Abstract
The anthrax vaccine candidate AV7909 is being developed as a next generation vaccine for a post-exposure prophylaxis (PEP) indication against anthrax. AV7909 consists of the Anthrax Vaccine Adsorbed (AVA, BioThrax®) bulk drug substance adjuvanted with the immunostimulatory oligodeoxynucleotide (ODN) compound, CPG 7909. The addition of CPG 7909 to AVA enhances both the magnitude and the kinetics of antibody responses in animals and human subjects, making AV7909 a suitable next-generation vaccine for use in a PEP setting. The studies described here provide initial information on AV7909-induced toxin-neutralizing antibody (TNA) levels associated with the protection of animals from lethal Bacillus anthracis challenge. Guinea pigs or nonhuman primates (NHPs) were immunized on Days 0 and 28 with various dilutions of AV7909, AVA or a saline or Alhydrogel + CPG 7909 control. Animals were challenged via the inhalational route with a lethal dose of aerosolized B. anthracis (Ames strain) spores and observed for clinical signs of disease and mortality. The relationship between pre-challenge serum TNA levels and survival following challenge was determined in order to calculate a threshold TNA level associated with protection. Immunisation with AV7909 induced a rapid, highly protective TNA response in guinea pigs and NHPs. Surprisingly, the TNA threshold associated with a 70% probability of survival for AV7909 immunized animals was substantially lower than the threshold which has been established for the licensed AVA vaccine. The results of this study suggest that the TNA threshold of protection against anthrax could be modified by the addition of an immune stimulant such as CPG 7909 and that the TNA levels associated with protection may be vaccine-specific.
Keywords: Animal Rule, Correlate of protection, TNA threshold, Anthrax vaccine, CPG 7909 adjuvant, guinea pig, cynomolgus macaque
INTRODUCTION
Anthrax is considered a serious biological threat due to the highly lethal effects of exposure via the inhalational route and the relative ease of weaponizing Bacillus anthracis spores. While antimicrobials administered post-exposure can reduce the incidence or progression of anthrax disease, they do not protect against subsequent disease resulting from germination of residual spores that may remain in the body after the cessation of the recommended 60-day antibiotic regimen (1, 2). Such additional protection may be achieved by post-exposure vaccination. AVA, the only FDA-approved anthrax vaccine licensed for pre-exposure prophylaxis, was recently approved under the FDA Animal Rule for post-exposure prophylaxis (PEP) of disease following suspected or confirmed B. anthracis exposure (3, 4). The anthrax vaccine candidate AV7909, is composed of the AVA drug substance and the adjuvant CPG 7909, an immunostimulatory Toll-like receptor 9 (TLR9) agonist, and is being developed as a next generation anthrax vaccine candidate for PEP that is expected to confer protection earlier and to require fewer immunisations. CPG 7909 is an immunostimulatory oligonucleotide (short deoxyribonucleic acid [DNA] sequence) shown to be a potent vaccine adjuvant (5–11). CPG 7909 has been shown to induce both an enhanced antigen-specific antibody response and a natural killer T-cell response when used in combination with prophylactic or therapeutic vaccines (12–14).
The current pre-exposure regimen for AVA is a series of 3 intramuscular (IM) doses at 0, 1, and 6 months with subsequent booster at 12 and 18 months and annual boosters thereafter. The AVA PEP regimen of three subcutaneous doses (SC) at 0, 2, and 4 weeks, combined with the recommended 60-day course of antibiotics is considered one of the most effective medical countermeasures to prevent inhalational anthrax following widespread exposure in a biological attack (15). The AV7909 vaccine candidate administered as two IM immunisations two weeks apart may offer an improvement over the current licensed PEP vaccination regimen (7). To provide the highest level of additional protection following inhalation of anthrax spores, immunity to anthrax should be gained as rapidly as possible. To decrease logistical complexity in a mass immunisation scenario, a post-exposure vaccine regimen should include as few injections as possible.
Because it is not ethical to evaluate efficacy of anthrax vaccines in humans, and field trials are no longer feasible due to the rarity of naturally occurring anthrax in humans in the United States, the licensure of an anthrax vaccine for PEP must be achieved using the U.S. Food and Drug Administration (FDA) “Animal Rule”. Using this FDA guidance a vaccine may be licensed based on adequate and well-controlled animal studies when the results of those animal studies establish that the biological product is reasonably likely to produce clinical benefit in humans (16, 17). Rabbits and NHPs are preferred animal models for inhalational anthrax and have been widely used to study disease pathogenesis, examine bacterial characteristics such as virulence, and assess efficacy of vaccines and therapeutics (18, 19). A rabbit model of inhalational anthrax was used to set a TNA threshold for the AVA PEP indication. However, the rabbit model was found to be inadequate for AV7909 development because rabbits do not respond strongly to CPG adjuvants such as CPG 7909 that act via the TLR9 receptor (20). Therefore, a guinea pig model was developed as the small animal model for evaluation of AV7909 vaccine immunogenicity and efficacy. The guinea pig and cynomolgus macaque models of inhalational anthrax have been characterized extensively to support their use for licensure of AV7909, and the clinical signs of disease observed in these models are similar to those of inhalational anthrax in humans (21–23). Furthermore, studies in these animal models have demonstrated that serum TNA and anti-PA IgG titres are reliable predictors of survival following lethal B. anthracis challenge (24– 27). The TNA assay can selectively quantitate functional antibodies by measuring the ability of serum from immunized animals or human subjects to neutralize lethal toxin (LT). For protective antigen (PA)-based anthrax vaccines, pre-challenge TNA titres correlate with animal survival post-challenge and provide the means for deriving an antibody titre associated with a specific probability of survival in animals (3, 28). Since the TNA assay is species-independent (29), it can be used to directly compare functional immune responses across species, thereby providing a mechanism for bridging animal and human immunogenicity data to support licensure of the vaccine under the Animal Rule.
