Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Survival of patients with MF after ruxolitinib discontinuation is poor, with median survival of 14 months.
Low platelets at the start or end of therapy or clonal evolution while on therapy are associated with an even worse prognosis.
Abstract
Despite significant improvements in the signs and symptoms of myelofibrosis (MF), and possible prolongation of patients’ survival, some have disease that is refractory to ruxolitinib and many lose their response over time. Furthermore, patients with ≥3 mutations are less likely to respond to ruxolitinib. Here we describe outcomes after ruxolitinib discontinuation in MF patients enrolled in a phase 1/2 study at our center. After a median follow-up of 79 months, 86 patients had discontinued ruxolitinib (30 of whom died while on therapy). The median follow-up after ruxolitinib discontinuation for the remaining 56 patients was 32 months, with median survival after discontinuation of 14 months. Platelets <260 × 109/L at the start of therapy or <100 × 109/L at the time of discontinuation were associated with shorter survival after discontinuation. Of 62 patients with molecular data at baseline and follow-up, 22 (35%) acquired a new mutation while receiving ruxolitinib (14 [61%] in ASXL1). Patients showing clonal evolution had significantly shorter survival after discontinuation (6 vs 16 months). Transfusion dependency was the only clinical variable associated with clonal evolution. These findings underscore the need for novel therapies and suggest that clonal evolution or decreasing platelet counts while on ruxolitinib therapy may be markers of poor prognosis.
Introduction
The introduction of ruxolitinib as a standard therapy for intermediate- or high-risk myelofibrosis (MF) has transformed the treatment landscape for this debilitating disease. Long-term follow-up studies of its safety and efficacy confirm that it provides rapid and durable improvements in symptoms, splenomegaly, and quality of life and that it improves survival compared with placebo or best available therapy.1,2 Despite these significant improvements in the signs and symptoms of the disease and prolongation of life in some patients with advanced features, some have refractory disease and many lose their response over time.1 In COMFORT-I and COMFORT-II, ruxolitinib discontinuation rates were ∼50% by 3 years.3,4 In addition, we have reported that MF patients treated with ruxolitinib in a phase 1/2 trial who harbored 3 or more mutations of any type at the start of therapy were less likely to have a response to ruxolitinib.5 Whether additional mutations are acquired during therapy and contribute to relapse, disease progression, or outcomes after discontinuation of ruxolitinib has not been explored. Understanding outcomes after ruxolitinib failure is important for identifying patients who might benefit from specific interventions, such as allogeneic stem cell transplantation (ASCT) or novel investigational agents (eg, IDH1/2 inhibitors). Here, we report outcomes of patients with MF following ruxolitinib discontinuation at our center.
Methods
We conducted a retrospective chart review study of 107 patients with MF who received ruxolitinib at MD Anderson Cancer Center as part of a phase 1/2 open-label, multicenter, nonrandomized study; these patients were enrolled between June 2007 and September 2011.6 Detailed eligibility criteria and treatment schema were previously reported.4 Spleen response was assessed as defined by 2006 International Working Group for Myelofibrosis Research and Treatment criteria.7 This study was approved by the Institutional Review Board at MD Anderson Cancer Center and was conducted in accordance with the principles of the Declaration of Helsinki. The clinical trial is registered at clinicaltrials.gov (NCT00509899).
DNA was purified from bone marrow aspirate samples collected from patients before starting ruxolitinib therapy and within 6 months of ruxolitinib discontinuation as previously described.5 The entire coding sequences of 28 genes (ABL1, ASXL1, BRAF, DNMT3A, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, KIT, KRAS, MDM2, IKZF2, JAK2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TET2, TP53, and WT1) known to be mutated in myeloid hematologic malignancies were sequenced using the Illumina MiSeq platform as previously described.8 Testing for SRSF2 mutations was not performed. Testing for CALR mutations was performed separately, as previously described.9
Categorical variables were compared using χ2 or Fisher’s exact test and continuous variables were compared using Mann-Whitney or Kruskal-Wallis tests. Because platelets <100 × 109/L were an exclusion criterion for the study, we used the median platelet count at baseline (260 × 109/L) as the cut point for our analysis. We used a cut point of <100 × 109/L for follow-up, because this is a known negative prognostic factor in MF.10 Survival probabilities were calculated using the Kaplan-Meier method and were assessed from either the start of therapy or the date of ruxolitinib discontinuation to date of last follow-up or death. Patients who had ASCT were censored at the date of transplant. The log-rank test was used for survival comparisons. Cox regression was used to calculate hazard ratios. Median follow-up was calculated using the reverse Kaplan-Meier method. All P values are 2-tailed and were considered significant when <.05. Statistical analyses were performed using SPSS v.22 (IBM).
