Abstract
Background
Hereditary spastic paraplegias (SPG1-SPG33) are characterized by progressive spastic weakness of the lower limbs. A nucleotide deletion (1110delA) in the (SPG20; OMIM 275900) spartin gene is the origin of autosomal recessive Troyer syndrome. This mutation is predicted to cause premature termination of the spartin protein. However, it remains unknown whether this truncated spartin protein is absent or is present and partially functional in patients.
Objective
To determine whether the truncated spartin protein is present or absent in cells derived from patients with Troyer syndrome.
Design
Case report.
Setting
Academic research.
Patients
We describe a new family with Troyer syndrome due to the 1110delA mutation.
Main Outcome Measures
We cultured primary fibroblasts and generated lymphoblasts from affected individuals, carriers, and control subjects and subjected these cells to immunoblot analyses.
Results
Spartin protein is undetectable in several cell lines derived from patients with Troyer syndrome.
Conclusions
Our data suggest that Troyer syndrome results from complete loss of spartin protein rather than from the predicted partly functional fragment. This may reflect increased protein degradation or impaired translation.
The hereditary spastic paraplegias (HSPs) are a group of neurodegenerative disorders characterized by progressive lower extremity spasticity and weakness.1 More than 30 genetic loci (SPG1-SPG33) comprising autosomal dominant, autosomal recessive, or X-linked inheritances have been mapped, and 15 proteins have been identified.2–5 Troyer syndrome (SPG20) was originally described in an Old Order Amish population in Holmes County, Ohio, as an autosomal recessive, complicated HSP with distal amyotrophy, short stature, and dysarthria.6–8 Several years ago, Patel et al9 identified the causative mutation as a nucleotide deletion (1110delA) in the SPG20 (spartin) gene coding region, which leads to a frameshift and predicted 29-residue substitution at the C-terminus, with truncation of the 666-residue spartin protein by 268 residues (fs369-398x399). Old Order Amish families practice endogamy and live in self-defined groups that are genetically isolated from neighboring communities. Thus far, all reported cases are from the same community and harbor the same 1110delA mutation.8
Several studies describing the functions, protein interactions, and localizations of spartin protein have recently been published. Spartin protein is monoubiquitinated, interacts with the endocytic protein Eps15, colocalizes with epidermal growth factor (EGF)–positive endosomes, and functions in EGF receptor trafficking in HeLa cells.10,11 One distribution study12 reported that endogenous spartin is present in the trans-Golgi network, nucleus, and neurites, while another study13 localized overexpressed spartin to mitochondria through C-terminus interactions. It remains unknown whether the mutant spartin is expressed as a partially functional protein fragment that would harbor several known interaction motifs10,11 or is rapidly degraded. Herein, we present the clinical features of a previously undescribed family with Troyer syndrome outside of the original community, as well as the effects of the 1110delA mutation on protein stability in fibroblasts and lymphoblasts from affected individuals.
METHODS
SUBJECTS
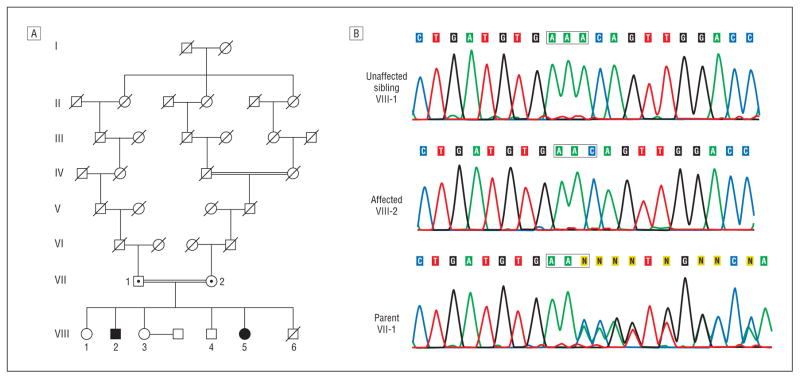
Subjects gave written informed consent to participate, and the study was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board. We tested for SPG20 mutations in a consanguineous Old Order Amish family from Geauga County, Ohio, in which 2 siblings exhibited features consistent with Troyer syndrome (Figure 1). These 2 individuals (VIII-2 and VIII-5) were homozygous for the 1110delA mutation and were examined in detail; clinical findings are presented herein and are summarized in the Table.
Figure 1.