A series of studies was performed to evaluate the immunogenicity and protective efficacy of AV7909 in guinea pig and NHP models of inhalational anthrax. The primary goal of these studies was to establish the relationship of TNAantibodies circulating in serum at the time of challenge with survival following lethal inhalation exposure to anthrax spores.
MATERIAL AND METHODS
Experimental Animals
Animal studies were performed at Battelle Biomedical Research Center (West Jefferson, OH), and all animal procedures were approved by Battelle’s Institutional Animal Care and Use Committee (IACUC). The studies were conducted in compliance with the Animal Welfare Act and followed the principles of the Guide for the Care and Use of Laboratory Animals from the National Research Council. Animal room temperatures (64–84°F) and relative humidity (30–70%) were maintained and recorded a minimum of twice daily. The light/dark cycle was approximately 12 hours each per day using fluorescent lighting.
Male and female Hartley guinea pigs (Cavia porcellus) weighing approximately 350–400 grams, were purchased from Charles River Laboratories (Saint Constant, Quebec Canada). Guinea pigs were single-housed in polycarbonate cages on stainless steel racks equipped with a watering system. Male and female Asian-origin cynomolgus macaques (Macaca fascicularis) weighing 2.29 to 4.11 kg (2.9–11.1 years of age) were procured from Covance Research Products (Alice, TX). All NHPs were tested and verified negative for tuberculosis, Simian Immunodeficiency Virus, Simian T-Lymphotrophic Virus-1, Macacine herpesvirus 1 (Herpes B virus), Simian Retroviruses 1 and 2 and Trypanosoma cruzi. NHPs were pair housed during quarantine and the pre-challenge period in stainless steel cages on racks equipped with automatic watering systems. Animals were individually housed when moved into the biosafety level 3 (BSL-3) laboratory seven days prior to challenge and while housed in the BSL-3 after challenge.
Test and Control Material
AVA (Emergent BioSolutions, Lansing, MI)] is prepared from cell-free culture filtrates of an avirulent, nonencapsulated strain of B. anthracis adjuvanted with Alhydrogel®. AV7909 final drug product was made by combining AVA bulk drug substance with CPG 7909 to achieve a concentration of 0.5 mg CPG 7909 per mL. AVA and AV7909 were serially-diluted from the human dose (0.5 mL) in sterile saline for use in animal studies. Adjuvant control groups were injected with 0.5 mL of a sterile saline containing 0.650 to 0.730 mg of Alhydrogel and 0.25 mg of CPG 7909. Saline control groups were injected with 0.5 mL of a sterile saline.
Study Design
Four independent studies were performed: two in guinea pigs and two in NHPs. In each study, groups of animals were immunized by intramuscular (IM) injection on Day 0 and 28 with 0.5 mL of various dilutions of AV7909 or AVA. The vaccine dilutions used and the number of animals used in each study are shown in Table 1. Guinea Pig Study 1 and NHP Study 1 controls received 0.5 mL of adjuvant control. Guinea Pig Study 2 and NHP Study 2 controls received 0.5 mL of saline. Equal numbers of male and female animals were used in all study groups. Animals were challenged via the inhalational route with aerosolized B. anthracis (Ames strain) spores on Day 70, and observed for mortality and clinical signs of disease for up to 21 (guinea pigs) or 28 (NHPs) days post-challenge.
Table 1.
Summary of AV7909 Efficacy: Survival
| Guinea Pig | NHP | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Study 1 | Study 2 | Study 1 | Study 2 | ||||||
|
| |||||||||
| Vaccine | Dilution | Survivala | Time to Deathb | Survivala | Time to Death b | Survivala | Time to Death b | Survivala | Time to Death b |
| 1:4 | 100 (16/16) d | NA | NT | NT | 100 (8/8) d | NA | 100 (10/10) d | NA | |
| 1:16 | 100 (16/16) d | NA | NT | NT | 88(7/8) d | 3.9 | 100 (9/9) d | NA | |
| 1:32 | NT | NT | 100 (24/24) d | NA | NT | NT | NT | NT | |
| AV7909c | 1:64 | 100 (16/16) d | NA | 100 (22/22) d | NA | 75 (6/8) d | 4.4 (1.4) | 80 (8/10) d | 4.8 (0.1) |
| 1:96 | NT | NT | 100 (23/23) c, | NA | NT | 30 (3/10) | 4.7 (1.9) | ||
| 1:128 | NT | NT | 96 (23/24 d | 5.9 | 75 (6/8) c | 4.8 (1.4) | 40 (4/10) | 5.2 (1.3 | |
| 1:192 | NT | NT | 83 (19/23)d | 4.5 (0.6) | NT | 10 (1/10) | 3.8 (1.5) | ||
| 1:256 | 6 (1/14) | 4.1 (1.0) | 77 (17/22) d | 4.6 (1.2) | 25 (2/8) | 5.0 (0.9) | 11 (1/9) | 5.4 (1.3) | |
|
|
|||||||||
| 1:16 | 67 (10/15) d | 7.5 (2.0) | NT | NT | 88 (7/8) d | 14.9 | NT | NT | |
| AVA | 1:96 | NT | NT | 50 (12/24) d | 6.0 (3.2) | NT | NT | NT | NT |
| 1:64 | NT | NT | NT | NT | NT | NT | 67 (6/9) d | 4.0 (0.9) | |
|
|
|||||||||
| Adjuvant/Salinec | N/A | 0 (0/15) | 2.8 (0.4) | 0 (0/12) | 2.6 (1.4) | 13 (1/8) | 3.8 (1.6) | 0 (0/10) | 3.7 (1.1) |
|
| |||||||||
| LD50 equivalents | 399 ± 111 | 336 ± 37 | 287 ± 87 | 178 ± 50 | |||||
% Survival (Number Survived / Total Challenged), Equal numbers of males and females were vaccinated on Day 0. The initial group size was 16 and 24 in Guinea Pig Study 1 and 2 respectively and 8 and 10 NHP Study 1 and 2 respectively. In several groups animals died prior to the challenge due to a non-study related illness or injury, and were not included any further analyses.