Results
Patient disposition at the start and end of therapy
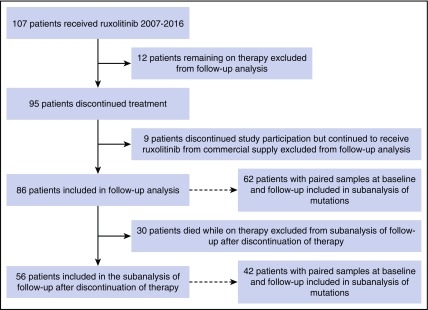
Of 107 patients enrolled, 95 (89%) had discontinued protocol participation as of September 2016; 9 of them discontinued study participation but continued to receive ruxolitinib commercially and were excluded from the analysis (7 of 9 lost to follow-up) (Figure 1). The median follow-up from the start of ruxolitinib therapy for the 86 patients who had discontinued therapy was 79 months (range, 76-82 months). Comparing clinical characteristics at the start and end of ruxolitinib treatment shows that patients had significantly lower platelet counts and hemoglobin levels and smaller spleen size at the time of ruxolitinib discontinuation (Table 1). In addition, significantly more patients were transfusion dependent, and significantly more had abnormal cytogenetics, including complex karyotype, at discontinuation. Reasons for discontinuation have been already discussed in detail elsewhere11 and included lack of response (N = 7) or intolerance (myelosuppression; N = 2), disease progression (including 6 with transformation to acute myeloid leukemia [AML]) or relapse (N = 36), other illness or patient choice (N = 11), or death (N = 30). Causes of death included multiorgan failure due to progressive disease (N = 12), myocardial infarction (N = 5), renal failure (N = 3), congestive heart failure (N = 2), sepsis (N = 2), stroke (N = 1), diverticular hemorrhage (N = 1), aortic aneurysm (N = 1), brain aneurysm (N = 1), metastatic pancreatic cancer (N = 1), and complications of portal hypertension (N = 1).
Figure 1.
CONSORT diagram. Patient enrollment, discontinuation, and analysis schema are shown.
Table 1.
Patient characteristics at the start and end of ruxolitinib treatment (N = 86)
| Parameter | Baseline, N (%) | At treatment discontinuation, N (%) | P |
|---|---|---|---|
| Age ≥ 65 y | 45 (52) | 58 (67) | <.0001 |
| Sex (female) | 36 (42) | 36 (42) | |
| Diagnosis | |||
| Primary | 56 (65) | 56 (65) | |
| Post ET | 9 (11) | 9 (11) | |
| Post PV | 21 (24) | 21 (24) | |
| Hgb < 10 g/dL | 39 (45) | 52 (61) | .002 |
| PLT, median (range), ×109/L | 262 (13-1183) | 91 (11-922) | <.001 |
| WBC ≥ 25 × 109/L | 29 (34) | 34 (40) | .493 |
| PB blasts ≥1% | 44 (51) | 51/80 (64) | .356 |
| PS > 0 | 73 (85) | 80 (93) | .053 |
| Spleen size,* median (range), cm | 20 (0-30) | 14 (0-36) | <.001 |
| Transfusion-dependent | 25 (29) | 36/84 (43) | .001 |
| JAK2 allele burden (range), % | 79 (20-99) | 65 (6-99) | .411 |
| Cytogenetic | |||
| Diploid | 22/53 (42) | 16/53 (30) | <.0001 |
| Abnormal | 31/53 (59) | 37/53 (70) | |
| Complex karyotype† | 7/53 (13) | 13/53 (25) | .007 |
BM, bone marrow; Hgb, hemoglobin; NA, not available; PB, peripheral blood; PLT, platelets; WBC, white blood cell count.
