Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Over the last decade, allogeneic HCT has been increasingly administered in the United States to adults aged 70 and older with hematologic malignancies.
Allogeneic transplant outcomes were reasonable; high comorbidity and ablative conditioning regimens were associated with inferior outcomes.
Abstract
In this study, we evaluated trends and outcomes of allogeneic hematopoietic cell transplantation (HCT) in adults ≥70 years with hematologic malignancies across the United States. Adults ≥70 years with a hematologic malignancy undergoing first allogeneic HCT in the United States between 2000 and 2013 and reported to the Center for International Blood and Marrow Transplant Research were eligible. Transplant utilization and transplant outcomes, including overall survival (OS), progression-free survival (PFS), and transplant-related mortality (TRM) were studied. One thousand one hundred and six patients ≥70 years underwent HCT across 103 transplant centers. The number and proportion of allografts performed in this population rose markedly over the past decade, accounting for 0.1% of transplants in 2000 to 3.85% (N = 298) in 2013. Acute myeloid leukemia and myelodysplastic syndromes represented the most common disease indications. Two-year OS and PFS significantly improved over time (OS: 26% [95% confidence interval (CI), 21% to 33%] in 2000-2007 to 39% [95% CI, 35% to 42%] in 2008-2013, P < .001; PFS: 22% [16% to 28%] in 2000-2007 to 32% [95% CI, 29% to 36%] in 2008-2013, P = .003). Two-year TRM ranged from 33% to 35% and was unchanged over time (P = .54). Multivariable analysis of OS in the modern era of 2008-2013 revealed higher comorbidity by HCT comorbidity index ≥3 (hazard ratio [HR], 1.27; P = .006), umbilical cord blood graft (HR, 1.97; P = .0002), and myeloablative conditioning (HR, 1.61; P = .0002) as adverse factors. Over the past decade, utilization and survival after allogeneic transplant have increased in patients ≥70 years. Select adults ≥70 years with hematologic malignancies should be considered for transplant.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers the best potential for prolonged disease control for many hematologic malignancies. Historically, older adults were not considered candidates out of concern for increased toxicity and mortality,1 thus excluding the majority of hematologic malignancy patients who may have gained benefit. In recent years, the development of reduced-intensity conditioning (RIC) and nonmyeloablative (NMA) regimens,2 coupled with improvements in supportive care measures3 and more accurate HLA typing methods,4 has broadened the application of HCT to include older adults. As such, 22% of HCT recipients for malignant diseases from 2007 to 2013 reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) were >60 years of age.5
The feasibility of HCT in adults >50 years of age, and even those >65 years, has been detailed in several reports.6-11 In a registry analysis of patients with acute myeloid leukemia (AML) in first remission or myelodysplastic syndrome (MDS) receiving RIC allogeneic transplants, no significant differences in outcomes were uncovered among HCT recipients aged 40 to 50 years versus those >65 years.7 Similar results have been reported in multicenter series from the United States and the European Society for Blood and Marrow Transplantation, where disease status and medical comorbidities, rather than chronological age, appear to predict for outcomes.6,8,11
Although most studies conclude that older age alone should not preclude transplant, very limited data exist regarding patients transplanted in their eighth decade.12 This population is of particular interest, as it has been shown that for each 5-year increase in age beyond 65 years, the proportion of cancer patients with comorbidity, disability, and/or geriatric syndromes increases by 3% to 5%,13,14 prompting recommendations to perform a comprehensive geriatric assessment for all cancer patients in this age group.15 Further, as the population continues to age, with 50% of all cancers and 70% of cancer mortality occurring in adults ≥65 years, the number of patients considered for HCT in their eighth decade will continue to rise.16
We therefore conducted an observational study of allogeneic HCT recipients ≥70 years reported to the CIBMTR between 2000 and 2013 with the aims of describing transplant utilization as well as baseline characteristics, outcomes, and prognostic factors for this understudied cohort of HCT recipients.
Methods
Data sources
The CIBMTR is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. The CIBMTR represents an international network of transplant centers that submit transplant-related data for patients. It has been collecting HCT outcomes data for >40 years and has an extensive database of detailed patient-, transplant-, and disease-related information with prospectively collected longitudinal data.17 CIBMTR data are collected in compliance with Health Insurance Portability and Accountability Act regulations and with all applicable federal regulations pertaining to the protection of human research participants, as determined by a continuous review by the National Marrow Donor Program institutional review board and the Medical College of Wisconsin.
The CIBMTR collects 2 levels of data: registration-level data are retrieved from Transplant Essential Data forms, and additional research level data are collected using Comprehensive Report Forms (CRFs). The latter is a subset of the former; patients for whom CRF-level data are collected are chosen through a selection algorithm, as these forms are longer and require more extensive data, including detailed disease information. Thus, the objective of this study included assessing transplant practices and activity in the United States using Transplant Essential Data–level data. Additional analysis with more detailed disease-specific information was performed in a subset of this population as described in statistical section below.
Patient population
Patients aged ≥70 years at time of first allogeneic HCT occurring between 2000 and 2013 and reported to the CIBMTR were included. Exclusion criteria included syngeneic donor transplant and patients reported to the CIBMTR who received HCT outside of the United States.