Time to death = Days (Standard Deviation)
Study 1 control animals received adjuvant (250 μg CpG 7909 + 0.650 to 0.730 mg Alhydrogel in 0.5 mL) control. Study 2 control animals received 0.5mL saline administered IM.
p < 0.05 compared to the adjuvant group determined by two-sided Fisher’s exact test with Bonferroni-Holm adjustment for multiple comparisons.
N/A, not applicable, NT, Not tested
Aerosol Challenge
B. anthracis Ames strain spores were prepared and characterized as described previously (2). Aqueous suspensions of B. anthracis spores were aerosolized by a 3-jet Collison nebulizer and delivered to the animals via a nose-only (guinea pigs) or head-only (NHPs) inhalational exposure system. The anthrax target challenge dose was 200 LD50 of B. anthracis Ames spores, using the LD50 values of 5.01 × 104 spores/animal for guinea pigs (21) and 6.18 × 104 spores/animal for cynomolgus macaques (23). Actual challenge doses achieved in the studies are shown in Table 1. The atmospheric concentration of spores in the exposure system was controlled by spore concentration in the nebulizer. Real-time verification of the atmospheric concentrations of spore in the exposure system was not possible; instead the concentration of spores per volume air in the nebulizer was established by prior performance tests without animals. Respiratory rates, tidal volumes, and minute ventilation during aerosol challenge were estimated by Guyton formula (30) for guinea pigs or measured by real-time plethysmography for NHPs. Exposure duration was based on the time required for each animal to achieve the designated volume of inspired air. The test system atmosphere was sampled using all-glass impingers (Model 7541, Ace Glass Inc. Vineland, NJ) and colony-forming units (spores)/mL in the impinger samples were enumerated to determine the concentration of spores during exposure. The estimated inhaled dose was calculated using the actual spore atmospheric concentration multiplied by the cumulative tidal volume inhaled by animals during exposure. The average mass-median aerodynamic diameter of challenge aerosol particles was 1.08–1.20 μm as determined with an Aerodynamic Particle Sizer (APS Model 3321, TSI Inc., Shoreview, MN), indicating the correct particle size to reach the alveoli.
Clinical Observations, Anesthesia, and Pathology
Signs of anthrax infection include, but are not limited to, respiratory distress, rough coat, abnormal posture, and changes in appetite and activity. Animals that were moribund, unresponsive, recumbent, or showed respiratory distress and all animals surviving the post-challenge observation period were euthanized. During aerosol challenge and blood collection, guinea pigs were anesthetized by intraperitoneal (IP) administration of a combination of ketamine (70 mg/kg) and xylazine (10 mg/kg). NHPs were anesthetized with 2 mg/kg Telazol, intramuscularly (IM), for blood collection and vaccinations and with 4 mg/kg Telazol, IM, for aerosol challenge and prior to euthanasia. Complete necropsies were performed on all animals that were found dead or were euthanized due to moribund condition (post-challenge) or that were euthanized after the post-challenge monitoring period. Tissues samples (brain, kidney, liver, lungs, mediastinal lymph nodes, spleen, and gross lesions) were fixed in 10% neutral buffered formalin and then were processed, and approximately 5 μm sections were prepared for routine hematoxylin and eosin staining.
Blood Collection
Pre- and post-challenge blood was collected, processed to serum and stored at ≤−70°C until immune assessment by TNA assay. Bacteremia assessment was performed on post-challenge blood samples to confirm anthrax infection.
Toxin-Neutralizing Antibody Assay
Anthrax toxin-neutralizing antibody levels in guinea pig and NHP serum samples were measured using TNA assay (31–33). The TNA assay is designed to measure and quantify the functional ability of serum to neutralize B. anthracis lethal toxin (LT) activity using a cell-based cytotoxicity assay. Serum-mediated neutralization of anthrax LT manifests as a suppression of cytotoxicity, and hence preservation of cell viability. The lower limit of quantitation (LLOQ) for guinea pig and NHP TNA NF50 is 0.063 and 0.105, respectively.
