Abstract
Adenosine Monophosphate-Activated Protein Kinase or AMPK is a highly-conserved master-regulator of numerous cellular processes, including: Maintaining cellular-energy homeostasis, modulation of cytoskeletal-dynamics, directing cell growth-rates and influencing cell-death pathways. AMPK has recently emerged as a promising molecular target in cancer therapy. In fact, AMPK deficiencies have been shown to enhance cell growth and proliferation, which is consistent with enhancement of tumorigenesis by AMPK-loss. Conversely, activation of AMPK is associated with tumor growth suppression via inhibition of the Mammalian Target of Rapamycin Complex-1 (mTORC1) or the mTOR signal pathway. The scientific communities’ recognition that AMPK-activating compounds possess an anti-neoplastic effect has contributed to a rush of discoveries and developments in AMPK-activating compounds as potential anticancer-drugs. One such example is the class of compounds known as Biguanides, which include Metformin and Phenformin. The current review will showcase natural compounds and their derivatives that activate the AMPK-complex and signaling pathway. In addition, the biology and history of AMPK-signaling and AMPK-activating compounds will be overviewed, their anticancer-roles and mechanisms-of-actions will be discussed, and potential strategies for the development of novel, selective AMPK-activators with enhanced efficacy and reduced toxicity will be proposed.
Keywords: AMPK, AMPK-activators, AMPK-signaling pathway, aspirin, BCA2, cancer, chemotherapy, drug discovery, metformin, natural compounds, salicylate
STRUCTURE AND LOCALIZATION OF AMPK
In essentially all eukaryotic cells, AMPK serves as a highly conserved sensor of intracellular energy status and homeostasis [1–4]. AMPK constitutes a heterotrimeric complex of proteins consisting of a catalytic AMPK-α subunit as well as two regulatory (AMPK-β and AMPK-γ) subunits (Fig. 1) [1, 5–9]. The AMPK α-subunit constitutes the kinase domain and contains a highly conserved Threonine at residue-172 (Thr172) located in the activation loop of AMPK-α, which serves as a critical phospho-target for upstream kinases, including Liver Kinase B1 (LKB1) [10] and Calcium-Activated Calmodulin-Dependent Kinase Kinase β (CAMKKβ) [11] which differentially modulate AMPK’s activity in response to diverse physiological and biological, conditions and stimuli. In all species from yeast to man, phosphorylation in the activation loop of regulatory AMPK-α subunit at Thr172, is required for full activation and subsequent induction of downstream AMPK signaling, and functional consequences [1–10].
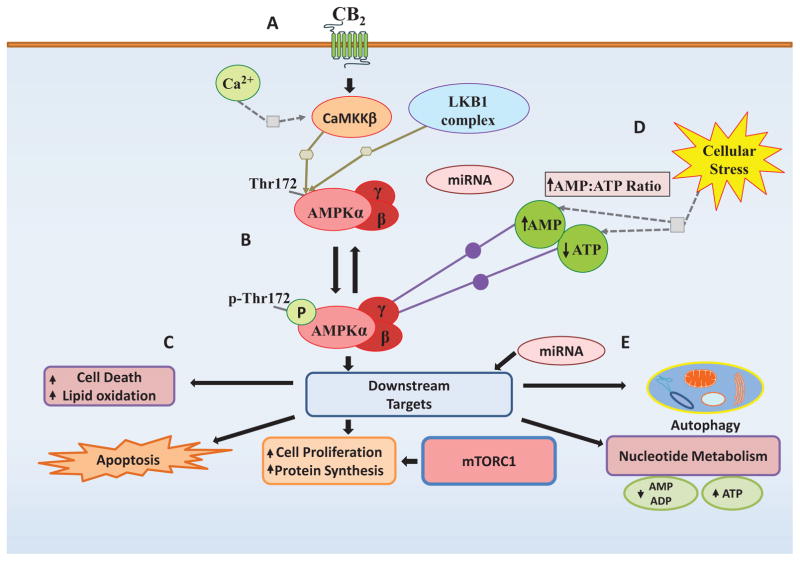
Fig. (1). Regulation of the AMPK Pathway.