Definitions and outcomes
Disease status at time of transplant followed CIBMTR disease risk classification18: early disease (acute leukemia in first complete remission, myelodysplastic syndromes with <5% blasts, or chronic-phase chronic myeloid leukemia [CML]), intermediate disease (second or greater complete remission acute leukemia, accelerated-phase CML), and advanced disease (acute leukemia not in remission, myelodysplastic syndromes with ≥5% marrow blasts, or blast-phase CML); lymphomas were classified according to chemotherapy sensitivity (sensitive or resistant at time of transplant). Patients with AML were also described in greater detail using CRF data to report disease characteristics of interest, including frequency of favorable, intermediate, and poor cytogenetic categories and presence or absence of FLT3 mutations. Conditioning regimen intensity followed CIBMTR working definitions (myeloablative vs RIC/NMA)19,20; due to data capture constraints, RIC and NMA were grouped together. Comorbidity was scored using the HCT comorbidity index; recipient HCT-comorbidity index scores have been routinely reported to the CIBMTR on all allogeneic HCT recipients since 2008. Performance status was captured by the Karnofsky Performance Scale (KPS). Disease Risk Index (DRI)21 was evaluated in the entire population and categorized in a subset of patients with all available elements. HLA-matched unrelated donors required 8/8 matching at HLA-A, HLA-B, HLA-C, and HLA-DRB1 at high resolution. Mismatched related donors included haploidentical donors as well as single-antigen and allele mismatches (ie, 7 of 8). Neutrophil engraftment was defined as neutrophil count above 500/µL for 3 consecutive days. Graft-versus-host disease (GVHD) was defined as acute GHVD (grades II-IV or III-IV according to maximum organ stage) or chronic GVHD.
The primary outcome for this study was overall survival (OS), defined as death from any cause; patients for whom death was not observed were censored at the time of last follow-up. Transplant-related mortality (TRM) was defined as death in the absence of disease relapse or progression. The composite end point of progression-free survival (PFS) required either disease relapse or progression or death; patients alive without such events were censored at the time of last follow up. Primary cause of death for each patient was reported by the treating center.
Statistical analysis
Descriptive statistics summarized the baseline patient, disease, and transplant-related characteristics according to two time cohorts during the study period (2000 to 2007, 2008 to 2013). The proportion of transplants performed in patients 70 years of age and older relative to all transplants performed in the United States was computed. Univariate summary measures for all outcomes were calculated and compared over the two time periods. Probabilities of OS and PFS were calculated using the Kaplan-Meier estimator, and comparisons between groups were performed using log-rank and point-wise tests. Probabilities of neutrophil engraftment, GVHD, TRM, and disease relapse/progression were calculated by cumulative incidence function accounting for competing risks. Comparisons of cumulative incidence across time cohorts were performed via Gray’s test.
Prognostic factor analysis was performed on 3 patient subsets. The first analysis included all patients ≥70 years transplanted between 2008 and 2013 with the intention of focusing on a contemporary cohort of patients with all disease indications who received comorbidity assessment by the HCT-comorbidity index (which was not available prior to 2008). For this subset, a multivariable Cox proportional hazards regression model was built using stepwise variable selection to evaluate patient, disease, and transplant variables associated with OS. The variables analyzed were age (70-74 and ≥75 years), gender, HCT-comorbidity index (0-2 and ≥3),22 KPS (modeled at ≥80% and ≥80%), disease, disease status, donor type (HLA-matched sibling, HLA- mismatched related, and HLA matched unrelated donor, divided according to donor age [<30 and ≥30 years] or mismatched unrelated donor, unrelated donor with HLA-matching unknown, and umbilical cord blood), conditioning regimen intensity19,20 graft source (bone marrow, mobilized peripheral blood stem cells, and umbilical cord blood), year of transplant, donor-recipient gender match, donor–recipient cytomegalovirus serologic status, and GVHD prophylaxis (calcineurin inhibitor and methotrexate, calcineurin inhibitor and mycophenolate mofetil, and calcineurin inhibitor with other combinations and other regimens).
The second subset analysis explored variables associated with OS and TRM among patients ≥70 years with controlled disease entering transplant. This subset included patients with early or intermediate-risk AML (ie, patients in complete remission 1 [CR1] or CR2 as opposed to active disease), MDS with <5% blasts, and chemosensitive non-Hodgkin lymphoma (NHL) who underwent HCT between 2008 and 2013. This more homogenous subset included the most common disease indications for HCT among older adults and enabled analysis of variables associated with TRM without confounding from less common transplant disease indications. Multivariable Cox regression models were constructed by testing the covariates as above with the exception of disease status as all patients in this subset had early or intermediate disease at transplant.
The third subset focused on exploring outcomes by comorbidity and disease status in patients with AML and MDS undergoing transplant between 2008 and 2013. For this subset, probabilities of OS and TRM were calculated as above.
For multivariable analysis performed in the first and second subsets, all covariates associated with outcome at P < .05 were retained in the final models and considered significant. Tests of proportionality for each covariate and of interaction between covariates were performed; no violations of proportionality or significant interactions were found. All analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Results
HCT utilization for adults 70 years and older over time
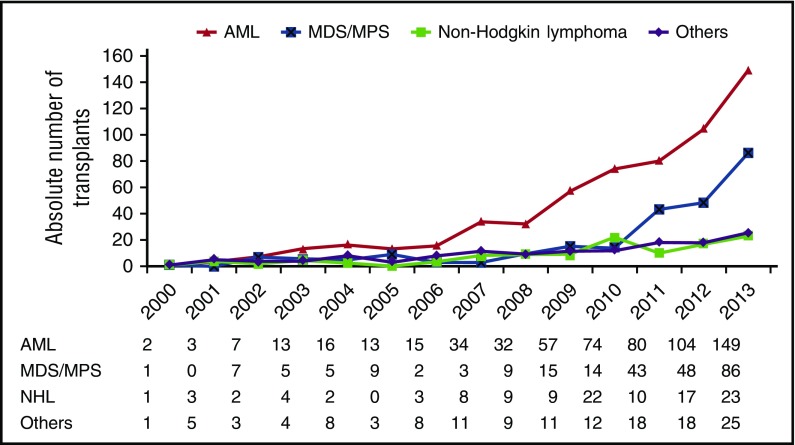
A total of 1106 patients ≥70 years underwent first allogeneic HCT in the United States and were reported to the CIBMTR between 2000 and 2013. The absolute number of transplants for adults ≥70 years increased substantially over that time, from 5 in 2000 to 283 in 2013. The proportion of allogeneic HCT recipients ≥70 years also increased over time, from 0.1% in 2000 to 3.85% in 2013. Further, the absolute number of transplant programs performing allogeneic HCT in patients ≥70 years increased from 65 centers in 2000-2007 to 93 centers in 2008-2013. Transplant for AML and MDS accounted for the main indications across time periods (Figure 1).