Bacterial Burden Assessment
Whole blood samples in volumes of 30–40 μL were inoculated onto blood agar plates and the plates were incubated at 37°C for a minimum of 48 hours. A plate containing at least one colony with morphology consistent with B. anthracis was considered positive for B. anthracis.
Statistical Analysis
Pairwise two-sided Fisher’s exact tests were performed to determine if the proportion of surviving was significantly different among the groups. Logistic regression was performed to determine the relationship between animal survival and pre-challenge titres (Day 69 for guinea pigs, and Day 70 for NHPs). Individual TNA NF50 values below LLOQ were replaced with the LLOQ value for statistical analysis.
RESULTS
Efficacy of AV7909 in Animal Models
Animals were immunized two times, four weeks apart with dilutions of AV7909, AVA, or control and were challenged on day 70 post-first immunisation. Survival following inhalational exposure to a lethal dose of B. anthracis Ames spores is shown in Table 1. Actual exposure doses received by the guinea pigs and NHPs were within the estimated range of variability expected for aerosol exposure. All untreated control guinea pigs and 17 out of 18 control NHP died confirming the lethality of the exposure doses. The cynomolgus model is historically known to have a low percentage (< 10%) of control survivors following exposure to lethal B. anthracis Ames spore doses (34). AV7909 conferred protection in a dose-dependent manner, with higher vaccine dose levels affording a statistically significant (Table 1) increase in survival compared to the control group.
Relationship between Survival and TNA Titres
Animal were immunized with a broad series of vaccine dilutions. This was critical for generating a wide range of immune responses allowing for a more accurate assessment of the relationship between immune response and survival. Data from the first study in each species was used to inform the design of second study including appropriate dilutions to use and number of animals at each dilution. The kinetics of the TNA response induced by AV7909 vaccination of guinea pigs and NHP are shown in Figure 1. All animals had TNA NF50 levels below the LLOQ prior to vaccination. After vaccination, a dose-dependent increase in TNA NF50 was observed in both animal models. The peak immune response, following immunization at 0 and 28 days, occurs between day 35 to 42. In the first guinea pig study, titres were measured on day 21 instead of day 28. We noted that NHPs do not respond as well to the lower CpG dose levels present in higher dilutions of AV7909, and thus the immunostimulatory effect of CPG 7909 is not readily observed at higher dilutions, although NHPs respond to the 250ug CPG 7909 dose level present in a full human dose of AV7909 (unpublished data).
Figure 1. Group geometric mean TNA NF50 response to vaccination with AV7909.
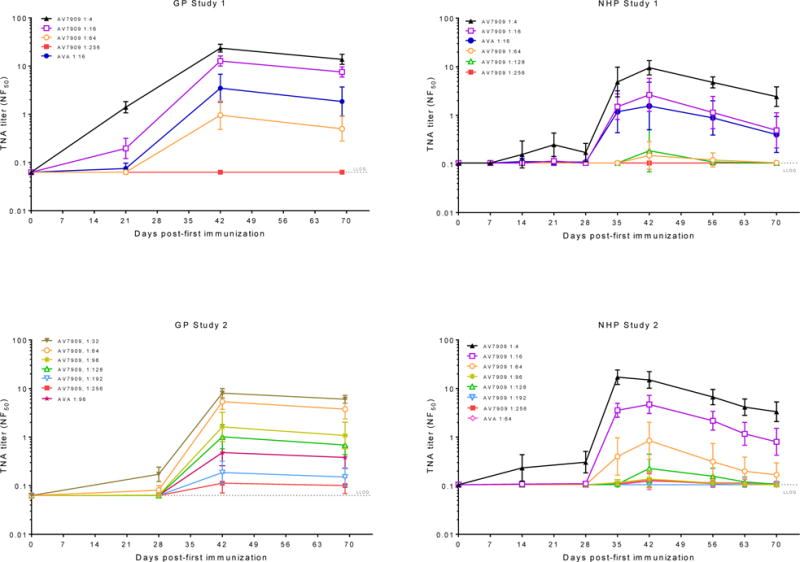
Guinea pigs (GP) or NHP were immunized with dilutions of AV7909 or AVA on days 0 and 28. TNA NF50 geometric means with 95% confidence intervals by study day are shown. In the first guinea pig study, titres were measured on day 21 instead of day 28. The number of animals in each dose group for the studies is shown in Table 1. TNA was below the lower limit of quantitation (LLOQ) at all time points in the control groups (data not shown). LLOQ is indicated by the dotted line (.…).
The relationship between TNA NF50 levels and the survival of AV7909 vaccinated animals following challenge was evaluated using logistic regression analysis (Figure 2). A strong correlation was observed between TNA NF50 levels just prior to challenge and survival following challenge. The logistic regression model allowed estimation of pre-challenge TNA NF50 titres associated with various probabilities of survival (Table 2). A 70% probability of survival was associated with TNA NF50 titres of 0.064 and 0.063 in guinea pig Study 1 and 2, respectively, and 0.154 and 0.107 NHP Study 1 and 2, respectively. Regression analysis of the combined results for each species indicated that the TNA NF50 titres associated with a 70% probability of survival were 0.063 and 0.180 for guinea pigs and NHPs, respectively (Table 2). The dose response curves for three of the four studies are very steep (Figure 2). As a result, survival rates greater than 70% also are associated with NF50 values that are just above the LLOQ for the TNA assay (Table 2). It was also observed that, unlike AVA-vaccinated groups where 57% of animals of the animals that died had measurable titres, only a single AV7909 immunized animal with measurable TNA titres died in the four studies (Figure 3). These results further support the finding that, there is a high probability that, any antibody response to AV7909 vaccination is sufficient to confer protection.