The AMPK signaling pathway is a master regulator of cellular energy homeostasis and metabolism AMPK is regulated by diverse mechanisms and stimuli that provide a variety of opportunities for pharmacological intervention. (A) Indirect Activation: Upstream AMPK activators. LKB1 and CaMKKβ principally regulate AMPK. Stimulation of these pathways upstream of AMPK can activate AMPK signaling indirectly. (B) Direct AMPK Activators. AMPK can be directly activated by a nucleotide analog (such as AMP and ZMP) binding to the regulatory subunit and inducing a conformational change that enhances AMPK phosphorylation. (C) Downstream AMPK signaling pathways. The AMPK pathway can also be activated by stimulation of downstream signaling partners. This can mimic and/or enhance the effects of AMPK activation in response to normal physiological stimuli. (D) Indirect inducers of metabolic stress. Many compounds and stimuli induce metabolic stress, which results in the release of Ca2+ ions, and fluctuation in the cellular AMP/ATP ratios. These metabolites play a profound role in regulation of AMPK activation and can significantly enhance AMPK signaling under these conditions. (E) miRNA modulation of AMPK pathway. The AMPK pathway is the target of multiple miRNAs, which regulate the expression of key regulators in the pathway. Changes in miRNA expression resulting from pharmacological intervention or changes in blood glucose levels can release the suppression on the system resulting in enhance AMPK expression and activation.
Two genes encode the mammalian AMPK α-catalytic subunit (AMPK-α1 and -α2), designated PRKAA1 & PRKAA2. The regulatory subunits of AMPK consist of two β-subunit genes (AMPK-β1 and β2), designated PRKAB1 and PRKAB2, and three AMPK-γ subunit genes (AMPK-γ1, -γ2 and -γ3), designated PRKAG1, PRKAG2 and PRKAG3 [1–6,10]. Some of the AMPK-α subunit isoforms have been shown to exhibit tissue-specificity as well as distinct functions. Specifically, the two catalytic AMPK-α subunits have been reported to demonstrate distinct responses to AMP binding and LKB1 association [6–7]. AMPK-α2 has shown preferential nuclear localization relative to the AMPK-α1 [12, 13]. However, it has been reported that the AMPK-α1 subunit is located in the nucleus under specific experimental conditions. Subunit-specific AMPK activities have also been observed for the AMPK-β subunits [9]. Specifically, myristoylation of the AMPK-β isoforms is essential for full activation of the kinase, as well as localization to specific membranes [1, 7]. Despite these findings the significance of AMPK localization or co-localization with LKB1 needs to be further elucidated and better understood in order to develop effective strategies for targeted therapies. Furthermore, it is unclear whether any of these variations are cancer-specific, or if any novel compounds may be able to target the isoforms or regulatory phenomena preferentially.
REGULATION OF AMPK ACTIVITY
AMPK Regulation By Changes in Adenine Nucleotide Balances
AMPK activation and signaling is an intensely studied field. AMPK activation takes place via a two-pronged mechanism (Fig. 1, and also see cited reviews for details [1–5, 8, 14–17]). These studies indicate that the AMPK complex displays a complex interaction with the myriad of adenine nucleotide states (AMP:ADP:ATP) and is differentially regulated through their binding to specific regulatory sites on the complex at different subunits as well as upstream regulation by the specific kinases (LKB1 and CAMKKβ). These studies have shown that AMPK activity is increased 5-fold by a moderate decrease in intracellular ATP, which is associated with a proportionate, concurrent and corresponding increase in intracellular AMP levels [18]. Further increases in cellular AMP levels may result in the direct binding of AMP to the AMPK-γ regulatory subunit. In fact, the AMPK-γ subunit contains three sites, which bind adenine-nucleotides with varying degrees of affinity [18]. The binding of AMP to the AMPK-γ regulatory subunit leads to a change in conformation that result in increased AMPK-α phosphorylation, in addition to decreased dephosphorylation at Thr172. As described, phosphorylation of AMPK-α at Thr172 is associated with a >100-fold increase in AMPK activity [1–5, 8, 14–18]. Additional studies have demonstrated that ADP may also be able to bind to the AMPK-γ subunit [12]. This suggests that intracellular ADP levels may also play a physiological role in AMPK activation in response to different types of cellular stresses and metabolic states [9, 19–21]. These findings demonstrate the complexity of cellular energy status signaling through AMPK.
