adult cohort experimentally infected with RSV strain Memphis-37b. A. Viral loads dated from the first sustained non-zero viral load per individual study subject infection for the adult experimental infection cohort. All samples from the study subjects are represented. Closed circles represent those samples successfully deep sequenced and analyzed for diversity. B. Total diversity (measured with the Shannon entropy index in units of bits), aggregated for all study subject samples. The dashed blue line reports the diversity determined from deep sequencing the Memphis-37b inoculum. C. The number of variants with first appearance by time point, aggregated for all study subject samples, is represented in closed circles. The variants with their first appearance that are present in known or predicted human T-cell or B-cell epitope regions are represented in open diamonds.
Plotting the aggregate number of variants with first appearance by time from infection revealed a large initial wave of variation peaking at day 3, a subsequent steady decrease in variation, a single time point with an elevated number of new variants at day 6, and a second peak at day 10 (Figure 1C). The diversity rise at day 6 was mostly due to study subject G14, and the rise at day 10 was predominantly attributable to study subject G13 (Figures 1C, 2). While all variants observed in each subject are presented in Supplemental Table 1, here we report our detailed findings for subjects G13 and G14 because they showed enduring viral responses.
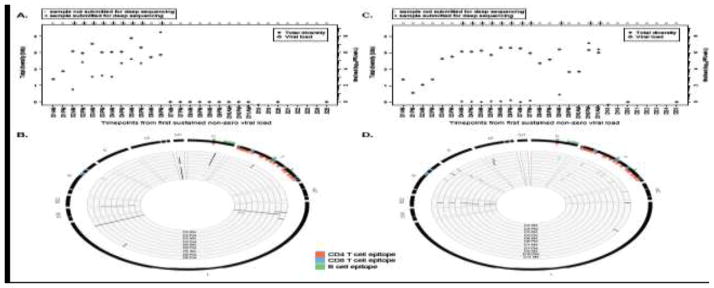
Figure 2.
Analysis of results for two study subjects in the adult experimental infection cohort. A. Plot of total diversity (closed circles) for those samples with deep sequence data (indicated by “+”) and viral load (open diamonds) over time for study subject G14. Time points without closed circles reflect absence of deep sequencing data with which to calculate diversity. B. Circos plot of locations of variant sites for study subject G14. The innermost circle represents the earliest time point with deep sequencing data and evaluation of diversity, with surrounding circles incrementing by time points with diversity data. The outermost circle represents the RSV-A genome, with known and predicted epitope sites identified (orange=CD4 T cell epitopes; blue=CD8 T cell epitopes; green=B cell epitopes). C and D. Similar to figures A and B but for subject G13.
Evaluation of the dynamics of diversity and viral load in individuals G13 and G14 showed prolonged high viral loads. In subject G14, the diversity rose until day 6, at which point the viral load became abruptly undetectable (Figure 2A). Analysis of the sites of diversity in the genome indicated that the number of diverse sites was greatest at the last time point during which the virus was detectable, with sites appearing in a previously-recognized epitope site in the N protein as well as a site in the F protein and two sites in L protein (Figure 2B) that were not within known human T or B cell epitopes. In subject G13, limited diversity was observed until days 10–11, after a brief decline in viral load (Figure 2C). Analysis of the genomic positions of diversity revealed that the diversity at these time points was predominantly in known and predicted B and T cell epitopes; moreover, the variants that emerged within subject G13 at the day 10 evening sample were undetectable at the day 11 morning sample, and were replaced by variants in other epitope sites (Figure 2D).
Nearly all the variants observed in the adults remained minor populations, other than at L-176, the site with variation in the inoculum. Only two observed variants (at amino acid positions M2-193 and L-2072 in subjects G8 and G14, respectively) became dominant, illustrating the inability of consensus sequencing to detect and characterize true diversification dynamics.
We also evaluated a cohort of 62 infants experiencing their first RSV-A infection and with symptoms severe enough to require hospitalization. Of these, 55 were enrolled from the years 2001–2004 and 7 from 2013 (Supplemental Table 2). A total of 229 nasal aspirate samples collected over 20 days from onset of symptoms had non-zero viral load by quantitative PCR (Figure 3A). Directly sequencing from the original specimens themselves, without passage of virus in tissue culture, we obtained deep sequencing data for 43 of the infants (69%), including 102/157 samples (65%) with viral load above 105 PFUe/mL. Phylogenetic analysis of the G protein gene sequence indicated that most viruses were in clade GA5, with two from 2001 and the two from 2013 in GA2 (Figure 4).