Figure 2. Logistic regression analyses for AV7909 GP and NHP Studies.
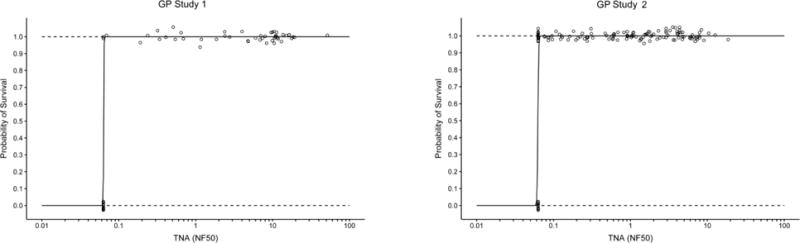
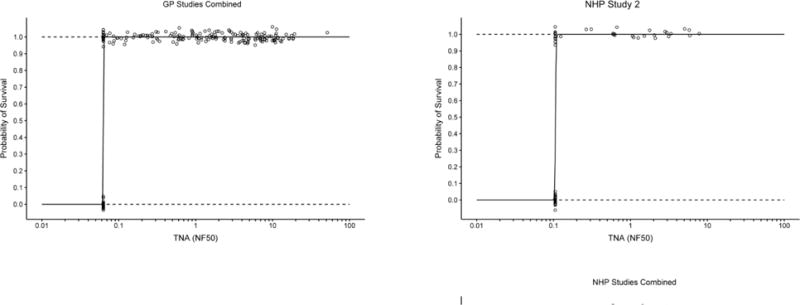
The logistic regression curves (solid line) with 95% CI (dashed lines) illustrate the changes in predicted survival probability with increasing immune responses. Individual animals (○) that survived or died are shown at the top and bottom of each figure, respectively. The combined data from Guinea Pig studies 1 and 2 and NHP studies 1 and 2 are shown in the two lower panels. Individual TNA NF50 values below the lower limit of quantitation (LLOQ) were replaced with the LLOQ value (LLOQ = 0.063 for GP TNA and LLOQ = 0.105 for NHP TNA). GP Study 1 N=62; GP Study 2 N= 138; NHP Study 1 N=39; NHP Study 2 N=68
Table 2.
Pre-Challenge TNA NF50 Titers Associated with Various Probabilities of Survival
| Guinea Pig TNA NF50 (95% Confidence Interval) |
NHP TNA NF50 (95% Confidence Interval) |
|||||
|---|---|---|---|---|---|---|
| Probability of Survival1 | Study 1 | Study 2 | Combined | Study 1 | Study 2 | Combined |
| 90% | 0.064 (0, 59) |
0.066 (0, 13,695) |
0.064 (0.003, 1.3) |
0.445 (0.092, 2.1) |
0.108 (0, 131) |
0.324 (0.146, 0.716) |
| 80% | 0.064 (0, 15) |
0.064 (0, 28) |
0.064 (0.009, 0.453) |
0.236 (0.092, 0.606) |
0.107 (0.001, 20) |
0.227 (0.131, 0.395) |
| 70% | 0.064 (0.001, 6) |
0.063 (0.009, 0.457) |
0.063 (0.018, 0.226) |
0.154 (0.081, 0.294) |
0.107 (0.002, 5.9) |
0.180 (0.121, 0.268) |
The probabilities of survival were derived from the regression analyses performed for each study.
Figure 3. Scatter plots for AV7909- and AVA-immunized survivors and non-survivors.
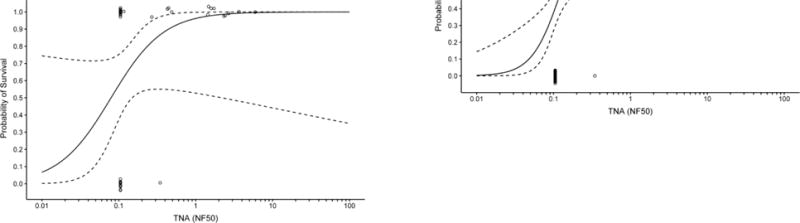
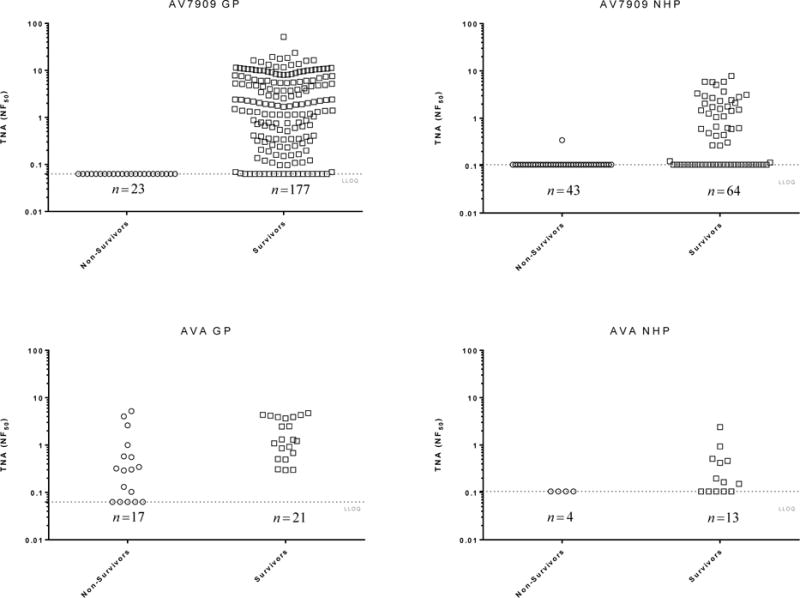
The combined data from Guinea Pig studies 1 and 2 and NHP studies 1 and 2 are shown. Circles, ○, represent individual TNA NF50 pre-challenge titres (day 69 for guinea pigs (GP) and day 70 for NHP) of non-survivors; squares, □, represent the individual TNA pre-challenge titres of survivors. Groups of animals were immunized with dilutions of AV7909 on day 0 and 28 and were challenged on day 70 post first-immunisation. The dotted horizontal line represents the limit of quantitation (LLOQ) of the assay.