The ability of AMPK-γ to bind different adenine nucleotide states (ATP:ADP:AMP) enables AMPK to act as a complex and sensitive sensor for regulation of cellular energy status [7, 16, 18]. Normal cellular catabolism maintains an ATP:ADP ratio of approximately 10:1. Under conditions where the cells are not subject to any energetic stress this ration of 10:1 ATP: ADP drives the adenylate kinase reaction towards ADP synthesis (ATP + AMP → 2ADP). The net result is the maintenance of relative low levels of AMP [12, 15–16, 18–19]. The normal ratios of ATP:ADP:AMP in an unstressed cell are approximately 100 to 10 to 1 [16, 18]. The specificity of the AMPK-γ subunits nucleotide-binding sites’ is similar for all three adenine nucleotide states (AMP, ADP, and ATP). The AMPK-γ subunits preferentially bind free ATP over ATP that is in complex with magnesium (ATP-Mg). However, approximately 90% of cellular ATP exists in complex with magnesium. Therefore, the relative concentrations of ADP and free ATP in a cell are comparable, resulting in their proportional competition for AMPK-γ nucleotide-binding pockets. Under normal conditions, in “unstressed cells” the concentrations of AMP are lower than cellular ADP and free ATP by 10-fold or more do to the cells natural tendency towards the adenylate kinase reaction (ATP + AMP → 2ADP). During periods of energetic stress, the ratio of AMP:ATP/ADP rises markedly. This results in a shift of the adenylate kinase reaction towards producing more AMP (2ADP → ATP + AMP) [12, 16–17]. Under conditions such as energetic stress, and a shift in the kinetics of the adenylate kinase reaction, the ratio of AMP rises sufficiently to compete with ATP and ADP at the AMPK-γ subunits’ nucleotide-binding sites, resulting in the activation (phosphorylation) of AMPK. As mentioned above, stoichiometric phosphorylation of AMPK-α at Thr-172 results in a greater than 100-fold induction of AMPK activity [12, 16–17]. The net effects of increased phosphorylation are further increased by as much as 10-fold by concurrent allosteric activation, resulting from ATP binding. This multi-leveled mechanism allows for a massive increase in AMPK activity in response to small changes in energy homeostasis, making AMPK an extraordinarily sensitive and responsive molecular switch reacting to the slightest shift in the energetic status of the cell [12, 16–17, 22–32].
AMPK Activation By Upstream Kinases
In addition to regulation by adenine nucleotide status, AMPK is also regulated by upstream kinases including LKB1. LKB1 is the primary upstream kinase of AMPK and it phosphorylates the AMPK-α subunit at Thr-172 in mammalian cells (Fig. 1) [13, 21, 33]. LKB1 is encoded by the gene STK11 and is classified as a serine/threonine kinase. LKB1 has been shown to play multifaceted roles in cell: proliferation, polarity, metabolism, and survival. LKB1 exists in an apparently constitutively active state under normal physiological conditions. This high basal activity of LKB1 is critical and required for phosphorylation of AMPK-α at Thr-172 in response to AMP and/or ADP binding to the AMPK-γ subunit, AMPK is a key downstream effector of LKB1 and carries out many of LKB1’s key tumor suppressive functions [19, 21, 33, 34]. These include indirect inhibition of mTORC1 signaling by phosphorylation of the tumor suppressor gene Tuberous Sclerosis 2 (TSC2) [35, 36] as well as direct inhibition via targeting the regulatory subunit of mTORC1 signaling Raptor [37–38]. Detailed AMPK signaling will be discussed in the next section.
LKB1 also phosphorylates a number of AMPK-related kinases in addition to AMPK-α1 and -α2. Thus, many of the phenotypes of LKB1-deficient tumors may not result from disrupted AMPK-α signaling [13]. Recent studies have identified ROS as an upstream activator of AMPK, which appears to be LKB1-independent [39]. Therefore, although closely linked, AMPK and LKB1 display distinct differences in signaling that may account for their divergent roles relating to inhibition of tumorigenesis.
Although LKB1 is the major regulator of AMPK activity, CAMKKβ (CAMKK2) can also phosphorylate Thr172 of AMPK-α, in response to calcium flux in a mechanism that is independent of LKB1 [16, 40]. Interestingly, CAMKKβ is the closest mammalian-kinase homolog to LKB1. In addition, this calcium-flux-dependent activation of AMPK can act independent of adenine nucleotide status, thereby allowing AMPK to respond to stimuli independent of energy state [41–44]. By this mechanism AMPK can respond to many stimuli (Agonists) that increase intracellular Ca2+. These stimuli include: Thrombin in endothelial cells, T-cell receptor binding to Antigens, ghrelin (The hunger hormone) activity in hypothalamic neurons, as well as endogenous and exogenous cannabinoids binding at the cannabinoid receptor (CB2) (Fig. 1) [41–43, 45]. Additional studies have suggested the MAPKKK family member TAK1/MAP3K7 may also phosphorylate AMPK-α at Thr172 but it is unclear whether this requires LKB1 [9, 12, 42–48]. These findings reveal that a complex network of regulatory molecules and stimuli can modulate AMPK activity with a large degree of cross talk between AMPK and AMPK-related kinases, therefore further studies will be require to understand how this master regulator functions in normal and cancer biology and how specific targeted therapies and drugs may be developed to target this system (Fig. 1).