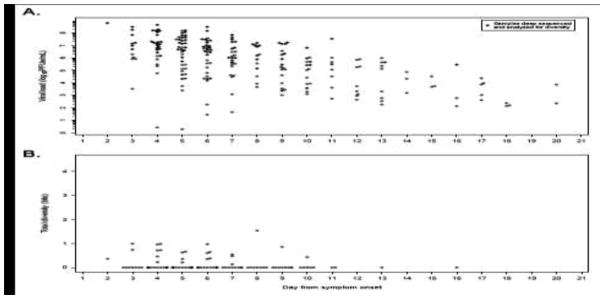
Figure 3.
Results from the hospitalized infant cohort. A. Viral loads timed from the reported onset of symptoms. Closed circles represent those samples successfully deep sequenced and analyzed for diversity. B. Total diversity (measured by Shannon entropy), aggregated for all hospitalized infant study subject samples.
Figure 4.
Maximum likelihood phylogeny of RSV-A using the second hypervariable region of the G protein from representative consensus sequences for the infant cohort, along with other representative samples from the literature. Samples from the study subjects presented here are highlighted in grey background. Genotype is indicated in the bar on the right. Asterisks indicate bootstrap values > 75%. See GenBank BioProject number PRJNA227457 for the sequences of the samples obtained in this study.
We detected much less diversity in the infants as compared to the adults (Figures 1B and 3B), with a total of 14 divergent sites in infants as compared to 71 within the adults (Supplemental Figure 5). Within the infant dataset, there is a trend towards overrepresentation of variants in G protein (p=0.06), with each of the G protein variants appearing in previously recognized epitope sites. Whereas diversity in adults appeared in multiple previously identified T and B cell epitopes, viral diversity in the infants was only present at one CD4 T cell epitope and two B cell epitopes.
DISCUSSION
Here, we present the first analysis of within-host longitudinal RSV diversity in acute human infections, evaluating samples from experimentally infected adults and naturally infected infants. In the adults, our estimate of an infection bottleneck of 25 virions is lower than the bottleneck sizes of other viruses that cause acute infections. The bottleneck size for Ebolavirus is an average of 100 virions, with a range of around 1–1000 virions (20), and the mean bottlenecks for influenza H1N1/2009 and H3N2 are 90 virions and 144 virions, respectively, with a standard deviation of 55 virions for both (28) (Supplemental Figure 2).
As the adults were infected by the same virus preparation, the observed viral diversity not shared with the inoculum is likely attributable to differences among the hosts, rather than pathogen. It may be that undetected diversity within the inoculum contributed to the observed diversity, with decreasing diversity over time reflecting the impact of purifying selection, in keeping with observations from human influenza virus challenge studies (29). However, the appearance of new variants peaks on day 3 (Figure 1BC) and then again later in the infection in study subjects G13 and G14 (Figure 2). This observation contrasts with the hypothesis’ prediction of steadily decreasing diversity over time. Given prior observations of viral diversification in response to humoral and cellular immunity, we hypothesize that each wave of observable diversity within the experimental adult infections is driven by host immune pressure.
During times of greatest viral load decline, the variant sites predominate in NS2 and M2 genes and not in previously recognized human epitope sites in the majority of the adult specimens. In contrast, the observations that the diversity at d10 in study subject G13 is predominantly in known and predicted B and T cell epitope sites and that the epitope sites vary from one time point to the next suggest sequential episodes of functionally effective immune pressure followed by attempted viral escape. The diversity observed in epitope sites in this study subject may reflect increasingly active adaptive immune responses, which drove viral disappearance at subsequent time points. For the remainder of the study subjects, alternative immune mechanisms, including innate immune mechanisms, may be primarily responsible for early viral load reductions. NS2 is known to be involved in modulating human host interferon responses (30), and a T cell epitope in M2 has been demonstrated in a mouse model of RSV infection to be a major target of CD8+ T cells (31). It is possible that viral diversity may be occurring in as yet unidentified human T and B cell epitopes within these regions. Alternatively, NS2 and M2 may be involved in adapting to the human host after passage in cell culture. Additionally, the recent observations that the RSV-innate immune response of apoptosis and viral-induced epithelial cell detachment (32, 33) may be principally causing early RSV viral load reduction suggest other pressures that may act on the virus. Further investigation into these mechanisms and the inferred heterogeneity in host response will be critical for understanding RSV pathogenesis and immune responses.