DISCUSSION
The relationship between toxin-neutralizing antibodies induced by AV7909 and protection of immunized animals from death due to anthrax was evaluated in accordance with the requirements of the Animal Rule. The essential elements of the FDA’s Draft Guidance for Industry “Product Development Under the Animal Rule ”(17) guided the design and execution of studies performed to evaluate the efficacy of the AV7909 vaccine. Guinea pig and NHP models of inhalation anthrax were used because they display characteristics that are very similar to human disease following inhalational anthrax spore exposure. Both animal models rapidly develop fulminant systemic disease, with the majority of deaths occurring within two to four days post-challenge. The course of disease in guinea pigs and NHPs is similar to that of humans. Histopathological findings in guinea pigs and NHP are also similar to those found in humans and include necrotizing lymphadenitis, splenitis, pneumonia, vasculitis, hemorrhage, congestion, and edema in multiple tissues and meninges (21–23).
The primary goal of these studies was to establish the relationship between circulating TNA antibodies and survival following lethal inhalation exposure to anthrax spores. Animal studies have demonstrated that serum TNA titre is a reliable predictor of protection against B. anthracis challenge (3, 24–27) and because the TNA assay is species-independent, it can be used to compare functional immune response across species. Thus, TNA data provide a mechanism for bridging animal and human immunogenicity data to support licensure of the vaccine under the Animal Rule.
As expected, among AV7909 vaccinated animals, there was a strong correlation between TNA levels just prior to challenge and survival. The estimated TNA NF50 values associated with a 70% probability of survival, 0.063 for guinea pig and 0.18 for NHP, were just above the LLOQ of the TNA assay. Analysis of the individual animal immune responses showed that the overwhelming majority of animals with detectable TNA titres survived B. anthracis challenge; only one animal (NHP) with a detectable TNA titre at the time of challenge died. The guinea pig- and NHP- derived TNA thresholds associated with a 70% probability of survival were comparable and further support the concept that these data can be used to conduct cross-species protective threshold estimations (3, 28). The TNA NF50 thresholds associated with a 70% probability of survival following AV7909 vaccination from the two studies conducted in guinea pigs and NHP were lower than the respective 0.56 and 0.29 TNA NF50 70% protective threshold values published for rabbits and NHP, respectively, immunized with AVA (3). Additional data obtained with AVA immunized rabbits indicates that the original rabbit study described in Ionin et al., likely overestimated the TNA threshold required for 70% protection in that animal model (unpublished data).
Although circulating TNA antibodies have been recognized as being predictive of protection against anthrax, it is understood that the circulating neutralizing antibodies at the time of challenge are not solely responsible for protection. An anamnestic antibody response due to immunological memory is one of the fundamental effector mechanisms of acquired immunity and plays a major role in protection against a variety of infections (35). The role of immunological memory in conferring protection by PA-based vaccines has been well established. Marcus et. al. (36) demonstrated the contribution of immunological memory to protective immunity conferred by a PA-based vaccine in guinea pigs. Quinn et al. (27) demonstrated that NHPs vaccinated with three doses of AVA (0, 1, and 6 months) were protected against inhalation anthrax for up to four years after the initial vaccination despite the fact that the circulating anti-PA IgG and TNA antibodies immediately prior to challenge were near or below LOQ. The authors concluded that long-term protection was afforded by PA-specific B and T memory cells capable of mounting a rapid protective anamnestic response following challenge with aerosolized B. anthracis Ames spores. Combining a CpG ODN with AVA was previously shown to increase the speed, magnitude and the antibody avidity of the anti-anthrax response in mice, rhesus macaques and humans (7, 8, 10). Tross and Klinman (37) showed in a mouse model that the addition of CpG ODN adjuvants to AVA enhanced protection both by stimulating a strong/persistent serum Ab response and by generating a high-affinity long-lived pool of memory B cells. The results from these studies suggested a potential mechanism by which a relatively low TNA NF50 threshold was associated with a high level of protection. Further investigation will be required to understand effect of CPG 7909 of antibody avidity memory B-cell populations. The potential contribution of early innate and T- cell mediated response to protective immunity has also been investigated in human subjects vaccinated with AV7909 (38). Biomarkers of early innate responses to CPG 7909 were confirmed, and adding a CpG adjuvant to a vaccine resulted in increased T cell effects relative to vaccine alone. The changes in biomarkers of early innate responses correlated with subsequent adaptive humoral immunity but not cellular immunity. The potential contribution of the T-cell response to enhanced humoral immunity (e.g. Th1 versus Th2-type T cell helper response) following AV7909 vaccination requires further investigation.
