Abstract
IMPORTANCE
Despite epidemiological and preclinical evidence suggesting that vitamin D and calcium inhibit colorectal carcinogenesis, daily supplementation with these nutrients for 3 to 5 years was not found to significantly reduce the risk of recurrent colorectal adenomas in a recent randomized clinical trial.
OBJECTIVE
To investigate whether common variants in 7 vitamin D and calcium pathway genes (VDR, GC, DHCR7, CYP2R1, CYP27B1, CYP24A1, and CASR) modify the effects of vitamin D3 or calcium supplementation on colorectal adenoma recurrence.
DESIGN, SETTING, AND PARTICIPANTS
We examined 41 candidate single-nucleotide polymorphisms (SNPs) in 2259 participants in a randomized, double-blind, placebo-controlled trial conducted at 11 clinical centers in the United States. Eligibility criteria included a recently diagnosed adenoma and no remaining colorectal polyps after complete colonoscopy. The study’s treatment phase ended on August 31, 2013, and the analysis for the present study took place from July 28, 2014, to October 19, 2016.
INTERVENTIONS
Daily oral supplementation with vitamin D3 (1000 IU) or calcium carbonate (1200 mg elemental calcium) or both or neither.
MAIN OUTCOMES AND MEASURES
The outcomes assessed were the occurrence of 1 or more adenomas or advanced adenomas (estimated diameter, ≥ 1 cm; or with villous histologic findings, high-grade dysplasia, or cancer) during follow-up. Treatment effects and genotype associations and interactions were estimated as adjusted risk ratios (RRs) and 95% confidence intervals (CIs). The effective number of independent SNPs was calculated to correct for multiple testing.
RESULTS
Among the 2259 participants randomized, 1702 were non-Hispanic whites who completed the trial and had genotype data for analysis (1101 men; mean [SD] age 58.1 [6.8] years). The effect of vitamin D3 supplementation on advanced adenomas, but not on adenoma risk overall, significantly varied according to genotype at 2 VDR SNPs (rs7968585 and rs731236) in linkage disequilibrium (D′ = 0.98; r2 = 0.6). For rs7968585, among individuals with the AA genotype (26%), vitamin D3 supplementation reduced risk by 64% (RR, 0.36; 95% CI, 0.19–0.69; P = .002; absolute risk decreased from 14.4% to 5.1%). Among individuals with 1 or 2 G alleles (74%), vitamin D3 supplementation increased risk by 41% (RR, 1.41; 95%CI, 0.99–2.00; P = .05; absolute risk increased from 7.7% to 11.1%; P < .001 for interaction). There were no significant interactions of genotypes with calcium supplementation.
CONCLUSIONS AND RELEVANCE
Our findings suggest that benefits from vitamin D3 supplementation for the prevention of advanced colorectal adenomas may vary according to vitamin D receptor genotype.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00153816
Although vitamin D and calcium are recognized for their importance to bone health, more recently attention has focused on the possibility that they may prevent cancer, especially colorectal cancer.1–3 Vitamin D is derived from limited dietary sources or cutaneous synthesis on exposure to sunlight.4 It is metabolized in a highly regulated multistep process to 1,25-dihydroxyvitamin D, a key hormone regulating calcium homeostasis.4 The hormone 1,25-dihydroxyvitamin D regulates gene expression after binding to the vitamin D receptor (VDR), a classic nuclear hormone receptor widely expressed in tissues throughout the body.5,6 Antineoplastic actions of vitamin D and calcium are suggested by preclinical research, and plausible mechanisms include induction of cell differentiation and apoptosis and inhibition of cell growth and proliferation.2,7,8 However, the evidence from observational studies in humans is susceptible to bias and confounding and provides limited evidence for causality.9–11
To address this gap, we recently conducted a randomized, double-blinded, placebo-controlled trial of colorectal adenoma chemoprevention by daily supplementation with vitamin D3 (1000 IU) and/or calcium carbonate (1200 mg elemental calcium) for 3 to 5 years among participants aged 45 to 75 years, after removal of all baseline colorectal adenomas (the Vitamin D/Calcium Polyp Prevention Study).12 Unexpectedly, supplementation did not reduce the risk of recurrent colorectal adenomas,12 and there was no evidence that specific subgroups of individuals experienced protective effects, with the exception of suggestive evidence that calcium supplementation might reduce risk in individuals with a lower body mass index.12
Another possibility is that these supplements may be effective in certain subgroups of individuals defined by their genetic makeup. Notably, single-nucleotide polymorphisms (SNPs) in vitamin D pathway genes are associated with circulating 25-hydroxyvitamin D, or 25(OH)D, levels.13–16 In prior work, we investigated whether 41 candidate SNPs in vitamin D and calcium pathway genes modified the efficacy of vitamin D3 supplementation to increase circulating 25(OH)D levels.17 We found significant interactions between vitamin D3 supplementation and SNPs in VDR, CYP2R1, and CYP24A1 on the magnitude of the 25(OH)D increase.17 In the present study, we investigated whether these same 41 SNPs modify the effects of vitamin D3 or calcium supplementation on risk of recurrent colorectal adenomas.