Evaluable for spleen size at baseline (82/86) and evaluable at discontinuation (68/86).
Data reported for 50 patients with cytogenetic information at baseline and follow-up. At baseline, 39/85 patients had abnormal cytogenetics (8 of which were complex karyotype). Complex karyotype is defined as 3 or more unrelated abnormalities.
Outcomes after discontinuation of therapy
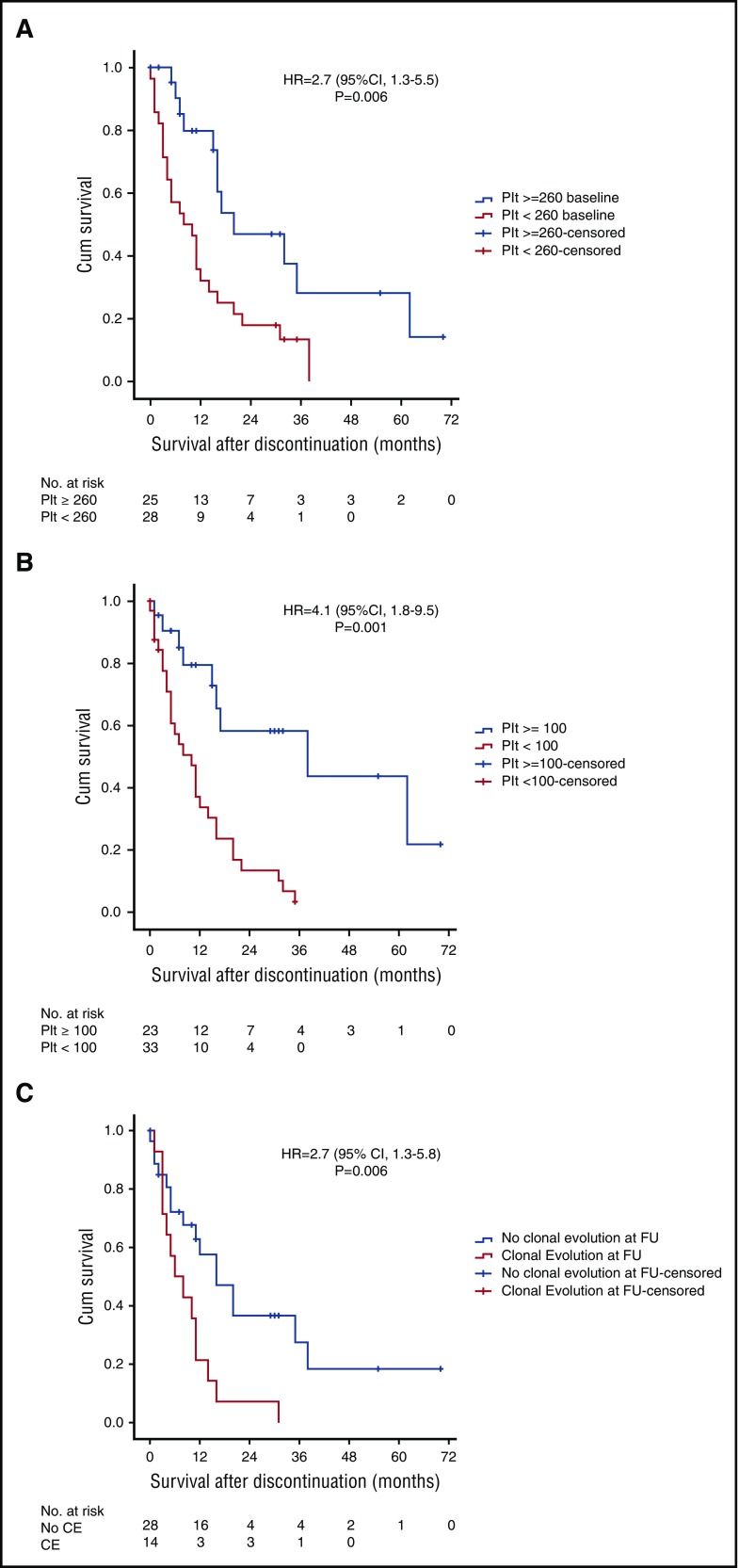
Thirty patients (35%) died while taking ruxolitinib. Therefore, follow-up data after ruxolitinib discontinuation were available for 56 (65%) patients. At the time of discontinuation, 6 (11%) patients had transformation to AML, 37 (66%) had progressive disease, and 13 (23%) had stable disease. Treatments received after ruxolitinib discontinuation included hydroxyurea (n = 15), investigational agents (n = 13), splenectomy (n = 9), ASCT (n = 7), hypomethylating agents (n = 6), induction chemotherapy (n = 3), and anagrelide (n = 1). Eight patients received >1 line of therapy after stopping ruxolitinib. The median follow-up time after discontinuation of ruxolitinib was 32 months (range, 26-38 months), with a median survival time of 14 months (95% confidence interval [CI], 10-18 months). Survival after discontinuation did not differ by reason for discontinuation (data not shown). Platelets <260 × 109/L at the start of ruxolitinib therapy or <100 × 109/L at the time of discontinuation were the only clinical variables associated with shorter survival after discontinuation (Figure 2A-B).