Infants demonstrated less viral diversity than adults. This was true even when using a time-delimited subset of the adult samples that were matched to the infant samples on the basis of the timing of illness onset for the infants (see Methods, Supplemental Figure 6). Additionally, the infants had higher peak viral loads and longer duration of shedding (Figures 1 and 3), an adult vs. pediatric clinical virology difference that has been observed previously (34). These different patterns may be a consequence of differing immunity. The infants’ dates of birth and the dates of the local RSV epidemics suggest that the infections were their first with RSV (see Materials and Methods). These infants therefore lack pre-existing adaptive immunity; this is in contrast with the adults, who have likely had multiple prior RSV infections. The trend towards significant overrepresentation in the infant infections of diversity in the surface G protein suggests that humoral immunity may play an important role in early infant immune response to RSV and is consistent with findings from a recent study (35). Alternatively, the limited diversity in infants compared to the adults at corresponding time points may reflect other factors, including inadequate correction for the differences in timing of sample collection and variation attributable to the distinct viruses that infected the infants (Figure 4) and the Memphis 37 viral preparation used to infect the adults. Further, recent findings have suggested that bottleneck sizes may vary across transmission events (36); as such, differences in the effective viral population size in experimental versus natural inoculums and in the size of the bottleneck in the challenge model as compared to natural transmission in infants could also account for the observed differences. Thus, these differences suggest important future directions for investigation into the forces that influence within-host diversity over time.
These results have general implications for the study of immune response to viral infections and reconstruction of transmission and epidemic spread. First, longitudinal within-host characterization of viral populations provides a lens with which to view the impact of immune pressure for rapidly mutating pathogens and hence establishes a method to examine the factors contributing to (a) initial viral diversification and immune control; (b) variation among hosts; (c) the relationship between variable responses and clinical outcome. As such, this approach may assist in identifying functional immune epitopes for targeted vaccines. Additionally, it may aid in development of predictive metrics for specific immune responses linked to severe disease outcomes or inadequate immune protection from vaccination or natural infection.
Second, deep sequencing of a single time point is inadequate to represent true viral diversity over the course of an infection and in populations with diverse immune responses. Analyses reliant on estimates of within-host viral diversity, such as for reconstruction of transmission and relating within-host viral diversification with population-wide diversity, would benefit from consideration of how viral diversity changes over time as a function of individual hosts.
Overall, this study demonstrates the utility of longitudinal viral sampling over the course of individual infections in understanding the evolutionary dynamics of RNA viruses during acute infections of individual human hosts. In conjunction with assays defining host immunity, including HLA typing and B and T cell profiling, these analyses advance understanding of the factors contributing to heterogeneous responses to infections.
Supplementary Material
Highlights.
Most adults cleared RSV quickly, with diversity overrepresented in NS2 and M2.
Delayed viral clearance was associated with diversity in B and T cell epitopes.
Infant infections showed much less diversity.
Our data suggest overlapping mechanisms for early control of acute viral infections.
These may differ between age groups and host immune responses.
IMPORTANCE.
While studies of acute infections by RNA viruses have shown viral evolution at a population level, little is known about viral diversification during individual infections. Here, we investigated the diversification of respiratory syncytial virus (RSV), a common cause of acute respiratory infections, in experimentally infected immunocompetent adults and naturally infected infants without preexisting humoral immunity. Using deep sequencing, we show that RSV diversification is heterogeneous, with early changes in viral NS2 and M2 genes and later diversity in known and predicted B- and T-cell epitopes, as well as greater diversity in adults as compared to infants. These findings suggest multiple overlapping mechanisms for early control of acute viral infections that may differ by age group and host immune responses.
