Abstract
Approximately one-third of individuals in the United States experience unsatisfactory bowel habits, and dietary intake, especially one low in fiber, could be partly responsible. We hypothesized that intake of a fermentable fiber (starch-entrapped microspheres, SM) that has a delayed, slow fermentation profile in vitro would improve bowel habit while exhibiting prebiotic capacity in those with self-described unsatisfactory bowel habits, all with minimal side effects. A total of 43 healthy volunteers completed a 3-month, double-blind, parallel-arm randomized clinical trial to assess the ability of a daily dose (9 g or 12 g) of SM versus psyllium (12 g) to improve bowel habit, including stool consistency and frequency, and modify gut milieu through changes in stool microbiota and short-chain fatty acids (SCFAs), while remaining tolerable through minimal gastrointestinal symptoms. All outcomes were compared before and after fiber treatment. Stool frequency significantly improved (P = .0003) in all groups after three months, but stool consistency improved only in both SM groups compared to psyllium. In addition, all groups self-reported a similar improvement in overall bowel habit with fiber intake. Both SM and psyllium resulted in minimal changes in microbiota composition and SCFA concentrations. The present study suggests that supplementation with a delayed and slow-fermenting fiber in vitro may improve bowel habit in those with constipation, but further investigation is warranted to determine capacity to alter microbiota and fermentation profiles in humans. This trial was registered at ClinicalTrials.gov as NCT01210625.
Keywords: prebiotics, dietary fiber, microbiota, constipation, clinical trial
1. Introduction
The number of individuals who experience unsatisfactory bowel habits are increasing, with up to one-third of the population experiencing constipation [1], a condition that is defined by any of the following: fewer than three bowel movements per week, straining, hard stool, and a sense of incomplete evacuation [2]. The reason for this increase is not completely clear, but likely involves several environmental factors including dietary intake of foods that are high in fat and refined carbohydrates and low in fiber such as fruit, vegetables, and complex carbohydrates [3]. Lack of adequate fiber intake may contribute to constipation-predominant unsatisfactory bowel habit because of fiber's fecal bulking capacity or lack of substrate for fermentation, the byproducts of which (e.g., short-chain fatty acids [SCFAs]) support healthy colonic epithelial cells[4]; these attributes are dependent on the specific attributes of the fiber.
Fiber supplements (primarily fiber capsules or fiber powders) commonly used for those with unsatisfactory bowel habit, including constipation, primarily consists of one of several specific dietary fibers, with psyllium being one of the commonly recommended fiber supplements [5]. Psyllium has been reported in a number of studies to be a non-fermentable bulking fiber that functions in the colon by binding water and increasing viscosity [6,7]. Some evidence exists to support the supplementation of fibers such as psyllium to improve constipation, but data are limited [8]. In addition, several challenges and obstacles have been encountered for widespread use of fibers in general as their intake often can result in significant side effects such as gas, bloating, and gastrointestinal pain, especially in those that have unsatisfactory bowel habit [9]. These side effects could be due, in part, to the fermentation capacity of the fiber. While fermentable fibers may beneficially alter the intestinal milieu, fermentation is often rapid, causing gas production and bloating [10,11]. These side effects could lead to poor compliance and intake of less than an optimal dose. Therefore, investigation into an alternative fermentable, non-bloating fiber supplement for constipation relief is warranted.
To this end, we utilized our expertise in carbohydrate science to develop a novel, fermentable, starch-based, fiber-entrapped microsphere. Both in vitro and in vivo experiments reveal that these starch-entrapped microspheres (SM) are slowly fermented, allowing for delayed gas production and thus potentially increased tolerability as compared to conventional fibers and current prebiotics [12-15]. Thus, we hypothesized that intake of SM would promote gut health by improving bowel habit and exhibiting prebiotic capacity, both with minimal side effects. Therefore, the primary objective of the study was to determine the ability of two daily doses of SM to improve bowel habit, including stool consistency and frequency, and modify gut milieu (microbiota and SCFA), while remaining tolerable through minimal gastrointestinal symptoms (e.g. gas, bloating, abdominal pain) in those self-described unsatisfactory bowel habit (constipation-predominant). We utilized a randomized, double-blind, controlled trial with those with self-described unsatisfactory bowel habits using psyllium as a comparator to meet our objective.
2. Methods and materials
2.1. Participants and study design
This was a randomized, double-blind, controlled, three-arm, parallel-group, Phase I clinical trial conducted at Rush University Medical Center from January 2011 to July 2012. Individuals were included if they were 18-65 year-old males and females with self-described unsatisfactory bowel habits. Unsatisfactory bowel habit was defined by any of the following conditions: <3 bowel movements per week, hard stool requiring straining, or sense of incomplete defecation. Potential participants also had to report dissatisfaction with their bowel movements. Exclusion criteria included the following: 1) abnormal complete blood count (CBC) values, 2) abnormal liver function tests, 3) low serum albumin (<3 g/dL), 4) abnormal thyroid stimulating hormone (TSH), 5) elevated C-reactive protein (CRP >5), 6) gastrointestinal symptoms except for occasional heartburn (less than 3 times a week not medication-dependent) or occasional fresh blood in stool from hemorrhoids, 7) prior intestinal resection, 8) history of gastrointestinal diseases (except for hemorrhoids or hiatal hernia), 9) antibiotic use within the last 12 weeks prior to enrollment, 10) underweight (BMI <18.5 kg/m2), 11) significant cardiac or respiratory diseases, severe hypertension, insulin-requiring and/or poorly controlled diabetes (HbA1c >6), 12) significant psychological disorders, drug and/or alcohol abuse, 13) unwillingness to consent to the study, 14) plan to have a major change in dietary habits during the following five months from time of enrollment, and 15) pregnant and lactating women due to lack of information about safety of the product in this population.
The study was 14 weeks in duration, with a 2-week initial run-in phase to ensure stability of reported bowel habit and a 12-week treatment phase. Study participants participated in three face-to-face visits (visits 1-3) throughout the study. At visit 1, participants returned a completed 7-day food and bowel habit diary and provided fecal samples. During this visit, the health status of each study participant was assessed through a comprehensive questionnaire, physical examination, and blood draw for CBC, CMP, and TSH to determine eligibility to participate. If eligible, participants returned for visit 2 at the end of the two-week run-in period and provided a second completed 7-day food and bowel habit diary; the bowel habit diary was used to determine if the subject had unsatisfactory bowel habits as previously described. Eligible participants were randomly assigned to one of three groups: 12 g psyllium, 9 g SM, or 12 g SM. Randomization was concealed and assigned numbers were computer generated by the statistician who was not involved in participant recruitment or outcome assessment and data analysis. Participants were then provided with the fiber supplements and consumption instructions. After the completion of the 3-month treatment period, participants returned for visit 3 and provided food, gastrointestinal symptom, and bowel habit diaries completed daily during the last seven days of each of the three months of the study. During the visit, health status was again assessed, and the number of capsules not consumed was recorded and reconciled with a medication log to determine compliance. Participants were also encouraged to maintain their usual diets throughout the study period. During the 3-month treatment phase, phone calls were made weekly to remind all participants to take their supplements and to inquire about their health and any new symptoms.