Methods
Study Design and Population
We analyzed associations between SNP genotypes and colorectal outcomes among participants in the Vitamin D/Calcium Polyp Prevention Study,12 a randomized, double-blinded, placebo-controlled trial of vitamin D3 or calcium supplementation conducted at 11 academic medical centers and associated practices in the United States. Institutional review boards at each site approved the protocol (Supplement 1), and participants provided written informed consent. Eligible participants were aged 45 to 75 years with at least 1 large bowel adenoma removed within 120 days before enrollment and no known polyps remaining after complete colonoscopy. Exclusion criteria included familial colorectal cancer syndromes, serious intestinal disease, contraindications to study treatment, serum calcium outside the normal range, creatinine greater than 20% above the upper limit of normal, and 25 (OH)D levels below 12 ng/mL or above 90 ng/mL. The planned intervention duration was either 3 or 5 years, according to the colonoscopic follow-up recommended by the participants’ physicians. Sample size was determined by the hypothesized treatment effect on adenoma recurrence.
Study staff enrolled trial participants between July 2004 and July 2008. At enrollment, they provided information on medical history, medication and supplement use, and demographic and lifestyle factors. They agreed to avoid personal vitamin D or calcium supplement use during the trial. Race and ethnicity were assessed by participant self-report using National Institutes of Health reporting standards. After enrollment, participants entered a blinded placebo run-in period to exclude those unlikely to follow study procedures. Thereafter, participants were randomized in a partial 2 × 2 factorial design with equal probability to take 2 identical-appearing tablets daily containing 1000 IU of vitamin D3, 1200mg of calcium as carbonate, both, or placebo (full factorial randomization). Women could elect to be given calcium and randomized with equal probability to calcium alone or calcium plus vitamin D3 (2-group randomization). The coordinating center implemented web-based randomization in permuted blocks using computer-generated random numbers stratified by clinical center, sex, follow-up colonoscopy interval (3 or 5 years), and full factorial or 2-group randomization. Participants and all clinical, coordination, and laboratory staff were blinded to treatment assignments.
After randomization, participants were interviewed every 6 months by telephone regarding adherence to study treatment, medication and supplement use, dietary calcium and vitamin D intake, illnesses, and colorectal procedures. Serum levels of 25(OH)D were measured at baseline and year 1 using a radio immunoassay kit from Immunodiagnostic Systems. The study’s treatment phase ended on August 31, 2013.
Outcome Assessment
Study end points included all adenomas diagnosed at any colorectal endoscopy or surgical procedure 1 year or more after randomization and 6 months or less after the anticipated 3- or 5-year follow-up colonoscopy. Pathology slides were obtained for all excised colorectal lesions and reviewed by a single, blinded, study pathologist. Advanced adenomas were defined as those with more than 25% villous features, high-grade dysplasia or cancer, or an estimated diameter of 1 cm or larger.
SNP Selection and Genotyping
As previously reported,17 we genotyped 41 candidate SNPs in or near 7 vitamin D or calcium pathway genes (GC, DHCR7, CYP2R1, CYP27B1, CYP24A1, VDR, and CASR) previously associated with 25(OH)D levels or other health outcomes (eFigure 1 and eTable 1 in Supplement 2). Briefly, genomic DNA was genotyped using KASP technology (LGC Limited), iPLEX Gold (Sequenom) or predesigned TaqMan assays (Thermo-Ficher Scientific). Samples that could not be called on more than 4 of 41 SNPs were dropped; the sample success rate was 96.1%, and SNP call rates ranged from 96.7% to 99.8%. Concordance rates among blinded replicates were 100%. All SNPs were in Hardy-Weinberg Equilibrium in non-Hispanic whites (P ≥ .05; eTable 1 in Supplement 2).
Statistical Analysis
Only self-reported non-Hispanic whites were included in analyses to avoid spurious associations from population stratification. The primary outcome assessed was the occurrence of 1 or more adenomas, and the secondary outcome was the occurrence of 1 or more advanced adenomas. Multivariable generalized linear models for binary data were used to estimate risk ratios (RRs) and 95% confidence intervals (CIs) for treatment effects, genotype associations, and interactions. Genotypes were modeled additively providing perallele RRs except as indicated in post hoc analyses. Covariates included age and sex. Models including additional covariates did not appreciably change the effect estimates, so the most parsimonious models were used. To evaluate whether genotype modified vitamin D3 or calcium treatment effects, we used multiplicative interaction terms in the regression models and Wald tests. In addition, case-only interaction analysis was used, which may improve statistical power18,19 (see eMethods in Supplement 2). Likelihood ratio tests were used to evaluate treatment effect interactions. To evaluate associations with serum 25(OH)D levels, a dichotomous variable was used with “low” 25(OH)D defined as the lowest season-specific quintile, as previously described20 (see eMethods in Supplement 2).
Based on early evidence for the potential functional significance of genetic variation in the 3′ region of the VDR gene (eg, associations with bone mineral density, circulating osteocalcin levels, and intestinal calcium absorption21–23) and associations with risk of colorectal neoplasia,24,25 subgroup analyses of vitamin D3 treatment effects among participants defined by SNPs rs731236, rs7975232, and rs1544410, all in linkage disequilibrium, were prespecified. Subgroup analyses of other SNPs were performed post hoc. To account for multiple testing, we calculated the effective number of independent tests (n = 28) taking into account linkage disequilibrium among the 41 SNPs analyzed, which gave a threshold for statistical significance of P < .002 (0.05/ 28.00) for interaction26 for an overall P < .05 for each outcome by treatment group analysis.
Except as indicated, in analyses that included randomized treatments, participants were retained in their assigned treatment group regardless of adherence to study treatment and procedures. Analyses of calcium treatment included only full factorial participants. To assess the impact of optimal adherence, sensitivity analyses included only participants who took at least 80% of their study pills and also reported taking nonstudy supplements (containing >400 mg of calcium or >400 IU of vitamin D) on no more than 1 biannual questionnaire. All statistical tests were 2 sided; P < .05 was considered significant except as indicated. Analyses were conducted using SAS (version 9.3; SAS Institute Inc) or Stata software (version 14; StataCorp LP).