Acknowledgments
FUNDING STATEMENT
This project has been funded in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No.: HHSN272200900018C. YHG was supported by K08-AI104767. Internal funds from the DeVincenzo laboratory and the Foundation for Respiratory Virus Research were also used for sample processing and technique development. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EAF, Rudan I, Weber MW, Campbell H. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 4.Hall CB. Respiratory syncytial virus. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. Vol. 2. Churchill Livingstone Elsevier; Philadelphia, PA: 2010. pp. 2207–2221. [Google Scholar]
- 5.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus research. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. American journal of diseases of children. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 7.Tapia LI, Shaw CA, Aideyan LO, Jewell AM, Dawson BC, Haq TR, Piedra PA. Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLoS One. 2014;9:e90786. doi: 10.1371/journal.pone.0090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peret TC, Hall CB, Hammond GW, Piedra PA, Storch GA, Sullender WM, Tsou C, Anderson LJ. Circulation patterns of group A and B human respiratory syncytial virus genotypes in 5 communities in North America. J Infect Dis. 2000;181:1891–1896. doi: 10.1086/315508. [DOI] [PubMed] [Google Scholar]
- 9.Peret TC, Hall CB, Schnabel KC, Golub JA, Anderson LJ. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J Gen Virol. 1998;79(Pt 9):2221–2229. doi: 10.1099/0022-1317-79-9-2221. [DOI] [PubMed] [Google Scholar]
- 10.Grad YH, Newman R, Zody M, Yang X, Murphy R, Qu J, Malboeuf CM, Levin JZ, Lipsitch M, DeVincenzo J. Within-host whole-genome deep sequencing and diversity analysis of human respiratory syncytial virus infection reveals dynamics of genomic diversity in the absence and presence of immune pressure. J Virol. 2014;88:7286–7293. doi: 10.1128/JVI.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YI, DeVincenzo JP, Jones BG, Rudraraju R, Harrison L, Meyers R, Cehelsky J, Alvarez R, Hurwitz JL. Respiratory syncytial virus human experimental infection model: provenance, production, and sequence of low-passaged memphis-37 challenge virus. PLoS One. 2014;9:e113100. doi: 10.1371/journal.pone.0113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Saleeby CM, Suzich J, Conley ME, DeVincenzo JP. Quantitative effects of palivizumab and donor-derived T cells on chronic respiratory syncytial virus infection, lung disease, and fusion glycoprotein amino acid sequences in a patient before and after bone marrow transplantation. Clin Infect Dis. 2004;39:e17–20. doi: 10.1086/421779. [DOI] [PubMed] [Google Scholar]
- 13.DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, McBride S, Lambkin-Williams R, Jordan R, Xin Y, Ramanathan S, O’Riordan T, Lewis SA, Li X, Toback SL, Lin SL, Chien JW. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med. 2014;371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 14.DeVincenzo JP, El Saleeby CM, Bush AJ. Respiratory syncytial virus load predicts disease severity in previously healthy infants. J Infect Dis. 2005;191:1861–1868. doi: 10.1086/430008. [DOI] [PubMed] [Google Scholar]
- 15.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, Oxford J, Pareek R, Moore R, Walsh E, Studholme R, Dorsett P, Alvarez R, Lambkin-Williams R. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med. 2010;182:1305–1314. doi: 10.1164/rccm.201002-0221OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perkins SM, Webb DL, Torrance SA, El Saleeby C, Harrison LM, Aitken JA, Patel A, DeVincenzo JP. Comparison of a real-time reverse transcriptase PCR assay and a culture technique for quantitative assessment of viral load in children naturally infected with respiratory syncytial virus. J Clin Microbiol. 2005;43:2356–2362. doi: 10.1128/JCM.43.5.2356-2362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malboeuf CM, Yang X, Charlebois P, Qu J, Berlin AM, Casali M, Pesko KN, Boutwell CL, DeVincenzo JP, Ebel GD, Allen TM, Zody MC, Henn MR, Levin JZ. Complete viral RNA genome sequencing of ultra-low copy samples by sequence-independent amplification. Nucleic acids research. 2013;41:e13. doi: 10.1093/nar/gks794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Charlebois P, Gnerre S, Coole MG, Lennon NJ, Levin JZ, Qu J, Ryan EM, Zody MC, Henn MR. De novo assembly of highly diverse viral populations. BMC genomics. 2012;13:475. doi: 10.1186/1471-2164-13-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Charlebois P, Macalalad A, Henn MR, Zody MC. V-Phaser 2: variant inference for viral populations. BMC genomics. 