Figure 1.
Annual number of HCTs in patients 70 years and older by indication. MPS, myeloproliferative syndrome.
Patient and HCT characteristics
Baseline patient demographics and transplant characteristics stratified by HCT time period for all patients in the dataset are described in Table 1. The median age at time of HCT was 72 (range, 70-84 years). KPS was <80% in 9% of the patients. Comorbidity by HCT-comorbidity index (captured for recipients transplanted from 2008 to 2013) demonstrated high HCT-comorbidity index of ≥3 in 46% of patients; 16% had HCT-comorbidity index scores of ≥5. Among patients with available DRI, the majority received transplant with intermediate risk. The donor source shifted from related donors in 51% in the initial time period to 70% unrelated grafts in the later time period, driven mostly be greater use of matched unrelated donors over time. Although most patients received RIC/NMA conditioning regimens, myeloablative regimens were administered to 10% overall. The more widespread use of fludarabine plus busulfan–based regimens signified the largest change in conditioning regimens over time. Increasing numbers of patients entered transplant with controlled disease in the more recent time period.
Table 1.
Demographics of patients 70 years and older who received HCT from 2000 to 2013
| 2000-2007, n (%) | 2008-2013, n (%) | All patients, n (%) | |
|---|---|---|---|
| No. of patients | 207 | 899 | 1106 |
| No. of centers | 65 | 93 | 103 |
| Age at transplant, y | |||
| Median (range) | 72 (70-83) | 72 (70-84) | 72 (70-84) |
| 70-74 | 176 (85) | 807 (90) | 983 (89) |
| 75-79 | 27 (13) | 88 (10) | 115 (10) |
| ≥80 | 4 (2) | 4 (<1) | 8 (1) |
| Sex | |||
| Male | 151 (73) | 629 (70) | 780 (71) |
| Female | 56 (27) | 270 (30) | 326 (29) |
| KPS | |||
| <80% | 17 (8) | 87 (10) | 104 (9) |
| ≥80% | 119 (57) | 784 (87) | 903 (82) |
| Missing | 71 (34) | 28 (3) | 99 (9) |
| HCT-comorbidity index | |||
| 0 | — | 248 (28) | 248 (22) |
| 1-2 | — | 234 (26) | 234 (21) |
| ≥3 | — | 417 (46) | 417 (38) |
| Missing* | 207 | 0 | 207 (19) |
| Disease | |||
| AML | 103 (50) | 496 (55) | 599 (54) |
| ALL | 2 (<1) | 23 (3) | 25 (2) |
| CLL/PLL | 22 (11) | 54 (6) | 76 (7) |
| CML | 5 (2) | 6 (<1) | 11 (<1) |
| MDS/MPS | 32 (15) | 215 (24) | 247 (22) |
| Other acute leukemia | 1 (<1) | 8 (<1) | 9 (<1) |
| NHL | 23 (11) | 90 (10) | 113 (10) |
| Plasma cell disorder/multiple myeloma | 10 (5) | 2 (<1) | 12 (1) |
| Other malignancies | 3 (1) | 0 | 3 (<1) |
| Severe aplastic anemia | 4 (2) | 5 (<1) | 9 (<1) |
| PNH | 2 (1) | 0 | 2 (<1) |
| Disease status | |||
| Early disease | 45 (22) | 355 (39) | 300 (27) |
| Intermediate disease | 25 (12) | 93 (10) | 118 (11) |
| Advanced disease | 48 (23) | 230 (26) | 278 (25) |
| NHL sensitive | 10 (5) | 79 (10) | 89 (9) |
| NHL resistant | 6 (3) | 10 (1) | 16 (1) |
| Other (unknown/missing/NA) | 73 (35) | 132 (15) | 205 (19) |
| DRI† | |||
| Low | 6 (3) | 32 (4) | 38 (3) |
| Intermediate | 28 (14) | 152 (17) | 180 (16) |
| High | 20 (10) | 115 (13) | 135 (12) |
| Very high | 7 (3) | 18 (2) | 25 (2) |
| Other (missing/NA) | 146 (71) | 582 (65) | 728 (66) |
| Donor type | |||
| Matched related | 93 (45) | 204 (23) | 297 (27) |
| Mismatched related | 13 (6) | 67 (7) | 80 (7) |
| Matched unrelated | 52 (25) | 463 (52) | 515 (47) |
| Mismatched unrelated | 12 (6) | 68 (8) | 80 (7) |
| Unrelated HLA match unknown | 20 (10) | 46 (5) | 66 (6) |