Results
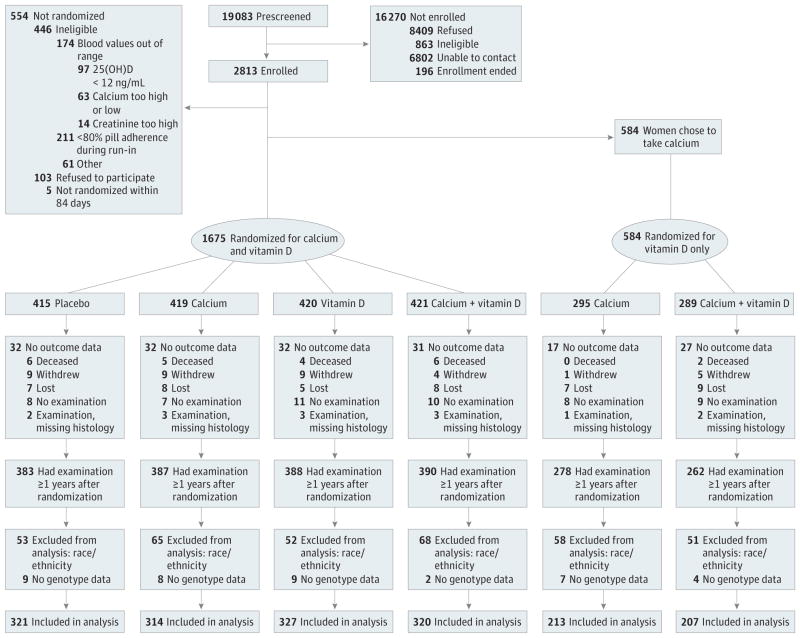
Of 2259 randomized participants, 2088 (92.4%) had outcome data from a colonoscopy 1 year or longer after randomization and were eligible for evaluation of study end points (Figure). Another 386 participants were excluded owing to either missing genotype data (n = 39) or self-identification as nonwhite race or Hispanic ethnicity (n = 347), leaving 1702 participants for genetic analyses. The population analyzed was 65% male and, on average, 58 years old (Table 1). Nearly 57% of participants had only 1 adenoma smaller than 1 cm at their qualifying colonoscopy, while almost 19% had at least 1 advanced adenoma. Baseline characteristics were similar between treatment groups (Table 1).
Figure.
Study Enrollment and Randomization Flowchart
Table 1.
Baseline Characteristics of Participants Included in Genetic Association Analysesa
| Characteristic | All (n = 1702) | No Vitamin Db (n = 848) | Vitamin Db (n = 854) | P Valuec | No Calciumd (n = 648) | Calciumd (n = 634) | P Valuec |
|---|---|---|---|---|---|---|---|
| Men, No. (%) | 1101 (64.7) | 548 (64.6) | 553 (64.8) | .96 | 560 (86.4) | 541 (85.3) | .58 |
| Age, mean (SD), y | 58.1 (6.8) | 58.2 (6.9) | 58.1 (6.7) | .63 | 58.3 (6.9) | 58.8 (6.9) | .22 |
| BMI, mean (SD) | 28.9 (5.1) | 28.9 (5.2) | 28.8 (5.1) | .80 | 29.0 (4.7) | 29.2 (5.2) | .45 |
| Current smoker, No. (%) | 148 (8.7) | 66 (7.8) | 82 (9.6) | .18 | 52 (8.0) | 56 (8.8) | .60 |
| Physical activity, MET-min/wk, mean (SD) | 3060 (2900) | 3076 (2851) | 3045 (2948) | .82 | 3200 (3081) | 3175 (2927) | .88 |
| Alcohol intake, mean (SD) drinks/d, No. | 0.82 (1.03) | 0.78 (1.00) | 0.87 (1.06) | .07 | 0.97 (1.1) | 0.91 (1.06) | .32 |
| Aspirin use ≥ 4 d/wk, No. (%) | 626 (36.8) | 305 (36.0) | 321 (37.6) | .49 | 271 (41.8) | 246 (38.8) | .27 |
| Dietary vitamin D intake, mean (SD), IU/d | 137 (99) | 141 (98) | 133 (100) | .08 | 138 (101) | 141 (98) | .66 |
| Dietary calcium intake, mean (SD), mg/d | 678 (311) | 694 (319) | 663 (302) | .04 | 681 (302) | 698 (320) | .35 |
| Multivitamin use, No. (%) | 971 (57.1) | 482 (57.0) | 489 (57.3) | .91 | 348 (53.7) | 331 (52.4) | .63 |
| Vitamin D supplement use, No. (%) | 231 (13.6) | 106 (12.5) | 125 (14.7) | .21 | 35 (5.4) | 36 (5.7) | .82 |
| Calcium supplement use, No. (%) | 337 (19.9) | 165 (19.5) | 172 (20.2) | .73 | 56 (8.7) | 52 (8.2) | .78 |
| Serum 25(OH)D, mean (SD), ng/mL | 25.4 (8.5) | 25.4 (8.7) | 25.5 (8.3) | .82 | 25.3 (8.2) | 25.5 (8.7) | .60 |
| One adenoma <1 cm, No. (%)e | 932 (56.5) | 472 (57.4) | 460 (55.7) | .50 | 344 (54.8) | 325 (53.2) | .58 |
| Advanced adenoma, No. (%)f | 312 (18.6) | 157 (18.8) | 155 (18.5) | .90 | 116 (18.3) | 119 (19.1) | .70 |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MET, metabolic equivalent of task.