2013;14:674. doi: 10.1186/1471-2164-14-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmett KJ, Lee A, Khiabanian H, Rabadan R. High-resolution Genomic Surveillance of 2014 Ebolavirus Using Shared Subclonal Variants. PLoS Curr. 2015:7. doi: 10.1371/currents.outbreaks.c7fd7946ba606c982668a96bcba43c90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbiza J, Taylor G, Lopez JA, Furze J, Wyld S, Whyte P, Stott EJ, Wertz G, Sullender W, Trudel M, et al. Characterization of two antigenic sites recognized by neutralizing monoclonal antibodies directed against the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1992;73(Pt 9):2225–2234. doi: 10.1099/0022-1317-73-9-2225. [DOI] [PubMed] [Google Scholar]
- 22.Cane PA. Analysis of linear epitopes recognised by the primary human antibody response to a variable region of the attachment (G) protein of respiratory syncytial virus. J Med Virol. 1997;51:297–304. doi: 10.1002/(sici)1096-9071(199704)51:4<297::aid-jmv7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Murata Y, Lightfoote PM, Falsey AR, Walsh EE. Identification of and human serum reactogenicity to neutralizing epitopes within the central unglycosylated region of the respiratory syncytial virus attachment protein. Clin Vaccine Immunol. 2010;17:695–697. doi: 10.1128/CVI.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SJ, Schmidt A, Beil EJ, Day ND, Branigan PJ, Liu C, Gutshall LL, Palomo C, Furze J, Taylor G, Melero JA, Tsui P, Del Vecchio AM, Kruszynski M. Characterization of the epitope for anti-human respiratory syncytial virus F protein monoclonal antibody 101F using synthetic peptides and genetic approaches. J Gen Virol. 2007;88:2719–2723. doi: 10.1099/vir.0.82753-0. [DOI] [PubMed] [Google Scholar]
- 25.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. https://www.R-project.org/ [Google Scholar]
- 27.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poon LL, Song T, Rosenfeld R, Lin X, Rogers MB, Zhou B, Sebra R, Halpin RA, Guan Y, Twaddle A, DePasse JV, Stockwell TB, Wentworth DE, Holmes EC, Greenbaum B, Peiris JS, Cowling BJ, Ghedin E. Quantifying influenza virus diversity and transmission in humans. Nat Genet. 2016;48:195–200. doi: 10.1038/ng.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobel Leonard A, McClain MT, Smith GJ, Wentworth DE, Halpin RA, Lin X, Ransier A, Stockwell TB, Das SR, Gilbert AS, Lambkin-Williams R, Ginsburg GS, Woods CW, Koelle K. Deep Sequencing of Influenza A Virus from a Human Challenge Study Reveals a Selective Bottleneck and Only Limited Intrahost Genetic Diversification. J Virol. 2016;90:11247–11258. doi: 10.1128/JVI.01657-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spann KM, Tran KC, Chi B, Rabin RL, Collins PL. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected] J Virol. 2004;78:4363–4369. doi: 10.1128/JVI.78.8.4363-4369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 32.Hall CB, McBride JT. Respiratory syncytial virus--from chimps with colds to conundrums and cures. N Engl J Med. 1991;325:57–58. doi: 10.1056/NEJM199107043250110. [DOI] [PubMed] [Google Scholar]
- 33.Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol. 2015;235:266–276. doi: 10.1002/path.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Englund JA, Baker CJ, Raskino C, McKinney RE, Lifschitz MH, Petrie B, Fowler MG, Connor JD, Mendez H, O’Donnell K, Wara DW. Clinical and laboratory characteristics of a large cohort of symptomatic, human immunodeficiency virus-infected infants and children. AIDS Clinical Trials Group Protocol 152 Study Team. Pediatr Infect Dis J. 1996;15:1025–1036. doi: 10.1097/00006454-199611000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Do LA, Wilm A, Van Doorn HR, Lam HM, Sim S, Sukumaran R, Tran AT, Nguyen BH, Tran TT, Tran QH, Vo QB, Dac NA, Trinh HN, Nguyen TT, Binh BT, Le K, Nguyen MT, Thai QT, Vo TV, Ngo NQ, Dang TK, Cao NH, Tran TV, Ho LV, Farrar J, Jong M, Chen S, Nagarajan N, Bryant JE, Hibberd ML. Direct whole-genome deep-sequencing of human respiratory syncytial virus A and B from Vietnamese children identifies distinct patterns of inter- and intra-host evolution. J Gen Virol. 2015;96:3470–3483. doi: 10.1099/jgv.0.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobel Leonard A, Weissman D, Greenbaum B, Ghedin E, Koelle K. Transmission Bottleneck Size Estimation from Pathogen Deep-Sequencing Data, with an Application to Human Influenza A Virus. J Virol. 2017 doi: 10.1128/JVI.00171-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.