| Umbilical cord blood | 7 (3) | 51 (6) | 58 (5) |
| Missing | 10 (5) | 0 | 10 (1) |
| Unrelated donor age, median (range), y | 36 (21-60) | 30 (19-61) | 31 (19-61) |
| 18-30 | 23 (27) | 278 (48) | 301 (46) |
| 31-40 | 24 (29) | 139 (24) | 163 (25) |
| 41-50 | 21 (25) | 96 (17) | 117 (18) |
| 51-61 | 5 (6) | 25 (4) | 30 (5) |
| Unknown | 11 (13) | 39 (7) | 50 (8) |
| Graft source | |||
| Bone marrow | 17 (8) | 93 (10) | 110 (10) |
| Peripheral blood | 183 (88) | 755 (84) | 938 (85) |
| Umbilical cord blood | 7 (3) | 51 (6) | 58 (5) |
| Donor–recipient sex match | |||
| Male–male | 95 (46) | 388 (43) | 483 (44) |
| Male–female | 25 (12) | 154 (17) | 179 (16) |
| Female–male | 50 (24) | 203 (23) | 253 (23) |
| Female–female | 30 (14) | 98 (11) | 128 (12) |
| Unknown | 7 (3) | 56 (6) | 63 (6) |
| Donor–recipient CMV status | |||
| Positive–positive | 16 (8) | 269 (30) | 285 (26) |
| Positive–negative | 7 (3) | 67 (7) | 74 (7) |
| Negative–positive | 37 (18) | 292 (32) | 329 (30) |
| Negative–negative | 13 (6) | 176 (20) | 189 (17) |
| Unknown | 134 (65) | 95 (11) | 229 (21) |
| Regimen intensity | |||
| Ablative | 11 (5) | 102 (11) | 113 (10) |
| RIC/NMA | 178 (86) | 796 (89) | 974 (88) |
| Unknown | 18 (9) | 1 (<1) | 19 (2) |
| Conditioning regimen | |||
| Flu/Bu ± other | 29 (14) | 368 (41) | 397 (36) |
| Flu/Mel ± other | 35 (17) | 137 (15) | 172 (16) |
| Flu/TBI ± other | 65 (31) | 274 (30) | 339 (31) |
| Bu based | 11 (5) | 17 (2) | 28 (3) |
| Flu/Cy ± other | 26 (13) | 40 (4) | 66 (6) |
| TBI/TLI ± other | 16 (8) | 49 (5) | 65 (6) |
| Other | 17 (8) | 14 (2) | 31 (3) |
| Unknown | 8 (4) | 0 | 8 (<1) |
ALL, acute lymphoblastic leukemia; Bu, busulfan; CLL, chronic lymphocytic leukemia; CMV, cytomegalovirus; Flu/Bu, fludarabine + busulfan; Flu/Mel, fludarabine + melphalan; Flu/TBI, fludarabine + total body irradiation; MPS, myeloproliferative syndrome; NA, not available; PLL, prolymphocytic leukemia; PNH, paroxysmal nocturnal hemoglobinuria; Flu/Cy, fludarabine + cyclophosphamide; TBI/TLI, total body irradiation or total lymphoid irradiation.
HCT-comorbidity index was not captured by the CIBMTR registry prior to 2008.
All elements required to generate the DRI were not available in all patients.
Supplemental Table A (available on the Blood Web site) includes additional disease and transplant-related details regarding AML patients transplanted between 2008 and 2013. In this subset of 120 patients with AML with available information, 70% had intermediate cytogenetics, 85% of patients with available FLT3 status were wild-type, and 69% were in morphologic complete remission at time of transplant.
Outcomes
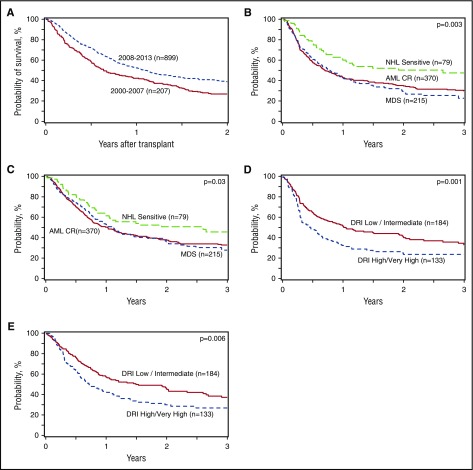
Unadjusted 1- and 2-year transplant outcome probabilities (neutrophil engraftment, grades II-IV and III-IV acute GVHD, chronic GVHD, TRM, relapse/progression, PFS, and OS) stratified by HCT time period are summarized in Table 2. Two-year PFS improved significantly from 2000-2007 to 2008-2013 (22% [95% confidence interval (CI) 16% to 28%] vs 32% [29% to 36%], P = .003), while TRM remained static (P = .54 over the entire time period). Two-year OS also improved significantly over time, from 26% [95% CI, 21% to 33%] in 2000-2007% to 39% [95% CI, 35% to 42%] in 2008-2013, P < .001 (Figure 2A). Overall and disease-free survival among patients with the most common indications for HCT from 2008 to 2013 and according to DRI in the same period are shown in Figure 2B-E).
Table 2.