Data were missing for participants in the following categories: activity (n = 24), diet (n = 99), multivitamins (n = 2), vitamin D supplements (n = 4), calcium supplements (n = 3), adenomas (n = 53), advanced adenomas (n = 27).
All participants were included in these vitamin D groups.
P values for 2-sample t tests for continuous variables and Pearson χ2 tests for categorical variables.
Women who chose to take calcium supplements were excluded from these calcium groups.
Adenomas at qualifying colonoscopy within 120 days prior to enrollment; missing data due to missing pathology results.
Advanced adenomas defined as those with cancer, high-grade dysplasia, more than 25% villous features, or with an estimated diameter of 1 cm or larger.
As previously reported for the study population as a whole,12 the interventions had no effect on adenoma risk over 3 to 5 years in this subset of participants (eTable 2 in Supplement 2). The proportions of participants with 1 or more adenomas or 1 or more advanced adenomas were similar between the treatment comparison groups. For any adenoma, the RR (95% CI) for vitamin D3 supplementation was 0.98 (0.87–1.09), and for calcium supplementation, 0.96 (0.85–1.09). For advanced adenomas, the RRs (95% CIs) were 1.00 (0.75–1.34) and 1.10 (0.80–1.51), respectively. There was no evidence for an interaction between vitamin D and calcium treatments. The length of follow-up (median, 45.2 months) was similar between the treatment comparison groups, as was participant adherence to pill taking and avoidance of nonstudy supplement use (eTable 2 in Supplement 2). The 7.1-ng/mL net increase in serum 25(OH)D level at 1 year among participants randomized to vitamin D3 (eTable 2 in Supplement 2) was similar to that reported for the study population as a whole.12
We investigated whether the effects of the study interventions on adenoma risk varied according to SNP genotypes in vitamin D and calcium pathway genes. Results for SNPs with any significant interaction before adjusting for multiple testing are listed in Table 2 (complete results in eTables 3 and 4 in Supplement 2). There were no significant interactions of genotypes with calcium supplementation, or with vitamin D3 supplementation for an outcome of any adenoma. However, the effect of vitamin D3 supplementation on risk of advanced adenomas significantly varied according to genotype at 2 VDR SNPs (rs7968585 and rs731236). Stratified results for these 2 3′ SNPs, which are in high linkage disequilibrium (D′ = 0.98 and r2 = 0.6; eFigure 2 in Supplement 2), are detailed in Table 3. Among individuals with the AA genotype at rs7968585 (25.8%; n = 436), vitamin D3 supplementation reduced risk of advanced adenomas by 64% (RR, 0.36; 95% CI, 0.19–0.69; P = .002), with an absolute risk reduction of 9.3%. In contrast, among individuals with 1 or 2 Galleles (74.1%; n = 1251), vitamin D supplementation increased risk by 41% (RR, 1.41; 95% CI, 0.99–2.00; P = .05), with an absolute risk increase of 3.4%. The interaction of vitamin D3 supplementation with rs7968585 genotype modeled dominantly was significant (IRR, 3.88; P < .001 for interaction). Likewise, there was a significant interaction between vitamin D3 supplementation and rs731236 genotype (Tables 2 and 3) (P = .001 for interaction), as well as nonsignificant interactions with rs795232 and rs1544410 (Table 2) (P = .002 for interaction), which are also in high linkage disequilibrium (eFigure 2 in Supplement 2). Case-only analyses yielded essentially identical findings (eTables 5 and 6 in Supplement 2): significant interactions for rs7968585, rs731236, and rs1544410, and a nonsignificant interaction for rs795232. Moreover, among the subset of optimally adherent participants (n = 1343), the estimated magnitudes of the interactions with vitamin D3 supplementation were greater for all 4 of these VDR SNPs (eTable 7 in Supplement 2).
Table 2.
IRRs (95%CIs) Between SNP Genotypes and Vitamin D3 or Calcium Supplementation for Risk of Colorectal Adenomasa
| Gene | SNP | Vitamin D (n = 1702) | Calcium (n = 1282) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Anyb | P Valuec | Advancedb | P Valuec | Anyd | P Valuec | Advancedd | P Valuec | ||
| GC | rs12512631 | 1.10 (0.93–1.29) | .26 | 0.60 (0.38–0.92) | .02 | 1.04 (0.87–1.24) | .70 | 1.07 (0.70–1.72) | .77 |
|
| |||||||||
| rs4588 | 0.82 (0.69–0.98) | .03 | 1.14 (0.71–1.83) | .62 | 0.86 (0.70–1.05) | .13 | 0.88 (0.53–1.47) | .62 | |
|
| |||||||||
| rs7041 | 0.81 (0.69–0.95) | .01 | 1.42 (0.92–2.18) | .11 | 0.90 (0.75–1.07) | .23 | 0.92 (0.57–1.47) | .73 | |
|
| |||||||||
| CYP24A1 | rs2296241 | 0.92 (0.78–1.08) | .29 | 0.68 (0.45–1.04) | .07 | 1.03 (0.87–1.23) | .70 | 0.50 (0.31–0.80) | .004 |
|
| |||||||||
| VDR | rs7968585 | 1.08 (0.92–1.26) | .33 | 1.99 (1.30–3.04) | .001 | 1.10 (0.93–1.31) | .27 | 0.99 (0.64–1.55) | .97 |
|
| |||||||||
| rs731236e | 0.94 (0.80–1.11) | .47 | 0.48 (0.31–0.75) | .001 | 0.79 (0.66–0.95) | .01 | 0.85 (0.54–1.36) | .51 | |
|
| |||||||||
| rs7975232e | 1.12 (0.96–1.31) | .15 | 1.96 (1.28–2.98) | .002 | 1.13 (0.95–1.34) | .16 | 0.96 (0.62–1.50) | .86 | |
|
| |||||||||
| rs1544410e | 0.94 (0.80–1.10) | .44 | 0.51 (0.32–0.79) | .002 | 0.83 (0.70–1.00) | .05 | 0.82 (0.52–1.30) | .40 | |
|
| |||||||||
| rs2239179 | 0.93 (0.79–1.09) | .39 | 0.60 (0.39–0.92) | .02 | 0.79 (0.66–0.95) | .01 | 0.70 (0.44–1.12) | .14 | |
|
| |||||||||
| rs10783219 | 0.86 (0.73–1.02) | .09 | 0.88 (0.57–1.35) | .56 | 1.23 (1.03–1.48) | .02 | 1.91 (1.17–3.12) | .01 | |
|
| |||||||||
| rs4516035e | 1.01 (0.86–1.18) | .91 | 1.15 (0.76–1.75) | .51 | 0.95 (0.80–1.13) | .56 | 0.56 (0.36–0.89) | .02 | |
|
| |||||||||
| rs7139166e | 1.02 (0.87–1.19) | .85 | 1.17 (0.77–1.77) | .48 | 0.94 (0.80–1.13) | .52 | 0.55 (0.35–0.87) | .01 | |
Abbreviations: IRR, interaction relative risk; SNP, single-nucleotide polymorphism.