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board of Rush University Medical Center (approved April 2010). Written informed consent was obtained from each participant before beginning the study. This trial was listed at ClinicalTrials.gov as NCT01210625.
2.2. Fiber treatments
Starch-entrapped microspheres were prepared as previously described [15]. In brief, a suspension of sodium alginate (2% w/v) and normal corn starch (9% w/v) was made in water and dropped through a 22-gauge hypodermic needle using a peristaltic pump (Tris, Teledyne Isco, Lincoln, NE, USA) into a calcium chloride bath (4% w/v). The microspheres were kept in the bath for 1 to 4 h, collected by filtration, thoroughly washed with water, and then dried in a warm air oven at 45°C for 24 hr. All materials that constitute the SM supplement are currently marketed in the United States for food use (GRAS classified: cornstarch, 21CFR§182.70; sodium alginate, 21CFR §184.1724), and the microspheres were manufactured and packaged under proper Standard Operating Procedures to ensure product safety. Psyllium was obtained from Natural Foods Inc (Toledo, OH) and was selected as the comparator due to its common use as a fiber supplement with gentle bulk-forming laxative capabilities. Both the SM and psyllium were encapsulated in 1 g quantities; depending on randomization, participants either consumed 9 g or 12 g each day. Participants were instructed to consume these 9 or 12 pills daily in three equal doses. A dose of 9 g and 12 g of SM was selected based on our pilot study in 10 participants with constipation indicating that after one month of supplementation, a 9 g dose improved bowel habit (improvement in stool consistency from 2 to 4 on the Bristol Stool Scale [16]; range of +6 to +10 on a visual analog scale ranging from -10 [worsening of bowel movements] to 10 [improvement of bowel movements] with 0 indicating no change), and increased stool butyrate concentration (P = .01; Fig. 1) without side effects. The psyllium group consumed 12 g per day to match the highest SM dose. Treatment compliance was addressed during the last seven days of each month during the 3-month treatment period. Compliance was set at 75% a priori; this was determined by assessing the number of days all capsules were taken by the 21 days recorded.
Fig. 1. Fecal butyrate concentrations (n=3) before and after 4 weeks of 9 g starch-entrapped microsphere supplementation in a one-group, open-label pilot study.
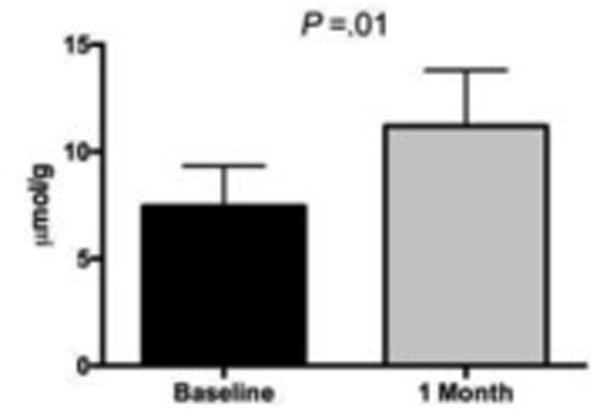
Butyrate concentrations analyzed by Mann-Whitney U Test and expressed as μmol/g of dry fecal weight. Values are means ± SD.
2.3. Assessment of gastrointestinal clinical outcomes
Structured questionnaires were used to assess impact of fiber supplementation on bowel habit and gastrointestinal symptoms. A Gastrointestinal Symptom and Severity Checklist (GSSC) containing 34 items was completed at all study visits to assess the presence of gastrointestinal symptoms. This tool asked participants to rank the severity of their symptoms based on an 11-point scale (score of 0-10 with 10 being most severe). In addition, a 10-item tool to assess side-effects of treatment was completed during the last week of each of the three months during the treatment period; these questions were a subset of the questions on the GSSC and included bloating, abdominal pain, indigestion, passing gas, belching, heartburn, acid reflux, regurgitation of food, early fullness, and after food fullness. At these same time points, stool frequency was assessed by having participants indicate the number of bowel movements daily, and stool consistency was quantified by the Bristol Stool Scale [16]. Abnormal stool consistency was operationalized as Types 1 and 2 (“constipated” stool), and 5-7 (“diarrheal” stool). The change in self-reported bowel habit was additionally assessed between the beginning and end of the treatment period by six questions asking about change in severity of constipation, stool frequency, completeness of bowel movements, stool consistency, ease of defecation, and overall well-being. Perceived improvement in physical and mental health was assessed through the SF-12v2 instrument (Medical Outcomes Trust; Boston, MA). The SF-12v2 consists of 12 questions with 3 to 5 response levels and is intended to asses both physical and mental functioning, represented by a Physical Component Summary (PCS-12) score and a Mental Component Summary (MCS-12) score, respectively. In addition, perceived improvements in constipation severity were assessed through six questions that addressed change in severity of constipation, stool frequency, completeness of bowel movements, stool consistency, ease of defecation, and overall well-being.
2.4. Stool short-chain fatty acid measurement
Stool samples were analyzed for short-chain fatty acids (SCFA) as previously described [13]. In brief, stool was centrifuged (12,000 rpm, 5 min), and a mixture of formic acid (20%), methanol, and 2-ethyl butyric acid (internal standard, 2 mg/ml in methanol) was added to the supernatant. A 0.5 ml sample was injected onto a GC column (Stabilwax-DA, length 15 m, inner diameter 0.53 mm, film thickness 0.1 mm; Varian Chrompack, Bergen op Zoom, The Netherlands) in a Chrompack CP9001 GC using an automatic sampler (Chrompack liquid sampler CP9050; Varian Chrompack). Quantification was accomplished by measuring the peak areas for acetate, propionate, butyrate, and the branched-chain fatty acids isobutyrate and isovalerate relative to 2-ethyl butyric acid. Short-chain fatty acids from visits 1 and 2 (two weeks apart) at baseline were averaged to address variability in the samples.