Pedigree and sequence analysis. A, Partial pedigree of Old Order Amish family with Troyer syndrome. Square indicates male; circle, female; central dot, obligate carrier and confirmed heterozygote; slash mark, deceased individual; double line, consanguinity; and solid symbol, homozygous for the 1110delA mutation. B, Sequencing chromogram. Sequence traces spanning the 1110delA mutation for an unaffected sibling, a homozygous affected subject, and a heterozygous parent.
Table.
Summary of Clinical Features in Patients With Troyer Syndrome
| Variable | VIII-2 | VIII-5 |
|---|---|---|
| Age, y | ||
| Onset | 1–2 | 1–2 |
| Evaluation | 53 | 45 |
| Delayed milestones | Yes | Yes |
| Impaired cognition | Yes | Yes |
| Emotional lability | Severe | Severe |
| Primitive reflexes | No | No |
| Spastic dysarthria | Severe | Severe |
| Hyperreflexia | ||
| Arms | Mild or moderate | Mild or moderate |
| Legs | Severe | Severe |
| Spasticity | ||
| Arms | Mild or moderate | Mild or moderate |
| Legs | Severe | Severe |
| Plantar responses | Extensor | Extensor |
| Distal amyotrophy | Severe | Severe |
| Skeletal abnormalities | Severe | Severe |
| Difficulty at school | Severe | Severe |
| Loss of vibration sense at toes | Minimal | Minimal |
| Cerebellar signs | Mild or moderate | Mild or moderate |
| Nystagmus | No | No |
| Choreoathetoid movements or dystonia | No | No |
| Dysphagia | Mild or moderate | Mild or moderate |
| Urinary difficulties | Mild or moderate | Mild or moderate |
| Constipation | Mild or moderate | Mild or moderate |
DNA ANALYSIS
Peripheral blood samples were obtained from the subjects, and DNA was extracted using standard techniques. Primers were designed to amplify the exon harboring the known 1110delA mutation, and the resulting polymerase chain reaction (PCR) products were directly sequenced.
PROTEIN ANALYSIS AND REVERSE TRANSCRIPTION–PCR
Skin fibroblasts from forearm punch biopsy specimens and lymphoblast cell lines from peripheral blood samples were prepared and maintained using standard techniques. Preparation of cell extracts and transfection of HeLa cells with Myc-spartin were performed as described previously.10,11 Site-directed mutagenesis to introduce the 1110delA mutation into Myc-spartin was performed using a commercially available kit (QuikChange; Stratagene, La Jolla, California). Immunoblotting was performed using mouse monoclonal anti-Myc epitope (Santa Cruz Biotechnology, Santa Cruz, California) and rabbit polyclonal antispartin antibodies.10,11
For reverse transcription–PCR, messenger RNA (mRNA) was extracted from cells using a commercially available reagent (TRI; Sigma-Aldrich Inc, St Louis, Missouri). First-strand complementary DNA (cDNA) synthesis was performed with 2.5 μg of mRNA using a commercially available system (SuperScript III First Stand Synthesis System; Invitrogen, San Diego, California). To amplify a spartin cDNA fragment, we used the following primers: sense, 5′-CTGGAAATTCTAGAGAAGGGTCTTGC-3′; and antisense, 5′-TTGTAGCATTGTATCAGGAAACATGTAG-3′. Cycling variables were 94°C for 2 minutes, then 40 cycles of 94°C for 30 seconds, 57°C for 30 seconds, and 72°C for 1 minute. To amplify an actin cDNA fragment, we used the following primers: sense, 5′-GCTCGTCGTCGACAACGGCTC-3′; and antisense, 5′-CAAACATGATCGGGTCATCTTCT-3′.
REPORT OF CASES
Subject VIII-2 is a 53-year-old man. He weighed 3118 g at birth. He walked at age 17 months and could almost run. Speech was delayed, and he attended school until about the fifth grade in the Amish system, when his teacher thought he would not benefit from further schooling. During a period of years, his gait and speech deteriorated, although it is less clear whether cognition has deteriorated. On physical examination, he was approximately 152 cm tall. He was alert and attentive but had frequent alternating bouts of inappropriate euphoria or crying. There was prominent spastic dysarthria. Tongue movements were slow and spastic without fasciculations. Eye movements were full, with saccadic pursuits but no nystagmus. There was mild pyramidal weakness in the lower extremities. In the upper and lower extremities, there was distal amyotrophy with weakness in multiple distal muscles but no fasciculations. Skeletal examination was notable for a severe pectus excavatum deformity and kyphoscoliosis, loss of teeth, pes cavus, and small feet. Hand joints were hyperextensible, particularly at the proximal interphalangeal joints and the wrist. There was ulnar deviation at the wrist, and feet were slightly inverted. There was a mild decrease in vibratory sensation at the toes. Reflexes were increased throughout, and plantar responses were extensor. On coordination testing, there was mild terminal dysmetria. Gait was wide based and spastic. He ambulated with difficulty and required assistance.