Figure 2.
Kaplan-Meier survival curves showing survival after ruxolitinib discontinuation. Comparison of survival after discontinuation by platelet counts at (A) baseline and (B) follow-up. (C) Comparison of survival after ruxolitinib discontinuation in those with and without clonal evolution. Cum, cumulative; FU, follow-up; HR, hazard ratio.
Molecular changes during therapy
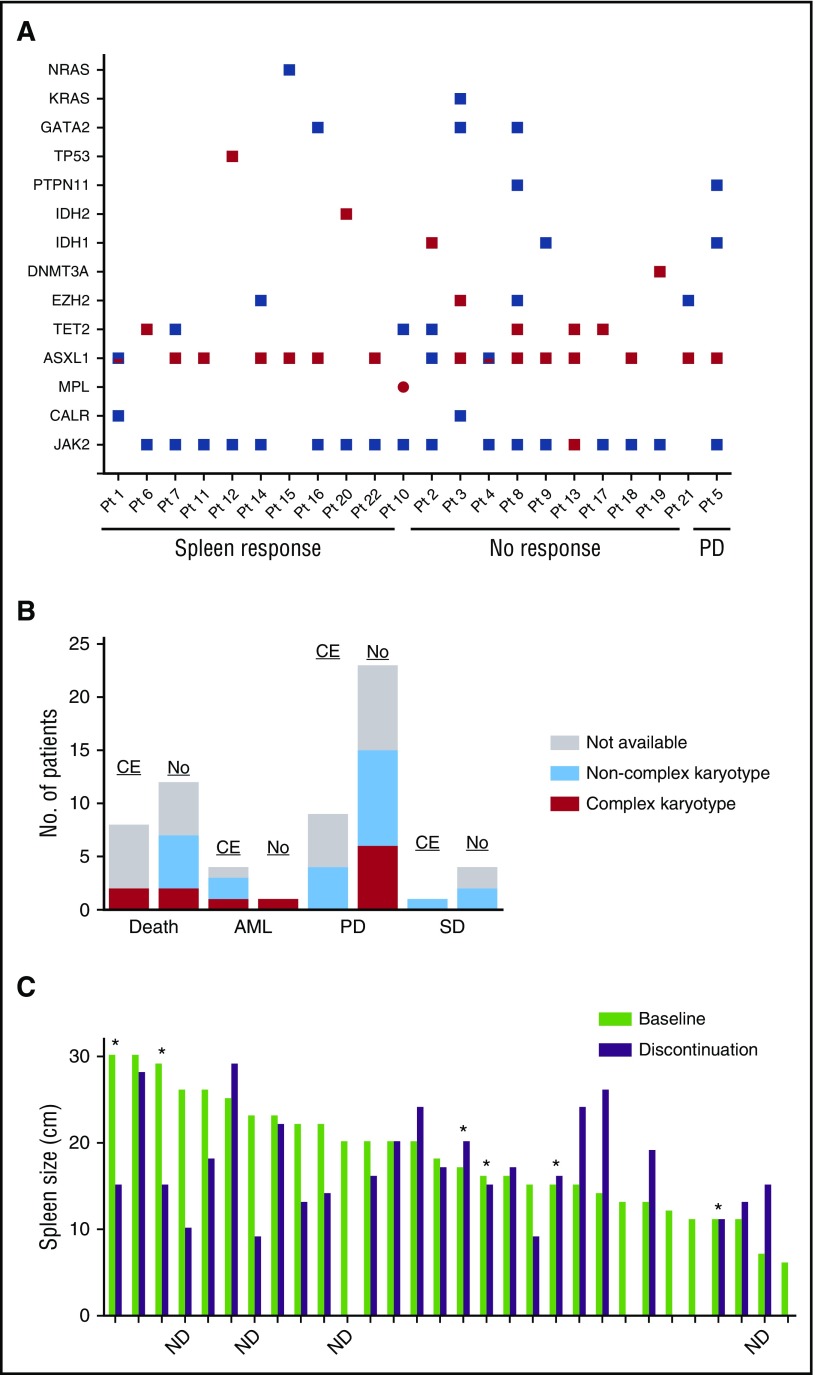
Paired samples collected at the start (baseline) of ruxolitinib therapy and at the time of discontinuation (follow-up) were available for 62 of 86 patients. Of these 62, 53 (85%) had a JAK2 mutation, 6 (10%) had CALR mutations, and 3 (5%) had MPL mutations. Details of other mutations at baseline were previously reported.5 The other most frequent mutations included ASXL1 (26%) and TET2 (15%) (Figure 3A; supplemental Table 1, available on the Blood Web site). Twenty-two of 62 (36%) patients had acquired a new mutation at follow-up (Figure 3A; supplemental Table 2). Of those, 8 (35%) died while on therapy. Acquired mutations were found most frequently in the ASXL1 gene (n = 15 patients; 68%), followed by TET2 (n = 4; 17%), EZH2 (n = 2; 9%), and TP53 (n = 2; 9%). New mutations in DNMT3A, MPL, IDH1, and IDH2 were each found in 1 patient (supplemental Table 2). Three patients had acquired a mutation in >1 gene at the time of discontinuation: ASXL1/EZH2 in patient 3, ASXL1/TET2 in patient 8, and ASXL1/TET2/JAK2 in patient 13 (Figure 3A). Eight of the 15 ASXL1 mutations were found at the same site (G646fs*11), and 14 of 15 were frameshift mutations. The allele frequencies of the driver mutations (in JAK2, CALR, MPL) at baseline and follow-up were not significantly different (supplemental Table 2). Complex karyotype (≥3 unrelated abnormalities) at the time of discontinuation was not associated with clonal evolution, as only 1 of 10 patients with complex karyotype at discontinuation had acquired a new mutation (Figure 3B).
Figure 3.
Correlation between clonal evolution and disease status. (A) Mutations at baseline and follow-up in patients who acquired a mutation while receiving ruxolitinib. Red squares denote an acquired mutation. Red/blue squares denote the acquisition of a second, different mutation in the same gene. Note that at the time of follow-up, patient (Pt) 3 had lost the GATA2 and KRAS mutations present at baseline. (B) Bar graph depicting disease status at the time of ruxolitinib discontinuation in patients with (CE) and without (No) clonal evolution. Bars are colored according to karyotype: gray, sample was not available; light blue, noncomplex karyotype; red, complex karyotype. (C) Bar graph depicting spleen size at baseline (light green) and at the time of ruxolitinib discontinuation (purple). Asterisks indicate patients who acquired a new mutation while on therapy. ND, mutation status at discontinuation not determined. PD, progressive disease; SD, stable disease.