2.5. Stool microbiota collection and interrogation
Participants collected stool using an anaerobic collection system (BD Gas Pak EZ Anaerobe Pouch System) and stored the sample in the refrigerator for a maximum of 24 hours before the scheduled visit. Once received, the stool samples were immediately stored at −80°C until used for microbiota interrogation. The microbial community was assessed for stool samples obtained at visit 2 (before treatment) and visit 3 (after completion of treatment). Genomic DNA was extracted from fecal samples using the FastDNA Spin Kit for Soil (MP Biomedicals, Solon, OH 44139 USA) according to the manufacturer's recommended protocol. The extracted DNA was quantified using the Qubit dsDNA BR Assay and the Qubit® 2.0 Fluorometer (Life Technologies, Grand Island, NY). Genomic DNA was PCR amplified using the primers 5′-GAGTTTGATCNTGGCTCAG-3′ and 5′-GTNTTACNGCGGCKGCTG-3′ targeting the V1-V3 region of bacterial 16S rRNA genes, and prepared for sequencing on a Roche 454 GS FLX pyrosequencing instrument at the Research and Testing Laboratory (RTL; Lubbock, TX), as described previously [17]. Raw SFF files were imported into the software package CLC genomics workbench (v8; CLC bio, Qiagen, Aarhus, Denmark). Data were quality trimmed (Q<0.03), and sequences shorter than 250 bases after trimming were removed from the dataset. Each sample was exported as a FASTA file, and rarefied to 2000 sequences per sample [18]. Samples were then processed using the QIIME software package (v1.8.0) [19]. Briefly, sequences were screened for chimeras using the USEARCH61 algorithm [20], and putative chimeric sequences were removed from the data set. Data were pooled and clustered into operational taxonomic units (OTU) at 97% similarity using the USEARCH61 de novo OTU picking algorithm. Subsequently, representative sequences from each OTU were extracted, and these sequences were classified using the assign taxonomy uclust algorithm, utilizing the Greengenes reference database (v13_8) [21]. A biological observation matrix (BIOM) containing all the grouped taxonomy of each individual OUT was generated [22]. Alpha-diversity (within sample) and beta-diversity (between-sample) metrics were calculated within the software package Primer6 (Primer-E Ltd., Plymouth, UK). Data from the entire dataset were visualized using non-metric multidimensional (NMDS) scaling using Bray-Curtis similarity. Analysis of similarity (ANOSIM) was performed on the similarity matrix to determine if there were significant effects of treatment (starch type group) or visit (before or after treatment) on patient fecal microbial community. Diversity indices were calculated based on the untransformed data, equalized to the same number of sequences [18]. Taxon-by-taxon analyses were performed to determine if the relative abundance of individual taxa were significantly different between treatments and sampling time points using the group significance algorithm implemented within QIIME. Taxa below a 1% threshold were removed from the group significance test (Kruskal-Wallis test), and a false-discovery rate (FDR) correction was applied. The amplicon sequence data from this study have been submitted to the NCBI Sequence Read Archive (SRA; https://urldefense.proofpoint.com/v2/url?u=http-3A__www.ncbi.nlm.nih.gov_Traces_sra_sra.cgi&d=CwIBaQ&c=XxU8ngzB_WPJXKyiin_6iQ&r=ZkwUjLY6PMt39iYSfGuwuIjGIktHOmd7xBfMiVmlpI&m=qsCZee47BbhooXFNIaJRZGrQQnwiLT8BeCVrhHiZV1g&s=GbBM30WUl3CBiuknSS83Dh1alncWJKn7dvv7MywznLQ&e=) under the BioProject (PRJNA385004) accession number SUB2628611.
2.6. Statistical analyses
Descriptive statistics were performed for normally distributed continuous measures using means and standard deviations, and non-normally distributed variables using medians and interquartile range. Categorical measures were reported using frequency distributions and medians and interquartile range. Descriptive measures were compared using one-way Analysis of Variance for continuous measures normally distributed, Kruskal-Wallis test for measures not normally distributed, and chi-square test statistic for percentages. For all outcomes, an intent-to-treat analysis was completed with the 43 participants that completed the treatment period. Change in gastrointestinal symptoms and quality of life (SF-12 scale) were assessed between and within the groups (9 g or 12 g SM and 12 g psyllium) at the three visits using a generalized estimating equations (GEE) approach with identity link function and an exchangeable correlation matrix. A p-value of <0.05 was set as the level of significance for all results. Data were analyzed with SAS software (version 9.1; SAS Institute, Cary, NC, USA); dietary intake data was entered using Food Processor version 10.9 (esha Research; Salem, OR).
The power for this study was based on a chi-squared test statistic to compare the proportion of participants with side effects between the psyllium control arm and the two treatment groups. Based on prior experience, the proportion of participants in the psyllium group with side-effects was expected to be approximately 80%, and we hypothesized that the SM supplement would reduce the side-effects by half, nearly 40%. Thus, we would have 90% power to detect this difference in proportions between the control and treatment arms with an error rate of 5%. If the differences in proportions were smaller, say, SM reduced side-effects by 60% of that in psyllium, then the power drops to 75%.
3. Results
3.1. Participant characteristics
Of the 68 participants enrolled, 63 returned for visit 2 after the 2-week run in, and 43 returned for the post-treatment visit at the end of the three months (visit 3) (Fig. 2). Of the 43 that completed the treatment period, 13 were in the 12 g psyllium group, 15 in the 9 g SM group, and 15 in the 12 g SM group (38.1%, 25.0%, and 31.8% attrition in each group, respectively); while the highest attrition occurred in the psyllium group, no significant differences in attrition existed between groups. The demographic characteristics of participants at baseline are listed by treatment group (n = 43) in Table 1. The mean (±SD) age of all participants was 42.6 ±11.9 years, 69.8% were female, 55.8% were African American, and average BMI was 30.1±7.6 kg/m2. A total of 80.0% of the participants had greater than 12 years of education, and 55.6% were employed. No differences in baseline characteristics existed between groups nor between those that were randomized to treatment (n = 63) and those that completed the study (n = 43). All analyses were performed with those completing the treatment period (returned for visit 3; n= 43).
Fig. 2. Participant screening, enrollment, and attrition.