Univariate analysis of posttransplant outcomes of patients aged 70 years and older and recipients of an allogeneic HCT from 2000 to 2013
| Total cohort | 2000-2007 | 2008-2013 | P* | |
|---|---|---|---|---|
| prob. (95% CI) | prob. (95% CI) | prob. (95% CI) | ||
| Neutrophil engraftment† | ||||
| No. evaluated | — | — | 890 | — |
| 28 d | — | — | 92 (90-93) | — |
| Acute GVHD, grade II-IV | ||||
| No. evaluated | 427 | 105 | 322 | .63 |
| 100 d | 32 (28-37) | 31 (23-41) | 33 (28-38) | .82 |
| Acute GVHD, grade III-IV | ||||
| No. Evaluated | 427 | 105 | 322 | .05 |
| 100 d | 13 (10-17) | 18 (11-26) | 12 (9-16) | .13 |
| Chronic GVHD | ||||
| No. evaluated | 1025 | 161 | 864 | .94 |
| 1 y | 32 (30-35) | 32 (25-40) | 32 (29-36) | .97 |
| 2 y | 37 (34-40) | 35 (28-43) | 38 (35-41) | .55 |
| TRM | ||||
| No. evaluated | 1086 | 192 | 894 | .77 |
| 1 y | 25 (23-28) | 26 (20-33) | 25 (22-28) | .73 |
| 2 y | 33 (30-36) | 35 (28-42) | 33 (29-36) | .54 |
| Relapse/progression | ||||
| No. evaluated | 1086 | 192 | 894 | .04 |
| 1 y | 32 (30-35) | 38 (31-45) | 31 (28-34) | .09 |
| 2 y | 37 (34-40) | 43 (36-50) | 35 (32-38) | .04 |
| PFS | ||||
| No. evaluated | 1086 | 192 | 894 | .001 |
| 1 y | 42 (39-45) | 36 (29-43) | 44 (40-47) | .04 |
| 2 y | 30 (27-33) | 22 (16-28) | 32 (29-36) | .003 |
| OS | ||||
| No. evaluated | 1106 | 207 | 899 | <.001 |
| 1 y | 50 (47-53) | 42 (35-49) | 52 (49-56) | .007 |
| 2 y | 36 (33-39) | 26 (21-33) | 39 (35-42) | <.001 |
Prob., probability.
P value for significance between the 2000-2007 and 2008-2013 cohorts.
Neutrophil engraftment was not consistently captured prior to 2008.
Figure 2.
Allogeneic HCT outcomes in adults 70 years and older. (A) OS after HCT in patients ≥70 years by year of transplant (2000-2007 vs 2008-2013). (B) Disease-free survival after HCT in patients ≥70 years with AML in remission, myelodysplasia, and chemotherapy-sensitive NHL from 2008 to 2013. (C) OS after HCT in patients ≥70 years with AML in remission, myelodysplasia, and chemotherapy-sensitive NHL from 2008 to 2013. (D) Disease-free survival after HCT in patients ≥70 years from 2008 to 2013 according to DRI. (E) OS after HCT in patients ≥70 years from 2008 to 2013 according to DRI.
The most frequent primary cause of death was relapse/progression of primary disease, which accounted for 46% of deaths. Infection and GVHD were each reported as the primary cause of death in 10%. Cause of death could not be clarified in 22% of the patients.
Prognostic factor analysis in modern cohorts
The first subset analysis focused on all patients ≥70 years transplanted between 2008 and 2013. Significant prognostic factors for inferior survival included high comorbidity defined by HCT-comorbidity index ≥3 (hazard ratio [HR], 1.27; 95% CI, 1.07-1.51; P = .006), receipt of cord blood as a donor source (HR, 1.97; 95% CI, 1.37-2.82; P = .0002) relative to HLA matched sibling donor, and use of a myeloablative conditioning regimen (HR, 1.61; 95% CI, 1.25-2.08; P = .0002) (Table 3). All other variables tested in the model, including age, disease status, disease, and KPS, were not found to significantly influence survival.
Table 3.
Multivariate models for overall mortality (1 − OS) and TRM in patients aged 70 years and older who received an allogeneic HCT
| Effect | No. | HR | 95% CI | P |
|---|---|---|---|---|
| Overall mortality | ||||
| HCT-comorbidity index | ||||
| 0-2 | 479 | 1.000 | — | — |
| ≥3 | 415 | 1.269 | 1.070, 1.504 | .0061 |
| Donor type | .0046* | |||
| HLA-matched, related | 202 | 1.000 | — | — |
| HLA-mismatched, related | 67 | 1.209 | 0.848, 1.724 | .2932 |
| HLA-matched, URD < = 30 y old | 233 | 0.933 | 0.726, 1.199 | .5874 |
| HLA-matched, URD > 30 y old | 210 | 1.144 | 0.898, 1.457 | .2754 |
| HLA-matched, URD of unknown age | 19 | 0.838 | 0.425, 1.649 | .6080 |
| HLA-mismatched, URD | 67 | 1.324 | 0.945, 1.854 | .1025 |
| HLA matching unknown, URD | 45 | 1.178 | 0.782, 1.775 | .4340 |
| UCB | 51 | 1.963 | 1.372, 2.809 | .0002 |
| Conditioning intensity | ||||
| RIC/NMA | 791 | 1.000 | — | — |
| Overall mortality for patients with early/intermediate AML/MDS and chemosensitive NHL | ||||
| Sex | ||||
| Male | 359 | 1.00 | — | — |
| Female | 165 | 1.30 | 1.03-1.65 | .03 |
| Conditioning intensity | ||||
| RIC/NMA | 473 | 1.00 | — | |
| MA | 50 | 1.60 | 1.13-2.28 | .009 |
| TRM for patients with early/intermediate AML/MDS and chemosensitive NHL | ||||
| Conditioning intensity | ||||
| RIC/NMA | 473 | 1.00 | — | — |
| MA | 50 | 2.06 | 1.32-3.22 | .001 |
The first model for overall mortality in a population from 2008 to 2013 and all hematologic malignances. The second multivariate analysis includes models for overall mortality for patients with early/intermediate AML/MDS and chemosensitive NHL and TRM for a subset of patients with early and intermediate AML and MDS who received an allogenic HCT from 2008 to 2013.
MA, myeloablative; UCB, umbilical cord blood; URD, unrelated donor.
Donor type variable was tested with 7 degrees of freedom and divided the group of matched unrelated donor according to donor age divided at the median.