In summary, AV7909 vaccination induced a rapid, highly protective TNA response in both guinea pigs and NHPs. The relatively low TNA levels associated with a high level of protection indicate that protection is conferred not only by the antibodies that are circulating at the time of challenge but also by the B and T-cell repertoire and other aspects of the immune response that are stimulated during an active immunisation with a vaccine and re-stimulated upon challenge.
HIGHLIGHTS.
-
❑
The AV7909 vaccine candidate induced a robust toxin-neutralizing antibody response in guinea pigs and NHPs and was highly protective against lethal aerosolized anthrax spore challenge
-
❑
On the day prior to challenge, TNA NF50 values that correlated with a 70% probability of protection were lower than those for AVA
Acknowledgments
This work was supported by NIAID Contract Number: HHSN272200800051C and NIAID Contract Number: HHSN272201000035C
We thank Tanya Nelson, Melissa Wynn, Tom Hickey, Andrea Harris, Jon Inglefield, Danielle Craig, Anita Constantinides, Tyler Laudenslager, Laurence Lemiale, Sukjoon Park for technical and programmatic support and Dianne Sweeney and Grace Lin for statistical support. We thank the management and staff of Battelle Biomedical Research Center for excellent technical support in execution of animal studies. We also thank Ed Nusum, Judith Hewitt, Larry Wolfraim, and Kimberly Taylor for review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. The Journal of hygiene. 1956;54(1):28–36. doi: 10.1017/s0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao LM, Bush K, Barnewall R, Estep J, Thalacker FW, Olson PH, et al. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrobial agents and chemotherapy. 2006;50(11):3535–42. doi: 10.1128/AAC.00090-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ionin B, Hopkins RJ, Pleune B, Sivko GS, Reid FM, Clement KH, et al. Evaluation of immunogenicity and efficacy of anthrax vaccine adsorbed for postexposure prophylaxis. Clin Vaccine Immunol. 2013;20(7):1016–26. doi: 10.1128/CVI.00099-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longstreth J, Skiadopoulos MH, Hopkins RJ. Licensure strategy for pre- and post-exposure prophylaxis of biothrax vaccine: the first vaccine licensed using the FDA animal rule. Expert review of vaccines. 2016;15(12):1467–79. doi: 10.1080/14760584.2016.1254556. [DOI] [PubMed] [Google Scholar]
- 5.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins RJ, Daczkowski NF, Kaptur PE, Muse D, Sheldon E, LaForce C, et al. Randomized, double-blind, placebo-controlled, safety and immunogenicity study of 4 formulations of Anthrax Vaccine Adsorbed plus CPG 7909 (AV7909) in healthy adult volunteers. Vaccine. 2013;31(30):3051–8. doi: 10.1016/j.vaccine.2013.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkins RJ, Kalsi G, Montalvo-Lugo VM, Sharma M, Wu Y, Muse DD, et al. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine. 2016;34(18):2096–105. doi: 10.1016/j.vaccine.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinman DM. CpG oligonucleotides accelerate and boost the immune response elicited by AVA, the licensed anthrax vaccine. Expert review of vaccines. 2006;5(3):365–9. doi: 10.1586/14760584.5.3.365. [DOI] [PubMed] [Google Scholar]
- 9.Mullen GE, Giersing BK, Ajose-Popoola O, Davis HL, Kothe C, Zhou H, et al. Enhancement of functional antibody responses to AMA1-C1/Alhydrogel, a Plasmodium falciparum malaria vaccine, with CpG oligodeoxynucleotide. Vaccine. 2006;24(14):2497–505. doi: 10.1016/j.vaccine.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Rynkiewicz D, Rathkopf M, Sim I, Waytes AT, Hopkins RJ, Giri L, et al. Marked enhancement of the immune response to BioThrax(R) (Anthrax Vaccine Adsorbed) by the TLR9 agonist CPG 7909 in healthy volunteers. Vaccine. 2011;29(37):6313–20. doi: 10.1016/j.vaccine.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 11.Gu M, Hine PM, James Jackson W, Giri L, Nabors GS. Increased potency of BioThrax anthrax vaccine with the addition of the C-class CpG oligonucleotide adjuvant CPG 10109. Vaccine. 2007;25(3):526–34. doi: 10.1016/j.vaccine.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 12.Krieg AM. Lymphocyte activation by CpG dinucleotide motifs in prokaryotic DNA. Trends Microbiol. 1996;4(2):73–6. doi: 10.1016/0966-842X(96)81515-0. [DOI] [PubMed] [Google Scholar]
- 13.Pisetsky DS. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5(4):303–10. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston PO. Comparison of the effect of different immunological adjuvants on the antibody and T-cell response to immunization with MUC1-KLH and GD3-KLH conjugate cancer vaccines. Vaccine. 1999;18(7–8):597–603. doi: 10.1016/s0264-410x(99)00316-3. [DOI] [PubMed] [Google Scholar]
- 15.Shepard CW, Soriano-Gabarro M, Zell ER, Hayslett J, Lukacs S, Goldstein S, et al. Antimicrobial postexposure prophylaxis for anthrax: adverse events and adherence. Emerg Infect Dis. 2002;8(10):1124–32. doi: 10.3201/eid0810.020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns DL. Licensure of vaccines using the Animal Rule. Current opinion in virology. 2012;2(3):353–6. doi: 10.1016/j.coviro.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Guidance for Industry Animal Models. Essential Elements to Address Efficacy Under the Animal Rule [Internet] 2015 Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf.