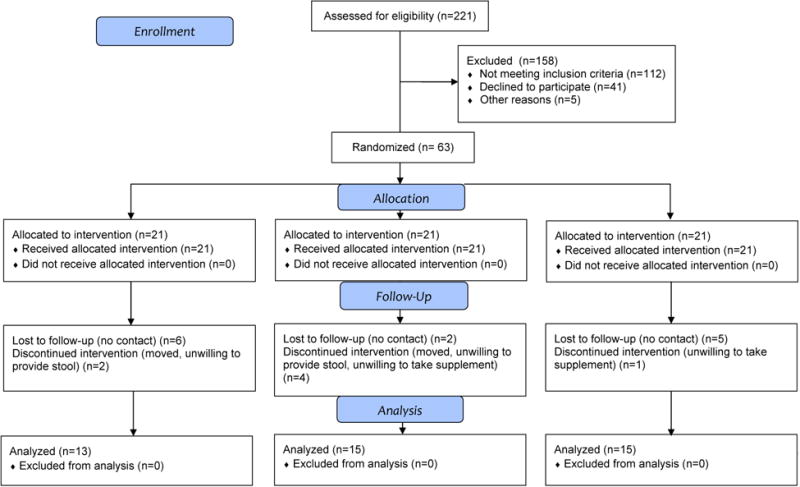
SM, starch-entrapped microspheres
Table 1. Baseline characteristics of study participantsa.
| Psylliumb (n = 13) | SM 9 g (n = 15) | SM 12 g (n = 15) | |
|---|---|---|---|
| Age, y | 48.2 ± 10.3 | 41.9 ± 13.6 | 38.3 ± 10.2 |
| Female (%) | 76.9 | 66.7 | 66.7 |
| Race (%)c | |||
| Black/African American | 61.5 | 40.0 | 66.7 |
| BMI (kg/m2) | 30.5±7.2 | 29.3 ± 8.7 | 30.5±7.2 |
| Educationd (%) | |||
| > 12 years | 91.7 | 69.2 | 80.0 |
| Employment status (%) | |||
| Employede | 58.3 | 46.2 | 63.6 |
| Dietary Fiber, g/1000 kcal | 12.7±3.6 | 12.1±4.6 | 13.2±6.1 |
Baseline characteristics based on data obtained from those completing the trial (n=43); data presented as means ± SD unless otherwise noted; no significant differences existed between groups at baseline by one-way analysis of variance or x2 test
Psyllium dose = 12 g
Races other than African American include white (n=12), Hispanic (n=14), and Asian (n=2).
Psyllium, n=12; 9 g SM, n=13; 12 g SM, n=10
Employed = full-time or part-time employment; those not employed included those that responded as unemployed, student, homemaker, or retired. Psyllium, n=12; SM 9g, n=13; SM 12g, n=11
Abbreviations: SM, starch-entrapped microspheres; kcal, kilocalories
3.2. Dietary intake and supplement compliance
Dietary intake (n = 42) was assessed through 7-day food records before and after the treatment period to ensure that any changes seen in bowel habit and gut milieu were due to the fiber supplementation and not changes in dietary intake. No significant differences in dietary intake existed, including dietary components that may modulate bowel habit when examined by both total intake and intake/1000 kilocalories. Specifically, total dietary fiber (Table 1), soluble fiber, and insoluble fiber did not differ between treatment groups within time point or change within groups over time (data not shown). Total fiber intake at baseline for the entire group was below recommendations at an intake of 12.6 g/1000 kilocalories of fiber per day, with soluble fiber intake at 1.0 g/1000 kilocalories. The average energy intake was 1,389 kilocalories a day, indicating that the participants likely underreported when documenting their dietary intake. None of the participants reported taking dietary supplements that would impact bowel habit such as prebiotics, probiotics, or supplemental dietary fiber. Of those that reported complete compliance data (n = 39), 85% were considered compliant. No differences in compliance existed between groups. All participants that completed the study were included in the analyses independent of compliance.
3.3. Tolerability and gastrointestinal symptoms
At baseline and each month of the 3-month treatment period, gastrointestinal symptoms were assessed to determine changes in these symptoms between groups over time and to determine tolerability of the fiber supplements. Of the 10 symptoms assessed, six were different between groups (bloating, abdominal pain, indigestion, passing gas, belching, heartburn; p<0.05) at baseline. Change in the summed score for each of these parameters between groups was not statistically significant. However, change between groups with treatment for several parameters including abdominal pain and bloating showed trends towards differences between groups (Fig. 3).
Fig. 3. Change in abdominal pain and bloating during supplementation at months 1-3 with either psyllium, 9 g SM, or 12 g SM.
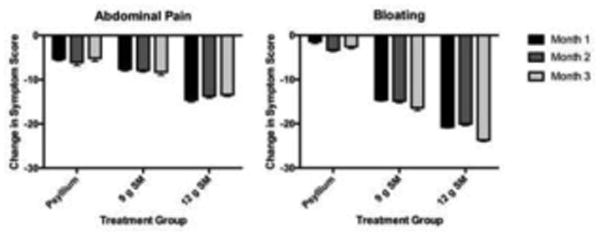
Change in symptoms scores were compared to baseline symptoms before treatment (Visit 1). Symptom change reported as summed scores from a 7-day symptom diary and analyzed by GEE. SM; starch-entrapped microspheres. Values are means ± SD.
3.4. Bowel habit
At baseline (visit 1), both 9 g and 12 g SM treatment groups had a lower percentage of those with normal consistency (Type 3 or 4) than psyllium, with 33.8% and 24.2% with normal stool consistency at baseline, respectively, versus 62.2% in the psyllium group (p < 0.012) (Table 2). At the end of the 3-month supplementation period, the percentage of participants who reported normal stool consistency were similar in all 3 groups; the percent with normal stool consistency was 56.7%, 48.0%, and 45.7% for psyllium, SM 9 g, and SM 12 g, respectively.
Table 2. Percent of participants with normal stool consistency at baseline and treatment enda.
| Treatment | Baseline | Month 3 |
|---|---|---|
| Psyllium | 62.2±7.0 | 56.7±8.7 |
| SM, 9 g | 33.8±7.5* | 48.0±10.3 |
| SM, 12 g | 24.2±8.8* | 45.7±9.8 |
Values are means ± SDs
Psyllium dose = 12 g
P < .05 for differences from psyllium at baseline; GEE Analysis
Abbreviations: SM, starch-entrapped microspheres
Mean bowel movement frequency increased with psyllium intake (p = 0.0003) from baseline to treatment end (9.8±0.1 bowel movements per week vs 14.0±0.2 bowel movements per week). The number of bowel movements per week in the 9 g and 12 g SM groups also increased from 9.8±0.1 to 11.9±0.2, and from 9.8±0.2 to 12.6±0.2 bowel movements, respectively; this increase was not significantly different than the increase in the psyllium group over time.