Table 3 highlights variables significantly associated with TRM and OS among the subset of 416 HCT recipients with early or intermediate AML or MDS or chemosensitive NHL transplanted between 2008 and 2013. Female relative to male recipients (HR, 1.30; 95% CI, 1.03-1.65; P = .029) and myeloablative conditioning as opposed to RIC/NMA (HR, 1.60; 95% CI, 1.13-2.28; P = .009) were associated with higher mortality. Conditioning regimen intensity was the only significant covariate associated with TRM (HR, 2.06; 95% CI, 1.32-3.22; P = .001) in this subset.
Supplemental Figure 1A-D demonstrates OS and TRM, respectively, for patients with AML or MDS who underwent transplantation between 2008 and 2013 based upon both disease risk at time of transplantation (early/intermediate disease vs advanced disease) and also by comorbidity score (ie, HCT <3 vs ≥3). High comorbidity in this population appeared to have a greater adverse impact on patients with advanced or active disease at time of transplant as opposed to patients with early or intermediate disease (ie, in remission) at the time of transplant.
Discussion
In the current observational study, we report on the utilization, characterization and prognostic factors associated with allogeneic HCT in adults ≥70 years. Our findings, derived from a large national cohort of patients transplanted in the United States over the last decade, demonstrate that the absolute number and proportion of allogeneic transplants performed for adults in their eighth decade has risen steadily on a yearly basis since 2000, such that patients ≥70 years now represent nearly 4% of allogeneic HCT recipients.
Several factors spurred the increase in transplant volume for this population. The majority of growth was in fact due to increasing numbers of patients with early or intermediate-risk AML or MDS as defined by the CIBMTR (ie, AML in CR1 or greater; MDS with <5% blasts) transplanted since 2008. Greater utilization of HCT for MDS beginning after 2010 coincides with the decision in the United States to cover this disease indication for Medicare beneficiaries participating in an approved clinical study through the Center for Medicare and Medicaid Services Coverage with Evidence Development.23 Additionally, the increasing use of unrelated donors (and, to some extent, haploidentical or cord blood donor stem cell sources) relative to sibling donors fueled transplant growth by substantially expanding donor availability. Perhaps the most important yet difficult to measure factor is the societal changes that have prompted physician and patient willingness to consider transplant. Similar trends in uptake for older patients have been reported in autologous transplants where donor sources are irrelevant, as well as in solid organ transplantation.24,25
Despite increased utilization of HCT for this older adult population, the relatively small number of allogeneic transplants confirms reports by others26,27 that the vast majority of adults aged ≥70 years with transplant-eligible hematologic malignancies are not undergoing HCT. For example, according to the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, there are ∼3000 new cases of AML diagnosed per year in adults aged 70 to 79 years in the United States (http://seer.cancer.gov/). Our data reveal that only 253 AML patients aged ≥70 years underwent allogeneic HCT between 2012 and 2013, representing only 4% of the population of newly diagnosed AML patients in their eighth decade of life. Transplant was even more rarely pursued in this age group for other disease indications, such as NHL (n = 113) and acute lymphoblastic leukemia (n = 25).
The lack of transplant outcome data in this age group has been a major impediment and previously has been restricted to reports including small numbers of these patients.11,27 We found that transplant survival has significantly improved over time for this older adult population, with 2-year PFS and OS estimates of 32% (95% CI, 29% to 36%) and 39% (95% CI, 35% to 42%), respectively, for HCT recipients transplanted in the more recent era of 2008-2013. As TRM and severe acute GVHD did not change over time, improvements were likely mediated in part by transplant in patients with less advanced disease. Consistent with this, a greater proportion of patients entered transplant with earlier-risk disease (recognizing many were missing disease risk in earlier years), and improvements occurred in PFS and cumulative incidence of disease relapse.
Given the methodological issues and biases associated with retrospectively comparing treatment approaches, prospective studies are required to effectively evaluate HCT versus non-HCT therapies in older adults for each disease indication. Few studies report outcomes of patients ≥70 years after nontransplant treatment to benchmark these results, and lack of consistent patient health information reported in these studies hinders identification of transplant-eligible subsets. In a population study of AML from the Netherlands, 69% of patients ≥70 years received some form of chemotherapy, but no patients were allografted, resulting in dismal 1- and 5-year survival rates of 15% and 2%, respectively, for patients diagnosed between 2007 and 2012.28 Thus, our study outcomes for AML patients transplanted between 2008 and 2013, revealing 2-year PFS and OS of 36% and 38%, respectively, appear extremely encouraging and suggest that select older patients achieve a substantial disease and survival benefit after HCT.
Concerns regarding treatment-related morbidity and mortality likely account for reluctance to offer transplant to older patients. Our findings that TRM has remained stable over the decade, with 2-year estimates at 30% to 35%, is similar to the findings of the registry analysis, where 2-year TRM ranged from 25% to 39% in adults ≥40 years with AML and MDS following RIC HCT, without significant differences across age groups.7 Registry summaries inclusive of heterogeneous disease status, donor type, and conditioning regimen may be less favorable than institutional protocol reports of more selected patients yet reflect present population-based practice patterns and results. Although supportive care for hematologic malignancy and transplant recipients has improved considerably in recent years, so much so as to allow the application of allogeneic HCT to patients ≥70 years, appreciable TRM remains a barrier to transplant utilization and success in this group. Impairments in adaptive immune response with aging are accentuated in the allogeneic transplant setting and likely contribute to the relatively high rates of transplant-related morbidity and mortality in older patients.29 Strategies to overcome thymic impairments with aging and allogeneic transplant are of considerable interest.30 Higher rates of TRM in those patients 70 years and older with advanced disease and high comorbidity suggest that transplant should be pursued with particularly caution in such patients.