- 18.Phipps AJ, Premanandan C, Barnewall RE, Lairmore MD. Rabbit and nonhuman primate models of toxin-targeting human anthrax vaccines. Microbiol Mol Biol Rev. 2004;68(4):617–29. doi: 10.1128/MMBR.68.4.617-629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goossens PL. Animal models of human anthrax: the Quest for the Holy Grail. Mol Aspects Med. 2009;30(6):467–80. doi: 10.1016/j.mam.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev. 2001;11(5):333–40. doi: 10.1089/108729001753231713. [DOI] [PubMed] [Google Scholar]
- 21.Savransky V, Sanford DC, Syar E, Austin JL, Tordoff KP, Anderson MS, et al. Pathology and pathophysiology of inhalational anthrax in a guinea pig model. Infect Immun. 2013;81(4):1152–63. doi: 10.1128/IAI.01289-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaucha GM, Pitt LM, Estep J, Ivins BE, Friedlander AM. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch Pathol Lab Med. 1998;122(11):982–92. [PubMed] [Google Scholar]
- 23.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, et al. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2003;83(8):1201–9. doi: 10.1097/01.lab.0000080599.43791.01. [DOI] [PubMed] [Google Scholar]
- 24.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, et al. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine. 2001;19(32):4768–73. doi: 10.1016/s0264-410x(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 25.Little SF, Ivins BE, Fellows PF, Pitt ML, Norris SL, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22(3–4):422–30. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Little SF, Ivins BE, Webster WM, Fellows PF, Pitt ML, Norris SL, et al. Duration of protection of rabbits after vaccination with Bacillus anthracis recombinant protective antigen vaccine. Vaccine. 2006;24(14):2530–6. doi: 10.1016/j.vaccine.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Quinn CP, Sabourin CL, Niemuth NA, Li H, Semenova VA, Rudge TL, et al. A three-dose intramuscular injection schedule of anthrax vaccine adsorbed generates sustained humoral and cellular immune responses to protective antigen and provides long-term protection against inhalation anthrax in rhesus macaques. Clin Vaccine Immunol. 2012;19(11):1730–45. doi: 10.1128/CVI.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fay MP, Follmann DA, Lynn F, Schiffer JM, Stark GV, Kohberger R, et al. Anthrax vaccine-induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci Transl Med. 2012;4(151):151ra26. doi: 10.1126/scitranslmed.3004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omland KS, Brys A, Lansky D, Clement K, Lynn F, Participating L. Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species. Clin Vaccine Immunol. 2008;15(6):946–53. doi: 10.1128/CVI.00003-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyton AC. Measurement of the respiratory volumes of laboratory animals. The American journal of physiology. 1947;150(1):70–7. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Soroka SD, Taylor TH, Jr, Stamey KL, Stinson KW, Freeman AE, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods. 2008;333(1–2):89–106. doi: 10.1016/j.jim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, et al. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg Infect Dis. 2002;8(10):1103–10. doi: 10.3201/eid0810.020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hering D, Thompson W, Hewetson J, Little S, Norris S, Pace-Templeton J. Validation of the anthrax lethal toxin neutralization assay. Biologicals : journal of the International Association of Biological Standardization. 2004;32(1):17–27. doi: 10.1016/j.biologicals.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Agents CoAMfACtB. Animal Models for Assessing Countermeasures to Bioterrorism Agents. In: National Research Council, editor. Institute for Laboratory Animal Research DoEaLS. Washington DC: National Academies Press; 2011. p. 116. [Google Scholar]
- 35.Siegrist CA. 2 - Vaccine immunology A2 - Plotkin, Stanley A. In: Orenstein WA, Offit PA, editors. Vaccines. Sixth. London: W.B. Saunders; 2013. pp. 14–32. [Google Scholar]
- 36.Marcus H, Danieli R, Epstein E, Velan B, Shafferman A, Reuveny S. Contribution of immunological memory to protective immunity conferred by a Bacillus anthracis protective antigen-based vaccine. Infect Immun. 2004;72(6):3471–7. doi: 10.1128/IAI.72.6.3471-3477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tross D, Klinman DM. Effect of CpG oligonucleotides on vaccine-induced B cell memory. J Immunol. 2008;181(8):5785–90. doi: 10.4049/jimmunol.181.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minang JT, Inglefield JR, Harris AM, Lathey JL, Alleva DG, Sweeney DL, et al. Enhanced early innate and T cell-mediated responses in subjects immunized with Anthrax Vaccine Adsorbed Plus CPG 7909 (AV7909) Vaccine. 2014;32(50):6847–54. doi: 10.1016/j.vaccine.2014.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]