When self-reporting improvement in bowel habit, participants in all three groups experienced a “minimally better” (4 of 5) or “significantly better” (5 of 5) change in all six parameters of bowel habit after supplementation, with no significant differences between groups for each of the six parameters (Table 3).
Table 3. Self-reported improvement in bowel habit after supplementation in participants consuming psyllium or starch-entrapped microspheres (SM)a.
| Psylliumb (n = 10) | SM 9 g (n = 12) | SM 12 g (n = 14) | |
|---|---|---|---|
| Constipation Severity | 5.0 (0.5) | 5.0 (2.0) | 5.0 (1.8) |
| Stool Frequency | 5.0 (0.5) | 4.5 (2.0) | 5.0 (1.0) |
| Bowel Movement Completeness | 5.0 (0.5) | 4.0 (2.0) | 5.0 (1.8) |
| Stool Consistency | 5.0 (0.0) | 4.5 (2.0) | 5.0 (1.8) |
| Ease of Defecation | 5.0 (0.3) | 5.0 (2.0) | 5.0 (1.0) |
| Overall Well-Being | 5.0 (0.3) | 4.0 (2.0) | 4.5 (2.0) |
based on a scale of 1-5 with 1 = Significantly worse, 2 = Minimally worse, 3=No change, 4=Minimally better, and 5= Significantly better; Data presented as median (IQR) as a calculated range; no significant difference in improvement between groups by GEE analysis
Psyllium dose = 12 g
3.5. Quality of life
Baseline SF12 scores assessed functional health through five parameters for both physical and mental health. Physical and mental components scores before and after treatment were 49.9 and 47.7, 49.2 and 51.2, and 51.8 and 44.1 for the psyllium, 9 g SM, and 12 g SM groups, respectively; the differences between these scores were not significant. In addition, the SF-12 scores approximated the SF-12 mean scores of the general US population (score of 50) for all parameters and all treatment groups.
3.6. Gut microbial community structure
No significant effect on alpha diversity was observed between the different treatment groups; for example, the average group Shannon index ranged from 2.20 to 2.64 between the six groups (three treatment groups by two visits [2 and 3]) (Figure 4). Likewise, two-way analysis of similarity (ANOSIM) (group and visit) did not indicate a significant difference in microbial community across the different groups or visits (Global R statistic = -0.005, p=0.559, 999 permutations). Analysis of similarity did indicate a significant effect of individual patient on gut microbial community (Global R statistic = 0.474, p=0.0001, 9999 permutations). We observed that in many individuals, the gut microbial community was fairly stable across the two visits, and within a clustering of overall Bray-Curtis similarity of 60 or higher (scale 0-100). In several individuals from each treatment, visit 2 and visit 3 gut microbial community structure was significantly divergent, but no systematic association with treatment was observed. No significant effect of compliance was observed.
Fig. 4. Microbial taxonomic composition of fecal samples based on 16S rRNA gene amplicon sequencing from genomic DNA extracts.
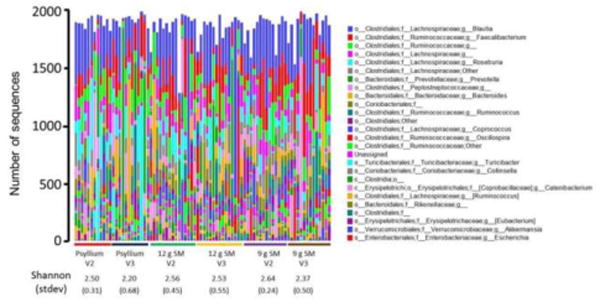
Raw sequence data (rarefied to 2,000 sequences/sample) were clustered (97%) and annotated as described in the text. Annotation data were summarized at the taxonomic level of genus, and the number of sequences from each taxon for each sample are presented as a bar plot. Samples are grouped by treatment group (psyllium, 9 g SM, 12 g SM) and visit (2 or 3). For each group, the average Shannon index and standard deviation is shown. SM; starch-entrapped microspheres
A taxon-by-taxon analysis did not reveal any individual taxa (across all taxonomic levels) with significant differences, as determined using Kruskal-Wallis nonparametric test, adjusted using the Benjamini-Hochberg false-discovery rate (FDR) correction. When examining taxa without correcting for FDR, differences in Coriobacteriaceaeae were observed (p =0.04); however, due to high variability, this change was not significant when corrected with FDR. The lack of taxa with significant differences appears to be the result of the incidence of many individuals with low relative variation between the two visits, while other individuals had extreme variation; this was observed across all three treatment groups. Thus, the variation across each sample size lead to the lack of significant effects across the study. For example, there was high variation in the relative abundance of bacteria from the genus Prevotella, ranging from no detected sequences (49 samples of 82) up to 1732 sequences (out of 2000). We note, that although not significant, the general trends in all three patient groups were towards higher relative abundance of Bacteroidetes from visit 2 (before treatment) to visit 3 (after treatment); part of this is due to the increase in average abundance of sequences from Prevotella (phylum Bacteroidetes) from visit 2 to 3 in all treatments. A majority of the Prevotella was P. copri, with few samples containing P. stercorea.
3.7. Short-chain fatty acids
Short-chain fatty acid analysis was performed in stool collected at baseline (visit 1 & 2) and treatment end (visit 3) for those that completed the treatment period. There were no differences in mean total SCFA or individual SCFA (propionate, acetate, and butyrate) between the three groups at baseline or from baseline to treatment end (Table 4). Similarly, branched-chain fatty acids (BCFA) and the ratio of SCFA:BCFA did not significantly change with treatment despite an increase in the ratio in the 9 g SM group. Trends in overall SCFA and butyrate decreases from baseline were seen in the psyllium group compared to both no change in SM groups. In addition, while only 28.6% of participants in the psyllium group experienced increases in SCFA from baseline to treatment end, 53.3% of participants in the 9 g SM group, and 43.8% of participants in the 12 g SM group experienced increases in SCFA with treatment.