To reduce TRM, we must consider improvements in patient selection and further refine the transplant process to allow for less toxicity and morbidity in older adults. Similar to others,11 we found that inferior survival was associated with high comorbidity, albeit with only a modest effect (HR, 1.27; 95% CI, 1.07-1.51). Patients ≥75 years did not fare significantly worse than those 70 to 74 years of age. Therefore, age alone or moderate degrees of comorbidity are insufficient to determine transplant eligibility. Enhanced discrimination may be obtained with the use of geriatric assessment prior to HCT.31 Geriatric assessment for evaluation and guided interventions warrant additional research to abrogate morbidity and mortality.15,32 More precise risk stratification for TRM, if not survival, may paradoxically expand the number of older patients eligible for transplant by delineating a larger pool of patients with acceptable transplant risks.
Similar to younger patients, disease relapse remains the primary cause of death in older transplant recipients. However, more intensive transplant conditioning via myeloablative regimens was associated with worse OS in the whole cohort and in the subset of early/intermediate AML/MDS and chemosensitive NHL. While new disease scoring systems such as the DRI- and minimal residual disease–based assessments have been used to evaluate relapse risk in younger HCT patients, the importance of these tools in older patients is less established.21,33 For example, independent of the DRI, older patients fare worse, and minimal residual disease measures have a less pronounced effect on relapse after NMA transplant for AML and have not been shown to affect survival in this population.33 This likely reflects the adverse biology of disease in older age in that even after adjustments for adverse disease features, older adults routinely demonstrate outcomes significantly inferior to those of younger adults.34,35 This underscores the need to move to transplant expeditiously after initial treatment–induced response as well as to explore posttransplant maintenance and adoptive immunotherapy approaches following RIC or NMA allogeneic HCT in older adults.
In summary, the utilization of allogeneic HCT in adults aged ≥70 years with hematologic malignancies has markedly increased over the past decade. Nearly 40% of adults in this age group are alive at 2 years following transplant, suggesting that this approach is feasible, offers promising disease control, and should be considered more frequently for patients in their eighth decade with transplant-eligible diseases.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The CIBMTR is supported primarily by National Institutes of Health public health service grant/cooperative agreement 5U24-CA076518 from the National Cancer Institute; the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; grant/cooperative agreement 5U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute; contract HHSH250201200016C with Health Resources and Services Administration (US Department of Health and Human Services); grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research. This work was also funded by grants from Actinium Pharmaceuticals, Inc.,* Alexion, and Amgen, Inc.*; by an anonymous donation to the Medical College of Wisconsin; by Astellas Pharma US, AstraZeneca, Atara Biotherapeutics, Inc., Be the Match Foundation, Bluebird Bio, Inc.,* Bristol Myers Squibb Oncology,* Celgene Corporation,* Cellular Dynamics International, Inc., Cerus Corporation, Chimerix, Inc.,* the Fred Hutchinson Cancer Research Center, Gamida Cell Ltd., Genentech, Inc., Genzyme Corporation, Gilead Sciences, Inc., Health Research, Inc., Roswell Park Cancer Institute, HistoGenetics, Inc., Incyte Corporation, Janssen Scientific Affairs, LLC, Jazz Pharmaceuticals, Inc.,* the Jeff Gordon Children’s Foundation, The Leukemia & Lymphoma Society, Medac, GmbH, MedImmune, The Medical College of Wisconsin, Merck & Co, Inc.,* Mesoblast,* MesoScale Diagnostics, Inc., Miltenyi Biotec, Inc.,* National Marrow Donor Program, Neovii Biotech NA, Inc., Novartis Pharmaceuticals Corporation, Onyx Pharmaceuticals, Optum Healthcare Solutions, Inc., Otsuka America Pharmaceutical, Inc., Otsuka Pharmaceutical Co, Ltd., Japan, PCORI, Perkin Elmer, Inc., Pfizer, Inc., Sanofi US,* Seattle Genetics,* Spectrum Pharmaceuticals, Inc.,* St. Baldrick’s Foundation, Sunesis Pharmaceuticals, Inc.,* Swedish Orphan Biovitrum, Inc., Takeda Oncology, Telomere Diagnostics, Inc., the University of Minnesota, and Wellpoint, Inc.* (asterisk indicates corporate members).
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US Government.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.M., A.A., M.C.P., R.B., M.M., X.Z., K.A., M.A., K.K.B., A. Bajel, F.B., M.B., A. Beitinjaneh, J.-Y.C., M.C., Y.-B.C., S. Chhabra, S. Ciurea, E.C., A.D., J.E., J.F., C.O.F., H.C.F., R.P.G., S.G., S.K.H., G.C.H., V.H., A.J., H.L., M.R.L., R. Martino, R. Maziarz, P.M., T.N., R.O., R.F.O., A.P., E.P., A.R.R., D.R., B.N.S., H.C.S., M. Sabloff, M. Seftel, S.S., M.L.S., J.S., B.M.W., and W.A.W. designed research; L.M., A.A., M.C.P., R.B., M.M., and X.Z. performed research; L.M., A.A., M.C.P., R.B., M.M., X.Z., K.A., M.A., K.K.B., A. Bajel, F.B., M.B., A. Beitinjaneh, J.-Y.C., M.C., Y.-B.C., S. Chhabra, S. Ciurea, E.C., A.D., J.E., J.F., C.O.F., H.C.F., R.P.G., S.G., S.K.H., G.C.H., V.H., A.J., H.L., M.R.L., R. Martino, R. Maziarz, P.M., T.N., R.O., R.F.O., A.P., E.P., A.R.R., D.R., B.N.S., H.C.S., M. Sabloff, M. Seftel, S.S., M.L.S., J.S., B.M.W., and W.A.W. analyzed and interpreted data; R.B., M.M., and X.Z. performed statistical analysis; and L.M., A.A., M.C.P., R.B., M.M., and X.Z. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lori Muffly, Stanford University, 300 Pasteur Dr, H0144B, Stanford, CA 94305; e-mail: lmuffly@stanford.edu.