The IRR is the ratio of the vitamin D3 supplementation relative risk per variant allele divided by that for the wild-type allele. Thus, an IRR value close to 1 indicates that genotype does not modify the effect of vitamin D3 supplementation on the outcome (adenoma or advanced adenoma); the further from 1 (higher or lower) the IRR is, the stronger the evidence that genotype modifies the effect of vitamin D3 supplementation. For IRRs greater than 1, the vitamin D3 supplementation relative risk is higher in individuals with the variant allele compared with the wild-type allele (eg, rs7968585). Conversely, for IRRs less than 1, the vitamin D3 supplementation relative risk is lower in individuals with the variant allele compared with the wild-type allele (eg, rs731236). Only SNPs with significant interaction before adjusting for multiple testing are listed. Full results are in shown in eTables 4 and 5 in Supplement 2; SNP locations are listed in eTable 1 in Supplement 2; and linkage disequilibrium r2 values are illustrated in eFigure 2 in Supplement 2.
Per-allele IRR between SNP genotype and vitamin D3 supplementation adjusted for age, sex, genotype, vitamin D3 treatment assignment, calcium treatment assignment, and interaction between genotype and calcium supplementation.
Wald test P value for interaction between SNP genotype and vitamin D3 or calcium supplementation; Accounting for multiple testing, threshold for statistical significance is P < .002.
Per-allele IRR between SNP genotype and calcium supplementation adjusted for age, sex, genotype, vitamin D3 treatment assignment, calcium treatment assignment, and interaction between genotype and vitamin D3 supplementation.
rs731236 (Taq1), rs7975232 (Apa1), rs1544410 (Bsm1); linkage disequilibrium r2 > 0.95: rs731236 and rs1544410; rs4516035 and rs7139166.
Table 3.
Association of Vitamin D3 Supplementation With Risk of Advanced Adenoma Stratified by 2 VDR Genotypes
| SNP | VDR Genotype | Participants, No. (%) | No. With Advanced Adenoma/Total No. (%) | RR (95% CI)a | P Valuea | P Valueb | |
|---|---|---|---|---|---|---|---|
| No Vitamin D | Vitamin D | ||||||
| rs7968585 (n = 1687) | AA | 436 (25.8) | 32/222 (14.4) | 11/214 (5.1) | 0.36 (0.19–0.69) | .002 | .001 |
| AG | 837 (49.6) | 31/422 (7.4) | 43/415 (10.4) | 1.41 (0.90–2.19) | .13 | ||
| GG | 414 (24.5) | 17/198 (8.6) | 27/216 (12.5) | 1.42 (0.79–2.53) | .24 | ||
| AG or GGc | 1251 (74.1) | 48/620 (7.7) | 70/631 (11.1) | 1.41 (0.99–2.00) | .05 | <.001 | |
| rs731236 (Taq1) (n = 1671) | TT | 631 (37.8) | 24/306 (7.8) | 38/325 (11.7) | 1.44 (0.89–2.35) | .14 | .001 |
| CT | 788 (47.2) | 37/402 (9.2) | 36/386 (9.3) | 1.02 (0.66–1.58) | .94 | ||
| CC | 252 (15.1) | 19/126 (15.1) | 4/126 (3.2) | 0.22 (0.07–0.63) | .005 | ||
Abbreviations: RR, relative risk; SNP, single-nucleotide polymorphism.
Relative risk for the effect of vitamin D3 supplementation on advanced adenomas and Wald test P values adjusted for age, sex, and calcium treatment assignment; threshold for significance is P < .05.
Wald test P value for interaction between SNP genotype and vitamin D3 supplementation on risk of advanced adenoma. Accounting for multiple testing, threshold for significance is P < .002. Note: a significant interaction test result indicates that there is evidence for heterogeneity between the effects of vitamin D treatment on risk of advanced adenoma outcomes in the different genotype subgroups. The individual estimates and their 95%CIs show how they differ. A significant test of interaction result does not imply that the estimates in each subgroup are necessarily statistically significant.
Post hoc analysis of dominant genetic model.