Table 4. Short-chain fatty acids before and after treatment in participants consuming psyllium or starch-entrapped microspheres (SM)a.
| Psylliumb (n = 13) | SM 9 g (n = 13) | SM 12 g (n = 13) | |
|---|---|---|---|
| Total SCFA, μmol/gc | |||
| Baseline | 100.1 (128.3) | 52.1 (29.2) | 76.7 (40.6) |
| Visit 3 | 67.4 (44.3) | 54.4 (35.2) | 76.9 (45.7) |
| Butyrate, μmol/g | |||
| Baseline | 25.6 (21.2) | 10.6 (7.5) | 14.4 (13.0) |
| Visit 3 | 13.4 (9.2) | 11.2 (8.5) | 11.8 (14.6) |
| Butyrate, mol% | |||
| Baseline | 20.2 (7.5) | 18.2 (10.4) | 20.6 (4.7) |
| Visit 3 | 17.0 (7.8) | 18.4 (5.1) | 19.4 (12.0) |
| Acetate, μmol/g | |||
| Baseline | 62.3 (33.1) | 32.5 (24.6) | 42.0 (18.7) |
| Visit 3 | 42.7 (26.5) | 34.4 (25.0) | 32.3 (13.4) |
| Propionate, μmol/g | |||
| Baseline | 19.0 (26.0) | 11.1 (5.9) | 16.7 (12.5) |
| Visit 3 | 14.7 (12.6) | 11.6 (7.6) | 13.4 (15.0) |
| Isobutyrate, μmol/g | |||
| Baseline | 1.1 (2.1) | 1.6 (1.0) | 1.8 (0.7) |
| Visit 3 | 1.6 (1.0) | 1.0 (1.0) | 1.7 (1.9) |
| Isovalerate, μmol/g | |||
| Baseline | 1.8 (2.3) | 2.4 (1.6) | 2.8 (1.0) |
| Visit 3 | 2.1 (0.9) | 1.7 (1.0) | 3.0 (3.1) |
| BCFA, μmol/g | |||
| Baseline | 2.8 (4.5) | 3.8 (2.6) | 4.6 (1.6) |
| Visit 3 | 3.8 (1.9) | 2.7 (2.2) | 4.8 (4.8) |
| SCFA:BCFA | |||
| Baseline | 32.5 (32.1) | 18.6 (18.1) | 16.3 (9.1) |
| Visit 3 | 18.4 (28.3) | 24.0 (24.2) | 11.0 (5.4) |
| Butyrate:SCFA | |||
| Baseline | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
| Visit 3 | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
Data are presented as median (IQR) as a calculated range; no significant difference in SCFA or BCFA between groups at baseline or treatment end by Kruskal-Wallis test
Psyllium dose = 12 g
Data are presented as μmol/g of dry fecal weight
Abbreviations: SCFA, short-chain fatty acid; BCFA, branched-chain fatty acid
4. Discussion
The potential impact of a fiber on health is well appreciated. Use of fiber was initially popularized for management of constipation; however, in addition to conventional bulking fibers that are marketed for treatment of constipation, fermentable fibers have recently also become available. These fermentable fibers are termed prebiotics and can be defined as “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health [23]. Researchers have demonstrated the key role of intestinal microbiota in several metabolic and inflammatory disorders and have highlighted the importance of availability of fermentable carbohydrates in the colon for maintaining healthy microbiota composition and function [24,25]. Thus, it is not surprising that fiber supplementation is a common recommendation by many health professionals and scientists alike; this is also evident as recent expenditures on fiber supplements in the US were approximately $300 million [26].
Coinciding with the recent surge of fermentable fiber research and availability, tolerability of fermentable fiber intake has become an area of concern. In fact, in some cases, individuals with gastrointestinal conditions (e.g., irritable bowel syndrome) are recommended to reduce their intake of foods containing certain prebiotics (e.g., low-FODMAP diet) [9]. This is, at least in part, due to the fast-fermenting nature of most prebiotics such as inulin and fructo-oligosaccharides (FOS), which are fermented by bacteria in the right-sided colon and cecum and do not reach the distal colon where the majority of bacteria are present. This fast-fermenting nature results in no benefit to or exacerbation of symptoms such as gas production and bloating [10,11]. Thus, it is plausible that onset and rate of fermentation of fermentable fiber products dictate the frequency and severity of associated side effects, and those fibers with delayed, slow fermentation rate should have fewer side effects. Indeed, our in vitro and ex-vivo stool fermentation studies in test tubes and in an artificial colonic simulator showed that the SM fiber product has a slow and delayed fermentation profile [12,13]. Thus, slow and delayed fermentable fibers have a potential to be non-bloating fibers, and if they can also positively impact BM, they would be particularly desirable. Therefore, this study aimed to determine if starch-entrapped microspheres are well-tolerated, improve bowel habit, and impact the colonic milieu through modulation of microbiota and SCFA compared to psyllium, a commonly used fiber.
Our hypothesis was only partially supported by the results of this study. We found that our study participants, who were primarily middle-aged females, reported improved bowel habit and stool frequency with intake of both types of fibers; these participants also had improved stool consistency with SM fiber at both doses. Although no statistically significant differences were seen in tolerability and gastrointestinal symptoms between psyllium and SM fibers, substantial differences in the change in specific bowel habits over time in the 9 g and 12 g SM groups existed compared to psyllium. This could in part be due to the differences in baseline values of these bowel habits; both the 9 g and 12 g SM groups had higher symptoms at baseline, thus causing more room for change over time. While not statistically significant, it should also be noted that attrition rate was higher and gastrointestinal symptoms less improved in the psyllium group. Our in vitro work compared gas production and fermentation profiles of various fibers, including psyllium and SM [12]. As psyllium generated a high amount of gas in the first four hours after incubation with microbiota, we expected to see less tolerability and more gastrointestinal symptoms in the psyllium group. However, our clinical study did not fully support our prediction as although there was a trend for potential benefit of SM fiber, the differences between SM fiber and psyllium was not marked.
Previous research supports improved stool consistency and increased stool frequency with psyllium intake in individuals with constipation [27,28]. In the current study, psyllium did not significantly improve stool consistency. While it is possible that SM is more effective at normalizing stool consistency compared to psyllium as seen in the current study, a larger sample will be needed to determine if SM is truly more effective than psyllium at improving stool consistency or if baseline differences in stool consistency contributed to the differing responses to the different fibers. A significant increase in stool frequency was seen equally in all three fiber groups in the current study. While our participants were not constipated by definition, the primary inclusion criterion was “unsatisfactory bowel habit.” This criterion allowed for inclusion of those with an average bowel movements of approximately 9-14 times per week as stool consistency, incomplete defecation, and ease of defecation satisfied the criteria of “unsatisfactory bowel habit.” Indeed, all three groups reported improved bowel habit at the end of three months. This improvement was expected in the psyllium group as over half of constipated participants had improvements in global constipation symptom scores in previous research [27]. We now show that SM fiber has a similar positive impact on “unsatisfactory bowel habit” when compared to psyllium fiber. Despite this improvement, no difference in SF12 scores was seen with treatment; this could have been because of relatively high subcomponent scores at baseline. Currently, limited data exists for the benefit of psyllium on quality of life in constipated individuals; a recent review in those with IBS concluded that psyllium did not increase quality of life [29].