Outcomes of patients with clonal evolution after ruxolitinib discontinuation
Of patients who were alive at the time of discontinuation (n = 56), 42 (75%) had molecular data at baseline and follow-up, 14 (33%) of whom acquired a new mutation (ASXL1 in 9 [60%]). Patients with clonal evolution had significantly shorter survival after discontinuation than those without clonal evolution (6 months [95% CI, 0.5-11.5] vs 16 months [95% CI, 7.9-24]; P = .006; Figure 2C). Red blood cell transfusion dependency at baseline was the only clinical variable associated with clonal evolution (48% vs 15%; P = .001; Table 2). The average ruxolitinib dose received did not differ between those with and those without clonal evolution (P = .45). Among the 42 evaluable patients, the majority (n = 32, 76%) discontinued ruxolitinib due to disease progression, 8 patients due to lack of response/intolerance, and 2 patients who chose to discontinue treatment. Twelve of 32 (38%) patients with disease progression (4 AML transformation), 2 of 6 (33%) with no response, and 1 of 2 (50%) with persistent anemia showed clonal evolution at the time of discontinuation. Forty of 42 (95%) patients were evaluable for a spleen response. Achievement of an International Working Group–defined spleen response while receiving ruxolitinib was not associated with longer survival after discontinuation (11 vs 12 months; P = .428) nor was it associated with clonal evolution (8/18 patients with clonal evolution had no spleen response vs 9/38 without clonal evolution; P = .132). Likewise, the degree to which a patient lost their spleen response was not associated with clonal evolution (Figure 3C).
Table 2.
Characteristics of patients with and without clonal evolution
| Start of ruxolitinib | P | Discontinuation | P | |||
|---|---|---|---|---|---|---|
| No clonal evolution (N = 40) | Clonal evolution (N = 22) | No clonal evolution (N = 40) | Clonal evolution (N = 22) | |||
| Age, y | 65 (43-80) | 68 (51-84) | .310 | 70 (44-84) | 70 (53-87) | .517 |
| Female | 16 (40) | 8 (36) | 1.00 | |||
| Diagnosis | .461 | |||||
| PMF | 26 (65) | 16 (73) | ||||
| PPV | 8 (20) | 5 (23) | ||||
| PET | 6 (15) | 1 (4) | ||||
| Median Hgb, g/dL | 10.9 (7.4-16.9) | 10.3 (7.2-14.3) | .576 | 9.6 (5.7-15.4) | 8.8 (6.1-12.1) | .233 |
| Hgb < 10 g/dL | 14 (35) | 11 (50) | .288 | 21 (53) | 14 (64) | .435 |
| Median WBC (range), ×109/L | 18 (3-52) | 25 (3-159) | .119 | 16.4 (2.8-225) | 31 (3.5-142) | .328 |
| WBC > 25 × 109/L | 10 (25) | 10 (46) | .155 | 15 (38) | 12 (55) | .285 |
| Median Plt (range), ×109/L | 262 (103-969) | 220 (101-761) | .162 | 105 (11-922) | 74 (20-430) | .188 |
| Plt < 250 × 109/L | 18 (45) | 13 (59) | .426 | |||
| Plt < 100 × 109/L | 0 | 0 | 20(50) | 16 (73) | .109 | |
| Median PBBL (range), % | 0 (0-8) | 1.0 (0-4) | .084 | 2 (0-70) | 2 (0-21) | .619 |
| Median LDH (range), IU | 1704 (661-4309) | 1974 (783-5006) | .410 | 2075 (196-4200) | 1617 (660-9711) | .953 |
| Median Spln (range), cm | 20 (0-29) | 16 (0-30) | .782 | 13 (0-29) | 15 (0-24) | .400 |
| TSF Dep, n | 7 (18) | 10 (46) | .035 | 14 (36) | 11 (50) | .416 |
| Abn cytogenetics, n | 18 (45) | 6 (29) | .275 | 17/25 (68) | 6/9 (67) | 1.0 |
| Complex karyotype,* n | 7 (18) | 0/21 | .084 | 9/26 (35) | 1/10 (10) | .223 |
| JAK2+, n | 35 (88) | 18 (82) | .709 | |||
| Median JAK2 (range), % | 60 (0-99) | 54 (0-98) | .872 | 54 (0-99) | 57 (0-98) | .698 |
| Median TTD (range), mo | 31 (2-100) | 23 (2-64) | .414 | |||
Abn, abnormal; BM, bone marrow; LDH, lactate dehydrogenase; PBBL, peripheral blood blasts; PET, post–essential thrombocythemia; Plt, platelets; PMF, primary myelofibrosis; PPV, post–polycythemia vera; Spln, spleen; TSF, transfusion; TTD, time to treatment discontinuation.