Subject VIII-5 is a 45-year-old woman. She weighed 2863 g at birth. She first walked at age 14 months but always had difficulties. She had developmental delay, particularly with gross motor function and speech. Gait and speech progressively deteriorated, but cognition has been stable. Although she has needed a wheelchair for the past 3 years, she was previously able to ambulate independently. On physical examination, she was approximately 125 cm tall. She was alert and attentive, and although she smiled appropriately at times, she had occasional inappropriate euphoria or crying. She had prominent spastic dysarthria. Tongue movements were slow and spastic. Eye movements were full, with saccadic pursuits but no nystagmus. There was mild pyramidal weakness in the lower extremities, and in the upper and lower extremities there was distal amyotrophy with weakness in multiple distal muscles. She had severe kyphoscoliosis, loss of teeth, pes cavus, and small feet. Hand joints were hyperextensible, particularly at the proximal interphalangeal joints and the wrist. Hands were notable for ulnar deviation at the wrist, and feet were slightly inverted. There was a mild decrease in vibratory sensation at the toes. Reflexes were increased throughout, and plantar responses were extensor bilaterally. On coordination testing, there was mild terminal dysmetria. She was unable to ambulate without 2-person assistance, and even then had a spastic, unsteady, wide-based gait.
The parents (VII-1 and VII-2) of these individuals were heterozygous for the mutation. Findings from neurologic examinations were normal, and there were no neurologic complaints.
RESULTS
A homozygous 1110delA mutation was found in the spartin gene by direct sequencing of DNA isolated from the subjects’ blood samples (Figure 1). This is the same mutation found in the original investigations identifying spartin gene mutations in a different Amish community; this family was not examined in those studies.6,8,9
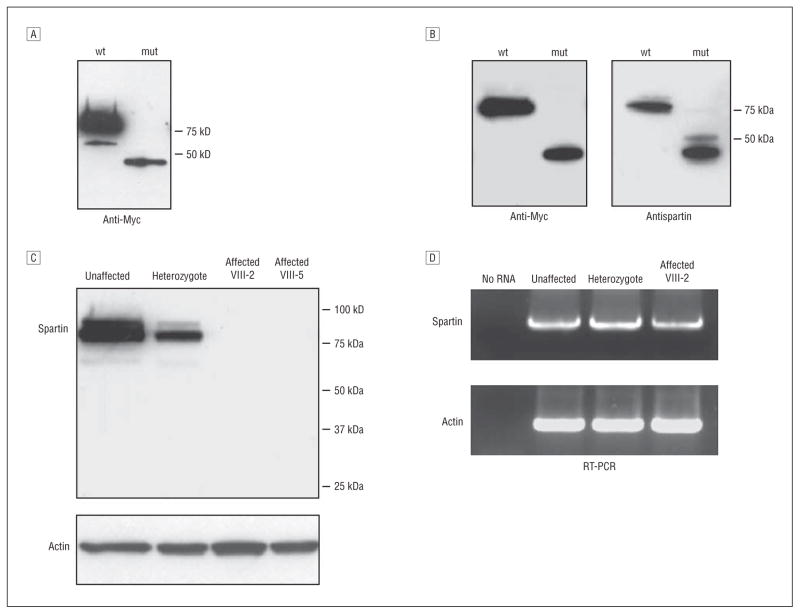
To confirm that the antispartin antibody can detect the mutant (fs369-398x399) form of spartin, we overexpressed wild-type and mutant spartin as Myc-tagged proteins in HeLa cells and performed immunoblotting with anti-Myc antibodies. Protein levels were much lower for the mutant spartin compared with the wild-type (Figure 2A). Therefore, 10-fold less DNA was transfected for the wild-type form to equalize expression levels, and immunoblotting with anti-Myc and antispartin antibodies demonstrated that our antispartin antibodies clearly detected the truncated protein (Figure 2B). This result was expected because the epitope to which this antispartin antibody was raised (residues 108-367) is fully present in the presumptive truncated protein.11 Next, we performed immunoblotting using lysates from primary skin fibroblasts. In the heterozygous carrier tested, the spartin protein level was about half that in the control subject. No protein was detected in cell lysates from either affected subject even at prolonged exposure times (Figure 2C). Similar results were found using cell lysates prepared from lymphoblasts (data not shown). Reverse transcription–PCR showed the presence of the spartin mRNA transcript in all individuals (Figure 2D), suggesting that the lack of spartin protein might be due to protein degradation. However, treatment of cells with the proteosomal inhibitor MG-132 (10 μM for 6 hours), with the lysosomal protease inhibitors leupeptin (100 μM for 6 hours) and ammonium chloride (20 mM for 6 hours), or with the calpain, cathepsin, and proteosomal inhibitor N-acetyl-leu-leu-norleucinal (100 μM for 6 hours)14 before harvesting did not stabilize the protein (data not shown), suggesting that degradation proceeds through other mechanisms or, alternatively, that lack of truncated spartin reflects impaired protein translation.