References
- 1.Armitage JO. Bone marrow transplantation. N Engl J Med. 1994;330(12):827-838. [DOI] [PubMed] [Google Scholar]
- 2.McSweeney PA, Niederwieser D, Shizuru JA, et al. . Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390-3400. [DOI] [PubMed] [Google Scholar]
- 3.van Besien K, Artz A, Stock W. Unrelated donor transplantation over the age of 55. Are we merely getting (b)older? Leukemia. 2005;19(1):31-33. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Klein J, Haagenson M, et al. . High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576-4583. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza A, Zhu X. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, 2016. Available at: https://www.cibtmr.org. Accessed 1 February 2017.
- 6.Chevallier P, Szydlo RM, Blaise D, et al. . Reduced-intensity conditioning before allogeneic hematopoietic stem cell transplantation in patients over 60 years: a report from the SFGM-TC. Biol Blood Marrow Transplant. 2012;18(2):289-294. [DOI] [PubMed] [Google Scholar]
- 7.McClune BL, Weisdorf DJ, Pedersen TL, et al. . Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schetelig J, Bornhäuser M, Schmid C, et al. . Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26(32):5183-5191. [DOI] [PubMed] [Google Scholar]
- 9.Lim Z, Brand R, Martino R, et al. . Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405-411. [DOI] [PubMed] [Google Scholar]
- 10.Wallen H, Gooley TA, Deeg HJ, et al. . Ablative allogeneic hematopoietic cell transplantation in adults 60 years of age and older. J Clin Oncol. 2005;23(15):3439-3446. [DOI] [PubMed] [Google Scholar]
- 11.Sorror ML, Sandmaier BM, Storer BE, et al. . Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidenreich S, Ziagkos D, de Wreede LC, et al. . Allogeneic stem cell transplantation for patients age ≥70 years with myelodysplastic syndrome: a retrospective study of the MDS Subcommittee of the Chronic Malignancies Working Party of the EBMT. Biol Blood Marrow Transplant. 2017;23(1):44-52. [DOI] [PubMed] [Google Scholar]
- 13.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006;24(15):2304-2310. [DOI] [PubMed] [Google Scholar]
- 14.Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age Ageing. 2015;44(1):162-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wildiers H, Heeren P, Puts M, et al. . International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtman SM. Therapy insight: therapeutic challenges in the treatment of elderly cancer patients. Nat Clin Pract Oncol. 2006;3(2):86-93. [DOI] [PubMed] [Google Scholar]
- 17.Pasquini MC, Wang Z, Horowitz M, et al. . 2013 Report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplant for blood and bone marrow disorders. In: Everly JE, Terazaki PI, eds. Clinical Transplants 2013. Los Angeles: The Terasaki Foundation Laboratory; 2014:187-198. [PubMed] [Google Scholar]
- 18.American Society for Blood and Marrow Transplant. ASBMT RFI 2016: Disease Classifications Corresponding to CIBMTR Classifications. Available at: http://asbmt.org/sites/default/files/asbmt_admin/ASBMT_RFI_2016_CIBMTR_Diseas.pdf. Accessed 1 February 2017.
- 19.Bacigalupo A, Ballen K, Rizzo D, et al. . Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giralt S, Ballen K, Rizzo D, et al. . Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armand P, Kim HT, Logan BR, et al. . Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atallah E, Horowitz MM, Logan B, et al. Outcome of patients 65 years and older with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation compared to patients 55–64 years of age. Blood 2015;126(23):193. [Google Scholar]
- 24.Abecassis M, Bridges ND, Clancy CJ, et al. . Solid-organ transplantation in older adults: current status and future research. Am J Transplant. 2012;12(10):2608-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auner HW, Garderet L, Kröger N. Autologous haematopoietic cell transplantation in elderly patients with multiple myeloma. Br J Haematol. 2015;171(4):453-462. [DOI] [PubMed] [Google Scholar]
- 26.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner AM, Kim HT, Coughlin E, et al. . Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2013;19(9):1374-1380. [DOI] [PubMed] [Google Scholar]
- 28.Dinmohamed AG, Visser O, van Norden Y, et al. . Treatment, trial participation and survival in adult acute myeloid leukemia: a population-based study in the Netherlands, 1989-2012. Leukemia. 2016;30(1):24-31. [DOI] [PubMed] [Google Scholar]
- 29.Krenger W, Blazar BR, Holländer GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood. 2011;117(25):6768-6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhry MS, Velardi E, Malard F, van den Brink MR. Immune reconstitution after allogeneic hematopoietic stem cell transplantation: time to T up the thymus. J Immunol. 2017;198(1):40-46. [DOI] [PubMed] [Google Scholar]
- 31.Muffly LS, Boulukos M, Swanson K, et al. . Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19(3):429-434. [DOI] [PubMed] [Google Scholar]
- 32.Artz AS, Chow S. Hematopoietic cell transplantation in older adults: deciding or decision-making? Bone Marrow Transplant. 2016;51(5):643-644. [DOI] [PubMed] [Google Scholar]
- 33.Walter RB, Gyurkocza B, Storer BE, et al. . Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29(1):137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mrózek K, Marcucci G, Nicolet D, et al. . Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg PL, Tuechler H, Schanz J, et al. . Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.