Finally, in an analysis analogous to that performed by Levin et al,20 we found that rs7968585 genotype also significantly modified the association of low serum 25(OH)D level with risk of advanced adenoma among participants not assigned to vitamin D3 supplementation (Table 4). Being in the lowest season-specific quintile of serum 25(OH)D level was associated with 69% lower risk (RR, 0.31; 95% CI, 0.08–1.19) among individuals with the AA genotype, but with 82% higher risk (RR, 1.82; 95% CI, 0.99–3.34) among those with 1 or 2 G alleles (P = .02 for interaction).
Table 4.
Association of Year-1 LowSerum 25(OH)D Level With Risk of Advanced Adenoma Stratified by VDR rs7968585 Genotype Among Vitamin D Placebo Participantsa
| Genotype | No. (%) | No. With Advanced Adenoma/Total No. (%) | RR (95% CI)b | P Valueb | P Valuec | |
|---|---|---|---|---|---|---|
| Lowest 25(OH)D Quintile | 4 Upper 25(OH)D Quintiles | |||||
| Total | 834 (100) | 17/165 (10.3) | 62/670 (9.3) | 1.17 (0.70–1.98) | .55 | NA |
| AA | 220 (26.4) | 2/41 (4.9) | 30/179 (16.8) | 0.31 (0.08–1.19) | .09 | .01 |
| AG | 416 (49.9) | 8/76 (10.5) | 22/340 (6.5) | 1.93 (0.85–4.35) | .12 | |
| GG | 198 (23.7) | 7/48 (14.6) | 10/150 (6.7) | 1.87 (0.71–4.96) | .21 | |
| AG or GGd | 614 (73.6) | 15/124 (12.1) | 32/490 (6.5) | 1.82 (0.99–3.34) | .06 | .02 |
Abbreviations: RR, relative risk; 25(OH)D, 25-hydroxyvitamin D.
Low serum 25(OH)D level is defined as being in the lowest season-specific quintile at the year-1 measurement.
Relative risk for association of advanced adenoma with low 25(OH)D level and Wald test P values adjusted for age; threshold for statistical significance is P < .05.
Wald test P value for interaction between single-nucleotide polymorphism genotype and low 25(OH)D level on risk of advanced adenoma; threshold for statistical significance is P < .05.
Post hoc analysis of dominant genetic model.
Discussion
Among participants in a randomized placebo-controlled trial, the effect of 1000 IU/d of vitamin D3 supplementation on the 3- to 5-year risk of advanced colorectal adenomas significantly varied according to genotype at 2 SNPs in high linkage disequilibrium located at the 3′ end of the VDR gene, rs7968585 and rs731236 (TaqI). For example, among individuals with the rs7968585 AA genotype, there was a 64% reduction in risk of new advanced adenomas due to vitamin D3 supplementation, but among those with 1 or 2 Galleles, there was a 41% increased risk. Two other 3′ VDR SNPs, rs7975232 (ApaI) and rs1544410(BsmI) in high linkage disequilibrium with rs7968585 and rs731236, also showed interactions, although they were not statistically significant after correcting for multiple testing. Sensitivity analyses restricted to optimally adherent participants supported these findings, since the interaction effect sizes for these SNPs increased in this group.
Our group previously reported that there was no association between adenoma risk and changes in 25(OH)D levels with supplementation.12 In addition, we found no concordance between SNPs that modified the effect of vitamin D3 supplementation on 25(OH)D levels in our prior analysis17 and those that modified the effect on adenoma outcomes in the present work. In fact, the rs7968585 variant was associated with a greater increase in 25(OH)D levels in our priorwork17 but with a higher risk of advanced adenomas in the present analysis. Thus, our results suggest that the effect of vitamin D supplementation on risk of advanced colorectal adenomas depends on VDR genotype rather than the magnitude of the change in circulating 25(OH)D levels.
The 3′ VDR SNPs studied here (rs731236, rs7975232 and rs1544410) have previously been associated with many nonskeletal health outcomes, including infectious and autoimmune diseases and cancer.27 In recent meta-analyses, rs1544410 (BsmI) was associated with lower risk of colorectal cancer28,29 but not of adenomas.30 We also found no significant interactions related to risk of 1 or more adenomas of any type, suggesting that the effects of vitamin D3 supplementation detected here might occur later in the carcinogenesis pathway than small tubular adenomas. In addition, the correlated SNP rs7968585 (which showed significant interactions in the present work) was previously reported to modify the association of circulating 25(OH)D levels with a composite outcome that included hip fracture, myocardial infarction, cancer, and all cause mortality.20 In that study, low serum 25(OH)D level was associated with higher risk of deleterious outcomes among individuals with the Gallele.20 Ostensibly, this seems opposite to our finding that vitamin D3 supplementation appeared to increase risk of advanced adenomas in individuals with the G allele, but it may reflect the effects of supplementation across the full range of basal circulating 25(OH)D levels in our study. In fact, we were able to replicate their finding in individuals who were not assigned to vitamin D3 supplementation, since low serum 25(OH)D level was associated with higher risk of advanced adenomas only among individuals with the G allele.