Complex karyotype is defined as 3 or more unrelated abnormalities.
Because the majority of acquired mutations were in ASXL1, we compared the characteristics of patients who acquired an ASXL1 mutation (n = 14) with those who did not (n = 48) at the start and discontinuation of ruxolitinib therapy. High WBC and low platelets at discontinuation were the only factors associated with acquisition of an ASXL1 mutation: 10 of 14 (71%) who gained an ASXL1 mutation had WBC > 25 × 109/L and 79% (n = 11/14) had platelets <90 × 109/L at discontinuation compared with 35% (n = 17/48) and 42% (n = 20/48) of the others (P = .03 and P = .031).
In total, 11 (12.6%) patients transformed to AML. Nine transformed at the time of discontinuation, and 3 transformed 9.5, 12.2, and 20.1 months after discontinuation. Median survival from the time of discontinuation was 5.8 months (range, 0-64 months). Median survival from the date of transformation was 3.9 months (range, 0-12 months). Of 8 (73%) patients with paired molecular data, 4 (50%) showed clonal evolution: 2 gained an ASXL1, 1 gained a TET2, and 1 gained a TP53 mutation. Survival after discontinuation ranged from 3 to 11 months.
Of the 7 (12.5%) patients who had stem cell transplant after discontinuation of ruxolitinib, 2 died of complications during transplant, 1 relapsed with AML and died 64 months after discontinuation, 1 died of progressive refractory diffuse large B-cell lymphoma, and 3 were alive at the date of last follow-up. Of 5 patients with paired molecular data, none had acquired a new mutation while receiving ruxolitinib.
Discussion
In this study, we found that survival after ruxolitinib discontinuation is poor, with a median survival of 14 months. We also found that low platelets at the start or end of therapy and the acquisition of mutations (clonal evolution) while on therapy may portend an even worse prognosis.
Thirty-five percent of patients analyzed acquired at least 1 additional mutation during treatment, 64% of which were in the ASXL1 gene. The acquisition of additional mutations during treatment with ruxolitinib poses the question of whether suppression of the initiating clone by ruxolitinib may have allowed the new clone to emerge. Acquired resistance to receptor tyrosine kinase inhibitors is a significant cause of treatment failure in chronic lymphocytic leukemia, AML, and chronic myelogenous leukemia.12 However, in this study, 2 observations argue against that idea: (1) the average dose of ruxolitinib received was not significantly different between those with and without clonal evolution, and (2) the allele frequency of the initial driver mutations (JAK2, CALR, MPL) had not significantly changed at the time of discontinuation. This observation is similar to the findings of Engle and colleagues, who found no change in the allele frequency of the JAK2V617F-containing clone in serial samples from a patient with MF that progressed to AML.13 Instead, the authors observed an expansion of an ASXL1-containing clone and the appearance of a RUNX1/IDH1 clone at the time of transformation, which disappeared at the time of AML remission/MF relapse. We also found that transfusion dependence at baseline (a marker of disease severity) was the only clinical parameter associated with clonal evolution. Although our findings suggest that the appearance/expansion of new clones serves as a marker of disease progression, we cannot rule out the possibility that the emerging clones developed due to selective pressure by ruxolitinib. A larger prospective study comparing the rate of clonal evolution in patients treated with and without ruxolitinib would be necessary to test this hypothesis. In addition, because we performed targeted sequencing of 29 genes, it is not known whether acquired mutations in other genes play a role in MF progression.