Figure 2.
Expression analysis of the spartin protein in primary fibroblasts. A, Expression of mutant (mut) spartin is lower than wild-type (wt) in heterologous cells. Extracts (20 μg of protein per lane) of HeLa cells overexpressing Myc-tagged wt and fs369-398x399 mut spartin were immunoblotted with anti-Myc antibodies. B, Antispartin antibodies detect overexpressed mut spartin protein. HeLa cells were transfected with expression vectors for wt spartin (10-fold less DNA to lower expression) and mut spartin and were then immunoblotted with anti-Myc and antispartin antibodies. C, Mutant spartin is degraded in affected subjects’ fibroblasts. Cell lysates of fibroblasts (20 μg of protein per lane) from unaffected heterozygous (VII-2 in Figure 1) and affected (VIII-2 and VIII-5) individuals were immunoblotted with antispartin antibodies. Actin levels were monitored by immunoblotting to ensure equal protein loading. D, Reverse transcription–polymerase chain reaction (RT-PCR) analysis of spartin messenger RNA expression in fibroblasts. Total RNA prepared from fibroblasts derived from the indicated heterozygous (VII-2) and affected (VIII-5) individuals was subjected to RT-PCR for spartin and actin. There were no significant differences in products obtained from unaffected, heterozygous, and affected individuals.
COMMENT
We identified a new family with Troyer syndrome, the first genetically confirmed cases of Troyer syndrome (to our knowledge) reported outside of the initial isolate.6,8 Most important, the mutation is the same, consistent with a common founder. Our study emphasizes multiple phenotypic aspects seen in the original population (Table). This is particularly important because previous patients with Troyer syndrome were all from within the same Old Order Amish community; therefore, other genetic factors may have influenced the observed clinical features. Members of a Wisconsin Amish family with Ohio ancestry described in a case report15 may have had Troyer syndrome, but other reports of possible Troyer syndrome in the literature seem less likely, and indeed, some of these subjects have tested negative for the spartin mutation.8 In our subjects, distal amyotrophy and emotional lability were prominent, as were skeletal abnormalities. The skeletal abnormalities comprised several different manifestations and may represent a particularly important distinguishing feature of this HSP.
Findings from several recent studies suggest a role of spartin in vesicle trafficking. Our results indicate that the disease pathogenesis likely reflects complete loss of spartin protein rather than expression of a partially functional protein. In fact, the truncated protein fs369-398x399 contains the MIT domain (present in microtubule-interacting and trafficking proteins),16 harbors a domain important for interaction with Eps15,10 and is monoubiquitinated when overexpressed (Figure 2B). Most important, if this fragment was present within the cell, it might retain partial function. Our study shows complete loss of the mutant protein in the fibroblasts of patients with SPG20 and markedly lower levels of mutant Myc-spartin overexpressed in heterologous cells. Therefore, spartin-null animal models may be most appropriate for further characterization of this disorder.
Acknowledgments
Funding/Support: This study was supported by the Intramural Research Program, National Institutes of Neurological Disorders and Stroke, National Institutes of Health (Drs Bakowska, Sumner, and Blackstone) and by the Elisabeth Severance Prentiss Foundation (Drs Wang and Xin).
Footnotes
Financial Disclosure: None reported.
Author Contributions: Study concept and design: Bakowska, Wang, Sumner, and Blackstone. Acquisition of data: Bakowska, Wang, Xin, Sumner, and Blackstone. Drafting of the manuscript: Bakowska and Blackstone. Critical revision of the manuscript for important intellectual content: Bakowska, Wang, Xin, Sumner, and Blackstone. Statistical analysis: Bakowska. Obtained funding: Wang and Blackstone. Administrative, technical, and material support: Bakowska, Wang, Xin, Sumner, and Blackstone. Study supervision: Wang and Blackstone.