Our results point to the importance of polymorphisms in the VDR gene but do not identify the mechanism involved. The 3′ VDR SNPs that modified the effect of vitamin D3 supplementation on risk for advanced adenomas may be markers for a single causal variant, since they are in high linkage disequilibrium. None of these polymorphisms modify the amino acid sequence of the VDR protein,31 but SNPs in the 3′ regulatory region of the gene may affect mRNA or protein levels or isoforms expressed, potentially altering interactions with complexes of coactivators or corepressors to modify VDR regulation of gene expression. Notably, the online tool F-SNP (see http://compbio.cs.queensu.ca/F-SNP) predicts that rs731236 regulates RNA splicing (no information is currently available for rs7968585)32,33 and at least 10 alternatively spliced VDR transcripts have been experimentally verified.34 In addition, rs731236 is located in a CpG site and modifies methylation at that CpG site as well as regionally,35 which is thought to regulate VDR mRNA levels via transcription of an lncRNA.34 Thus, there are biologically plausible mechanisms for functional effects of genetic variants at this locus that could potentially explain SNP-specific opposing effects of vitamin D3 supplementation via differential effects on the activation or repression of target genes. Future research could test for functional effects on VDR expression or response genes in cultured cells using CRISPR (clustered, regularly interspaced, short palindromic repeats) gene editing technology to introduce specific VDR variants.36 The extraordinary complexity of VDR gene regulation,34 and, in turn, the vast number of nonoverlapping VDR binding sites identified in genomewide analyses (>20000)37,38 highlight the potential for broad impact of VDR genetic polymorphisms on numerous processes related to carcinogenesis. Moreover, the large variation in biological response to vitamin D3 supplementation observed in isolated human peripheral blood monocytes also supports the plausibility of our findings.39,40
Strengths of our study include the randomized design with good adherence to study treatment and avoidance of outside supplementation, a high follow-up rate, central pathology review, and the large sample size. Also, our adjustment for multiple testing was conservative for the 3′VDRSNPs, which were prespecified analyses.
Limitations
The trial tested only 1 dose of vitamin D3 and for a limited time; the candidate SNP approach was not comprehensive; and power to detect interactions was limited for advanced adenoma outcomes for rare variants. Also, this analysis was restricted to non-Hispanic whites and individuals with a prior adenoma history and 25(OH)D levels of 12ng/mL or higher and so may not be generalizable to others. Since rs7968585 and rs731236 genotype frequencies vary by race and ethnicity,31 the effect of vitamin D3 supplementation is likely to vary in different populations. Future research is needed to assess the replicability of our findings as well as to identify the causal VDR variant(s) and its mechanism of action.
Conclusions
In summary, the results of this candidate gene study indicate that the effect of vitamin D3 supplementation on risk of advanced colorectal adenomas may vary according to common differences in the vitamin D receptor gene. These findings represent an important first step toward identifying potential clinically relevant effects of polymorphisms in the VDR gene that may influence which individuals benefit, or potentially experience harm, from vitamin D interventions. Further studies are needed to confirm our findings and to improve our understanding of mechanisms by which genetic variation in metabolic genes may influence the effects of vitamin D and calcium supplementation on health.
Supplementary Material
Key Points.
Question
Do common variants in vitamin D and calcium pathway genes modify effects of vitamin D3 or calcium supplementation on risk of colorectal adenomas?
Findings
In a randomized clinical trial, the effect of vitamin D3 supplementation on advanced adenomas (but not adenoma risk overall) significantly varied according to vitamin D receptor genotypes. Among individuals with the rs7968585 AA genotype, vitamin D supplementation reduced risk by 64%, while among those with 1 or 2 G alleles, risk was increased by 41%.
Meaning
This study provides some clarity on who may benefit from vitamin D3 supplementation for preventing advanced colorectal adenomas based on vitamin D receptor genotype.
Acknowledgments
Funding/Support: This work was supported by grants from the National Cancer Institute (CA159360 and CA098286). Study pills were provided without cost by Pfizer Consumer Healthcare. Dr Peacock was supported by the United Kingdom (UK) NIHR Biomedical Research Centre based at Guy’s and St Thomas’ NHS
Foundation Trust and King’s College London. Use of the Dartmouth Biospecimen Storage Facility was supported by the Center for Molecular Epidemiology COBRE program with a grant from the National Institute of General Medical Sciences (P20 GM104416).
Footnotes
Conflict of Interest Disclosures: Together with Dartmouth College, Dr Baron holds a patent for the chemopreventive use of calcium (US 6 251 439 B1). This is currently not licensed. No other disclosures are reported.
Role of the Funder/Sponsor: The study sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; the preparation, review or approval of the manuscript; nor the decision to submit the manuscript for publication.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the UK Department of Health.
Additional Contributions: The authors acknowledge the contributions of all of the Polyp Prevention Study Group Investigators and Study Coordinators, as well as the Informatics and Coordination staff at the Dartmouth Project Coordination Center. We are most grateful to the study participants, whose long-term dedication and commitment made this research possible.
Author Contributions: Dr Barry had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Barry, Bresalier, Baron.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Barry, Bostick.
Critical revision of the manuscript for important
intellectual content: All authors.
Statistical analysis: Barry, Peacock, Bostick.
Obtained Funding: Barry, Rees, Bostick, Bresalier, Baron.
Administrative, technical, or material support: Bresalier, Baron.
Study supervision: Rees, Bostick, Bresalier, Baron.
Review and classification of clinical events during the trial: Rees.