Our findings underscore the need for novel therapies for MF that is relapsed/refractory to ruxolitinib and provide further support for the prognostic relevance of mutations in epigenetic modifiers, which have been suggested to be markers of shorter overall and leukemia-free survival in MF.14,15 Earlier intervention with stem cell transplantation or investigational targeted therapies should be explored in patients treated with ruxolitinib showing signs of disease progression.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgment
This research was supported in part by a Cancer Center Support grant to MD Anderson Cancer Center from the National Cancer Institute, National Institutes of Health (P30 CA016672).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.V., E.J., and H.K. designed the study; K.J.N. analyzed the data and wrote the manuscript; K.P. and R.L. provided the molecular data; T.M. provided samples and assisted in the data analysis; and P.B., L.M., N.D., J.C., and H.K. provided patient care. All authors wrote and approved the final manuscript.
Conflict-of-interest disclosure: S.V. receives research funding from Incyte Corporation. The remaining authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: sverstov@mdanderson.org.
References
- 1.Verstovsek S, Mesa RA, Gotlib J, et al. ; COMFORT-I investigators. Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica. 2015;100(4):479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. . Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30(8):1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervantes F, Kiladjian JJ, Niederwieser D, et al. . Long-term safety, efficacy, and survival findings from Comfort-II, a phase 3 study comparing ruxolitinib with best available therapy (BAT) for the treatment of myelofibrosis (MF) [abstract]. Blood. 2012;120(21). Abstract 801. [Google Scholar]
- 4.Verstovsek S, Mesa RA, Gotlib J, et al. . Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel KP, Newberry KJ, Luthra R, et al. . Correlation of mutation profile and response in patients with myelofibrosis treated with ruxolitinib. Blood. 2015;126(6):790-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstovsek S, Kantarjian H, Mesa RA, et al. . Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363(12):1117-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tefferi A, Barosi G, Mesa RA, et al. ; IWG for Myelofibrosis Research and Treatment (IWG-MRT). International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for myelofibrosis research and treatment (IWG-MRT). Blood. 2006;108(5):1497-1503. [DOI] [PubMed] [Google Scholar]
- 8.Luthra R, Patel KP, Reddy NG, et al. . Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica. 2014;99(3):465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang SA, Hasserjian RP, Fox PS, et al. . Atypical chronic myeloid leukemia is clinically distinct from unclassifiable myelodysplastic/myeloproliferative neoplasms. Blood. 2014;123(17):2645-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangat N, Caramazza D, Vaidya R, et al. . DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29(4):392-397. [DOI] [PubMed] [Google Scholar]
- 11.Verstovsek S, Kantarjian HM, Estrov Z, et al. . Long-term outcomes of 107 patients with myelofibrosis receiving JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to matched historical controls. Blood. 2012;120(6):1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daver N, Cortes J, Ravandi F, et al. . Secondary mutations as mediators of resistance to targeted therapy in leukemia. Blood. 2015;125(21):3236-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engle EK, Fisher DA, Miller CA, et al. . Clonal evolution revealed by whole genome sequencing in a case of primary myelofibrosis transformed to secondary acute myeloid leukemia. Leukemia. 2015;29(4):869-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guglielmelli P, Biamonte F, Rotunno G, et al. ; COMFORT-II Investigators; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative (AGIMM) Investigators. Impact of mutational status on outcomes in myelofibrosis patients treated with ruxolitinib in the COMFORT-II study. Blood. 2014;123(14):2157-2160. [DOI] [PubMed] [Google Scholar]
- 15.Tefferi A, Guglielmelli P, Lasho TL, et al. . CALR and ASXL1 mutations-based molecular prognostication in primary myelofibrosis: an international study of 570 patients. Leukemia. 2014;28(7):1494-1500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.