Based on our prior in vitro work that indicates SM positively alters microbiota composition in the stool and increases production of butyrate through the entire colon [12-14], we hypothesized that SM fiber intake would lead to significant change in microbiota composition and increased SCFA production. However, this was not supported as we found that microbiota composition and SCFA were largely unchanged with SM fiber treatment. In addition, the impact of fiber supplement on microbiota in the current study was minimal in not just SM but all three fiber groups, with the primary impact on changes in Prevotella in select participants. The biological importance of changes in abundance of Prevotella is not clear. For example, select participants responded to a 3-day consumption of kernel-based bread as indicated by a higher Prevotella/Bacteroides ratio than non-responders [30], but several pathological states like HIV-infection are also associated with higher Prevotella abundance [31]. As individual species within the Prevotella genus were not identified, it is possible that changes in Prevotella after SM intake could be originating from increases in beneficial strains.
While total SCFA was not significantly different with supplementation in any group in the current study, the percent of individuals with increased SCFA with treatment was greater in both SM groups compared to psyllium. In addition, umol/g of butyrate, acetate, and propionate all tended to decrease with psyllium intake while remaining unchanged in the SM groups; however, we acknowledge that these changes were not significant. Salyers et al. suggests that psyllium may be fermentable due to the presence of arabinoxylans [32], but the complex nature of these highly branched arabinoxylans [33] may cause incomplete fermentation of psyllium with little or no effect on SCFA production. The lack of fermentibility or incomplete and limited fermentibility of psyllium is supported by our findings as well as prior studies in humans, in which intestinal gas is not increased with psyllium intake, indicating a lack of fermentable properties [6,34,35]. It should, however, be noted that our findings cannot definitely exclude fermentation capacity of psyllium or SM fiber because: (1) SCFA measurements are highly variable, and stool SCFA may not reflect true SCFA production in the intestine [36], and (2) as dietary intake can have strong short-term influences on microbiota [37], and not recording dietary intake the day before stool collection limited the ability to adjust for the influence of dietary intake on microbiota and SCFA. A larger sample size and assessment of microbiota composition and SCFA measurement at multiple time points are needed to determine if SM impacts microbiota composition and SCFA production in a way that differs from psyllium.
Strengths of this study include use of validated or structured questionnaires to assess outcomes; randomized, double blind controlled study design; and provision of dietary fibers at high doses to be able to determine if these fibers have beneficial impacts on bowel habit and gut health. Assessing dietary components that may impact bowel habit allowed us to attribute the changes in bowl habits to the fiber supplement and not changes in dietary intake. As the low-fiber background diet in this study is similar to the general population [38], the impact of the fiber supplements in this study may be generalizable to the population with unsatisfactory bowel habits.
Our primary limitation was that our study was underpowered to see differences in our outcomes between treatment groups. In the current study, enrollment proved to be difficult, and attrition was relatively high, with 68% of those starting the study retained until treatment end. As there were no significant differences in dropout rates between groups, it is unlikely that the participants dropped out due to difficulties with treatment. We believe that attrition was due in part to the treatment length of 12 weeks. In addition, lack of randomization effectiveness is a limitation. We attempted to use propensity scoring to correct for imbalances in baseline data, but as we did not see any differences in baseline values of other variables, we were unable to perform propensity scoring. Also, stool samples were stored without acidification, potentially reducing SCFA concentrations in the samples. Lastly, significant inter-individual variability in microbiota composition and stool SCFA levels compromised our ability to detect statistically significant impact of the fibers. Our study suggests that a crossover design might be more suitable than a parallel design for assessing impact of fibers with potential prebiotic capacity in order to overcome this inter-individual variability in microbiota composition and response to supplements.
In summary, recent advances in carbohydrate science are providing alternatives to conventional fibers such as psyllium, but commonly used fast-fermenting prebiotics like FOS and inulin may cause gastrointestinal side effects and not provide benefit to the entire colon when consumed. Development of a novel, slowly fermenting fiber has the potential to address the need for a fiber that produces SCFAs and beneficially modulates gut microbiota and gut health, while not creating side effects when consumed. Taken together, the current data support improved bowel habit with fiber intake for those with self-reported constipation. It appears that consumption of SM may have an advantage over psyllium for improvement in stool consistency, but definitive differences in this outcome, as well as in microbiota and SCFA between fiber types for those with self-reported constipation will need to be determined in a larger sample.
Acknowledgments
The authors would like to thank Nazia Kazmi (clinical coordinator) for recruiting study participants and assisting the investigators during the conduct of the study. This work was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Small Business Innovation Research Grant (DK088525-01). Ali Keshavarzian and Bruce Hamaker are holders of the patent for the starch-entrapped microspheres (patent is pending) and are part owners of Nutrabiotix LLC, the company that has the sole right of license of the product. Ali Keshavarzian and Bruce Hamaker were involved in study design and preparation and submission of the application for the SBIR grant but did not participate in participant assessment or data analysis. Both Ali Keshavarzian and Bruce Hamaker participated in preparation of the manuscript after the data were analyzed and finalized.