References
- 1.Zhang X, Giovannucci E. Calcium, vitamin D and colorectal cancer chemoprevention. Best Pract Res Clin Gastroenterol. 2011;25(4–5):485–494. doi: 10.1016/j.bpg.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 3.Pereira F, Larriba MJ, Muñoz A. Vitamin D and colon cancer. Endocr Relat Cancer. 2012;19(3):R51–R71. doi: 10.1530/ERC-11-0388. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92(2):77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 6.Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J Steroid Biochem Mol Biol. 2014;144(Pt A):5–11. doi: 10.1016/j.jsbmb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipkin M, Newmark H. Calcium and the prevention of colon cancer. J Cell Biochem Suppl. 1995;22:65–73. doi: 10.1002/jcb.240590810. [DOI] [PubMed] [Google Scholar]
- 8.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313(13):1311–1312. doi: 10.1001/jama.2015.1353. [DOI] [PubMed] [Google Scholar]
- 10.Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135(8):1940–1948. doi: 10.1002/ijc.28840. [DOI] [PubMed] [Google Scholar]
- 11.Shui I, Giovannucci E. Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol. 2014;810:33–51. doi: 10.1007/978-1-4939-0437-3_3. [DOI] [PubMed] [Google Scholar]
- 12.Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015;373(16):1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121(1–2):471–477. doi: 10.1016/j.jsbmb.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 14.Ahn J, Yu K, Stolzenberg-Solomon R, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dastani Z, Li R, Richards B. Genetic regulation of vitamin D levels. Calcif Tissue Int. 2013;92(2):106–117. doi: 10.1007/s00223-012-9660-z. [DOI] [PubMed] [Google Scholar]
- 17.Barry EL, Rees JR, Peacock JL, et al. Genetic variants in CYP2R1, CYP24A1, and VDR modify the efficacy of vitamin D3 supplementation for increasing serum 25-hydroxyvitamin D levels in a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(10):E2133–E2137. doi: 10.1210/jc.2014-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoury MJ, Flanders WD. Nontraditional epidemiologic approaches in the analysis of gene-environment interaction: case-control studies with no controls! Am J Epidemiol. 1996;144(3):207–213. doi: 10.1093/oxfordjournals.aje.a008915. [DOI] [PubMed] [Google Scholar]
- 19.Dennis J, Hawken S, Krewski D, et al. Bias in the case-only design applied to studies of gene-environment and gene-gene interaction: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(5):1329–1341. doi: 10.1093/ije/dyr088. [DOI] [PubMed] [Google Scholar]
- 20.Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308(18):1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison NA, Yeoman R, Kelly PJ, Eisman JA. Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor gene polymorphism and circulating osteocalcin. Proc Natl Acad Sci U S A. 1992;89(15):6665–6669. doi: 10.1073/pnas.89.15.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison NA, Qi JC, Tokita A, et al. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367(6460):284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 23.Wishart JM, Horowitz M, Need AG, et al. Relations between calcium intake, calcitriol, polymorphisms of the vitamin D receptor gene, and calcium absorption in premenopausal women. Am J Clin Nutr. 1997;65(3):798–802. doi: 10.1093/ajcn/65.3.798. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Newcomb PA, Ulrich CM, et al. Vitamin D receptor polymorphism and the risk of colorectal adenomas: evidence of interaction with dietary vitamin D and calcium. Cancer Epidemiol Biomarkers Prev. 2001;10(8):869–874. [PubMed] [Google Scholar]
- 25.Slatter ML, Yakumo K, Hoffman M, Neuhausen S. Variants of the VDR gene and risk of colon cancer (United States) Cancer Causes Control. 2001;12(4):359–364. doi: 10.1023/a:1011280518278. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005;95(3):221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 27.Jolliffe DA, Walton RT, Griffiths CJ, Martineau AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin Dmetabolites and non-skeletal health outcomes: Review of genetic association studies. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.12.007. S0960–0760(15)30153–9. [DOI] [PubMed] [Google Scholar]
- 28.Bai YH, Lu H, Hong D, Lin CC, Yu Z, Chen BC. Vitamin D receptor gene polymorphisms and colorectal cancer risk: a systematic meta-analysis. World J Gastroenterol. 2012;18(14):1672–1679. doi: 10.3748/wjg.v18.i14.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touvier M, Chan DS, Lau R, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20(5):1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 30.Lee JE. Circulating levels of vitamin D, vitamin D receptor polymorphisms, and colorectal adenoma: a meta-analysis. Nutr Res Pract. 2011;5(5):464–470. doi: 10.4162/nrp.2011.5.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Lee PH, Shatkay H. F-SNP: computationally predicted functional SNPs for disease association studies. Nucleic Acids Res. 2008;36(Database issue):D820–D824. doi: 10.1093/nar/gkm904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PH, Shatkay H. An integrative scoring system for ranking SNPs by their potential deleterious effects. Bioinformatics. 2009;25(8):1048–1055. doi: 10.1093/bioinformatics/btp103. [DOI] [PubMed] [Google Scholar]
- 34.Saccone D, Asani F, Bornman L. Regulation of the vitamin D receptor gene by environment, genetics and epigenetics. Gene. 2015;561(2):171–180. doi: 10.1016/j.gene.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Andraos C, Koorsen G, Knight JC, Bornman L. Vitamin D receptor gene methylation is associated with ethnicity, tuberculosis, and TaqI polymorphism. Hum Immunol. 2011;72(3):262–268. doi: 10.1016/j.humimm.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10(10):957–963. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuoresmäki P, Väisänen S, Neme A, Heikkinen S, Carlberg C. Patterns of genome-wide VDR locations. PLoS One. 2014;9(4):e96105. doi: 10.1371/journal.pone.0096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol. 2014;5:167. doi: 10.3389/fphys.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saksa N, Neme A, Ryynänen J, et al. Dissecting high from low responders in a vitamin D3 intervention study. J Steroid Biochem Mol Biol. 2015;148:275–282. doi: 10.1016/j.jsbmb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 40.Vukić M, Neme A, Seuter S, et al. Relevance of vitamin D receptor target genes for monitoring the vitamin D responsiveness of primary human cells. PLoS One. 2015;10(4):e0124339. doi: 10.1371/journal.pone.0124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.