Additional Contributions: Alison La Pean, MS, provided technical assistance, and Kurt Fischbeck, MD, provided additional support.
References
- 1.Harding AE. Hereditary spastic paraplegias. Semin Neurol. 1993;13(4):333–336. doi: 10.1055/s-2008-1041143. [DOI] [PubMed] [Google Scholar]
- 2.Crosby AH, Proukakis C. Is the transportation highway the right road for hereditary spastic paraplegia? Am J Hum Genet. 2002;71(5):1009–1016. doi: 10.1086/344206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid E. Science in motion: common molecular pathological themes emerge in the hereditary spastic paraplegias. J Med Genet. 2003;40(2):81–86. doi: 10.1136/jmg.40.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fink JK. Hereditary spastic paraplegia. Curr Neurol Neurosci Rep. 2006;6(1):65–76. doi: 10.1007/s11910-996-0011-1. [DOI] [PubMed] [Google Scholar]
- 5.Soderblom C, Blackstone C. Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias. Pharmacol Ther. 2006;109(1–2):42–56. doi: 10.1016/j.pharmthera.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Cross HE, McKusick VA. The Troyer syndrome: a recessive form of spastic paraplegia with distal muscle wasting. Arch Neurol. 1967;16(5):473–485. doi: 10.1001/archneur.1967.00470230025003. [DOI] [PubMed] [Google Scholar]
- 7.Auer-Grumbach M, Fazekas F, Radner H, Irmler A, Strasser-Fuchs S, Hartung HP. Troyer syndrome: a combination of central brain abnormality and motor neuron disease? J Neurol. 1999;246(7):556–561. doi: 10.1007/s004150050403. [DOI] [PubMed] [Google Scholar]
- 8.Proukakis C, Cross H, Patel H, Patton MA, Valentine A, Crosby AH. Troyer syndrome revisited: a clinical and radiological study of a complicated hereditary spastic paraplegia. J Neurol. 2004;251(9):1105–1110. doi: 10.1007/s00415-004-0491-3. [DOI] [PubMed] [Google Scholar]
- 9.Patel H, Cross H, Proukakis C, et al. SPG20 is mutated in Troyer syndrome, an hereditary spastic paraplegia. Nat Genet. 2002;31(4):347–348. doi: 10.1038/ng937. [DOI] [PubMed] [Google Scholar]
- 10.Bakowska JC, Jenkins R, Pendleton J, Blackstone C. The Troyer syndrome (SPG20) protein spartin interacts with Eps15. Biochem Biophys Res Commun. 2005;334(4):1042–1048. doi: 10.1016/j.bbrc.2005.06.201. [DOI] [PubMed] [Google Scholar]
- 11.Bakowska JC, Jupille H, Fatheddin P, Puertollano R, Blackstone C. Troyer syndrome protein spartin is mono-ubiquitinated and functions in EGF receptor trafficking. Mol Biol Cell. 2007;18(5):1683–1692. doi: 10.1091/mbc.E06-09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robay D, Patel H, Simpson MA, Brown NA, Crosby AH. Endogenous spartin, mutated in hereditary spastic paraplegia, has a complex subcellular localization suggesting diverse roles in neurons. Exp Cell Res. 2006;312(15):2764–2777. doi: 10.1016/j.yexcr.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Lu J, Rashid F, Byrne P. The hereditary spastic paraplegia protein spartin localises to mitochondria. J Neurochem. 2006;98(6):1908–1919. doi: 10.1111/j.1471-4159.2006.04008.x. [DOI] [PubMed] [Google Scholar]
- 14.Blagosklonny MV, An WG, Melillo G, Nguyen P, Trepel JB, Neckers LM. Regulation of BRCA1 by protein degradation. Oncogene. 1999;18(47):6460–6468. doi: 10.1038/sj.onc.1203068. [DOI] [PubMed] [Google Scholar]
- 15.Neuhäuser G, Wiffler C, Opitz JM. Familial spastic paraplegia with distal muscle wasting in the Old Order Amish: atypical Troyer syndrome or “new” syndrome. Clin Genet. 1976;9(3):315–323. doi: 10.1111/j.1399-0004.1976.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 16.Ciccarelli FD, Proukakis C, Patel H, et al. The identification of a conserved domain in both spartin and spastin, mutated in hereditary spastic paraplegia. Genomics. 2003;81(4):437–441. doi: 10.1016/s0888-7543(03)00011-9. [DOI] [PubMed] [Google Scholar]