Abbreviations
- SM
Starch-entrapped microspheres
- SCFA
Short-chain fatty acid
Glossary
- Unsatisfactory bowel habit
defined any of the following conditions: <3 bowel movements per week, hard stool requiring straining, or sense of incomplete defecation
- Prebiotic
a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microflora that confers benefits upon host well-being and health
- Short-chain fatty acid
fatty acids of less than six carbons created as metabolite of gastrointestinal microbial fermentation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- 1.Talley NJ. Definitions, epidemiology, and impact of chronic constipation. Rev Gastroenterol Disord. 2004;4(2):S3–S10. [PubMed] [Google Scholar]
- 2.Rome Foundation. Guidelines--Rome III Diagnostic Criteria for Functional Gastrointestinal Disorders. J Gastrointestin Liver Dis. 2006;15:307–12. [PubMed] [Google Scholar]
- 3.Markland AD, Palsson O, Goode PS, Burgio KL, Busby-Whitehead J, Whitehead WE. Association of low dietary intake of fiber and liquids with constipation: evidence from the National Health and Nutrition Examination Survey. Am J Gastroenterol. 2013;108:796–803. doi: 10.1038/ajg.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HY, Kelly EC, Lembo AJ. Current gut-directed therapies for irritable bowel syndrome. Curr Treat Options Gastroenterol. 2006;9:314–23. doi: 10.1007/s11938-006-0013-8. [DOI] [PubMed] [Google Scholar]
- 6.Levitt MD, Furbe J, Olsson S. The relation of passage of gas an abdominal bloating to colonic gas production. Ann Intern Med. 1996 Feb 15;124(4):422–4. doi: 10.7326/0003-4819-124-4-199602150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Zumarraga L, Levitt M, Suarez F. Absence of gaseous symptoms during ingestion of commercial fibre preparations. Aliment Pharmacol Ther. 1997 Dec;11(6):1067–72. doi: 10.1046/j.1365-2036.1997.00250.x. [DOI] [PubMed] [Google Scholar]
- 8.Yang J, Wang HP, Zhou L, Xu CF. Effect of dietary fiber on constipation: A meta analysis. World J Gastroenterol. 2012;18:7378–83. doi: 10.3748/wjg.v18.i48.7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 10.Collado Yurrita L, San Mauro Martin I, Ciudad-Cabanas MJ, Calle-Puron ME, Hernandez Cabria M. Effectiveness of inulin intake on indicators of chronic constipation; a meta-analysis of controlled randomized clinical trials. Nutr Hosp. 2014;30:244–52. doi: 10.3305/nh.2014.30.2.7565. [DOI] [PubMed] [Google Scholar]
- 11.Olesen M, Gudmand-Hoyer E. Efficacy, safety, and tolerability of fructooligosaccharides in the treatment of irritable bowel syndrome. Am J Clin Nutr. 2000;72:1570–5. doi: 10.1093/ajcn/72.6.1570. [DOI] [PubMed] [Google Scholar]
- 12.Kaur A, Rose DJ, Rumpagaporn P, Patterson JA, Hamaker BR. In vitro batch fecal fermentation comparison of gas and short-chain fatty acid production using “slowly fermentable” dietary fibers. J Food Sci. 2011;76:H137–42. doi: 10.1111/j.1750-3841.2011.02172.x. [DOI] [PubMed] [Google Scholar]
- 13.Rose DJ, Keshavarzian A, Patterson JA, Venkatachalam M, Gillevet P, Hamaker BR. Starch-entrapped microspheres extend in vitro fecal fermentation, increase butyrate production, and influence microbiota pattern. Mol Nutr Food Res. 2009;53(1):S121–30. doi: 10.1002/mnfr.200800033. [DOI] [PubMed] [Google Scholar]
- 14.Rose DJ, Venema K, Keshavarzian A, Hamaker BR. Starch-entrapped microspheres show a beneficial fermentation profile and decrease in potentially harmful bacteria during in vitro fermentation in faecal microbiota obtained from patients with inflammatory bowel disease. Br J Nutr. 2010;103:1514–24. doi: 10.1017/S0007114509993515. [DOI] [PubMed] [Google Scholar]
- 15.Venkatachalam M, Kushnick MR, Zhang G, Hamaker BR. Starch-entrapped biopolymer microspheres as a novel approach to vary blood glucose profiles. J Am Coll Nutr. 2009;28:583–90. doi: 10.1080/07315724.2009.10719790. [DOI] [PubMed] [Google Scholar]
- 16.O'Donnell LJ, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. Bmj. 1990;300:439–40. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol. 2011;76:301–10. doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- 18.Gihring TM, Green SJ, Schadt CW. Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ Microbiol. 2012;14:285–90. doi: 10.1111/j.1462-2920.2011.02550.x. [DOI] [PubMed] [Google Scholar]
- 19.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 21.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–72. doi: 10.1128/AEM.03006-05. 72/7/5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald D, Clemente JC, Kuczynski J, Rideout JR, Stombaugh J, Wendel D, Wilke A, Huse S, Hufnagle J, Meyer F, Knight R, Caporaso JG. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience. 2012;1:7. doi: 10.1186/2047-217X-1-7. 217X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137:830S–7S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 24.Cho I, Blaser MJ. The human microbiome: At the interface of health and disease. Nat Rev Gen. 2012;13:260–70. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soldavini J, Kaunitz JD. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Dig Dis Sci. 2013;58:2756–66. doi: 10.1007/s10620-013-2744-4. 10.1007/s10620-013-2744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IBIS World. Dietary FiberSupplement Manufacturing in the US: Market Research Report. [accessed 16.06.01]; http://www.prweb.com/releases/2012/3/prweb9267872.htm.
- 27.Attaluri A, Donahoe R, Valestin J, Brown K, Rao SS. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011;33:822–8. doi: 10.1111/j.1365-2036.2011.04594.x. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf W, Park F, Lof J, Quigley EM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther. 1995;9:639–47. doi: 10.1111/j.1365-2036.1995.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 29.Chouinard LE. The role of psyllium fibre supplementation in treating irritable bowel syndrome. Can J Diet Pract Res. 2011;72:e107–14. doi: 10.3148/72.1.2011.48. [DOI] [PubMed] [Google Scholar]
- 30.Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–82. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, Robertson CE, Frank DN, Wilson CC. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–94. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salyers AA, Palmer JK, Wilkins TD. Degradation of polysaccharides by intestinal bacterial enzymes. Am J Clin Nutr. 1978;31:S128–30. doi: 10.1093/ajcn/31.10.S128. [DOI] [PubMed] [Google Scholar]
- 33.Edwards S, Chaplin MF, Blackwood AD, Dettmar PW. Primary structure of arabinoxylans of ispaghula husk and wheat bran. Proc Nutr Soc. 2003;62:217–22. doi: 10.1079/pns2003202. doi:S002966510300034X. [DOI] [PubMed] [Google Scholar]
- 34.Wolever TM, ter Wal P, Spadafora P, Robb P. Guar, but not psyllium, increases breath methane and serum acetate concentrations in human subjects. Am J Clin Nutr. 1992;55:719–22. doi: 10.1093/ajcn/55.3.719. [DOI] [PubMed] [Google Scholar]
- 35.Marteau P, Flourie B, Cherbut C, Correze JL, Pellier P, Seylaz J, Rambaud JC. Digestibility and bulking effect of ispaghula husks in healthy humans. Gut. 1994;35:1747–52. doi: 10.1136/gut.35.12.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbeke KA, Boobis AR, Chiodini A, Edwards CA, Franck A, Kleerebezem M, Nauta A, Raes J, van Tol EA, Tuohy KM. Towards microbial fermentation metabolites as markers for health benefits of prebiotics. Nutr Res Rev. 2015;28:42–66. doi: 10.1017/S0954422415000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Department of Agriculture, Agricultural Research Service. Energy Intakes: Percentages of Energy from Protein, Carbohydrate, Fat, and Alcohol, by Gender and Age, What We Eat in American, NHANES 2009-2010. 2014:2014. [Google Scholar]