AMPK Suppression by Breast Cancer-Associated Gene 2 (BCA2)
BCA2 is a unique ring-finger-containing ubiquitin E3 ligase [49] that is overexpressed in >50% of breast tumors [50]. The downstream targets of BCA2 and its contributions to the overall breast cancer cell-survival advantage remains to be fully elucidated. Research from our laboratory has demonstrated that BCA2 is an endogenous inhibitor of AMPK activation in multiple human breast cancer cell lines tested [51]. Our lab has also shown that RNAi-based inhibition of BCA2 is associated with an increase in metformin (an indirect AMPK activator) efficacy in breast cancer cell lines [51]. Over-expression BCA2 inhibited both basal and inducible phosphorylation/activation of AMPK-α1, while BCA2-specific small interfering RNA (siRNA) enhanced Thr172 phosphorylation of AMPKα1 (pAMPKα1). The AMPK-suppressive function of BCA2 requires its E3 ligase-specific RING domain, suggesting that BCA2 targets some protein/proteins that control the phosphorylation or dephosphorylation of AMPKα1 via degradation of the regulatory protein, or by some other yet undetermined mechanism (Fig. 2A–B). Activation of AMPK by metformin triggered a growth inhibitory signal but also increased BCA2 protein levels, which correlated with AKT activation and could be curbed by an AMPK inhibitor, suggesting a potential feedback mechanism from pAMPKα1 to pAKT to BCA2 [51] (Fig. 2). Finally, BCA2 siRNA, and/or inhibition of its upstream stabilizing kinase, AKT, increased metformin-mediated growth inhibition in multiple breast cancer cell lines, thus demonstrating that inhibition of BCA2 expression and or activation enhances metformin’s efficacy. Overexpression of BCA2 has also been shown to decrease metformin activity. Our data suggest that metformin in combination with a BCA2 inhibitor may be a more effective breast cancer treatment strategy than metformin alone [51].
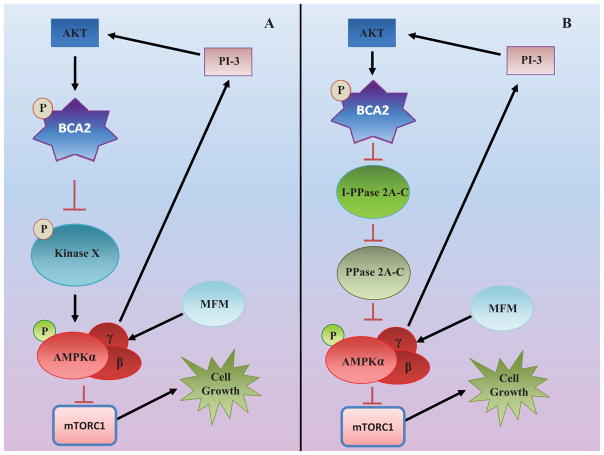
Fig. (2). Proposed schematic of the Role of BCA2 in modulation of AMPK activation.
At the basal level, AKT phosphorylates and stabilizes BCA2 which is responsible for ubiquitinating protein substrates, including some signaling proteins controlling phosphorylation (A) and dephosphorylation (B) of pAMPK-α (at Thr-172), and target them for degradation via ubiquitin-proteasome pathway, resulting in inhibition of AMPK signaling in breast cancer cells. The activation of AMPK by metformin, however, increases BCA2 protein levels, inciting cancer cell survival and restarting the regulatory loop via PI3K/AKT. Therefore, the balance of AMPK and PI3K/AKT/BCA2 may control breast cancer cell sensitivity to metformin therapy and the tumor cell life-death switch.
It has been reported that AMPK activation triggers two conflicting signaling pathways: the well-known, cancer-preventive/tumor-suppressive signaling via mTOR1 inhibition, as well as the less-known cancer-survival feedback pathway via increased levels of PI3K/AKT signal ([47–48, 52–54], Fig. 2). How that survival pathway affects metformin efficacy in patients is unknown and the detailed mechanisms by which metformin affect cancer risk and outcome remain poorly understood. Our most recently findings suggest that BCA2 is an endogenous AMPK inhibitor whose levels are increased by AMPK activators via a PI3K/AKT-mediated feedback loop ([51] Fig. 2). These findings place BCA2 at an essential intersection in the metformin/AMPK regulation map. We hypothesized that metformin triggers an AMPK-mediated survival signal which leads to stabilization of BCA2 protein [51, 55] that then inhibits AMPK activation and metformin efficacy via a yet unidentified feedback mechanism (Fig. 2).
It is possible that BCA2 inhibition up-regulates a protein, which may be either a direct kinase that may phosphorylate/activate pAMPKα at T172 or work upstream (Fig. 2A). It has also been shown that PP2A and PP2C complexes with AMPKα, and are responsible for dephosphorylation of AMPKα at Thr172, resulting in its inactivation ([4–6, 11, 55–57], Fig. 2B). It is therefore also possible that BCA2 regulates degradation of some protein(s) (e.g., a putative endogenous phosphatase inhibitor, I-PPase 2A-C in Fig. 2B) that control the activity of PP2A-C and the subsequent dephosphorylation of AMPKα at T172 [58].
In summary, cellular AKT works to stabilize BCA2 so that it is free to ubiquitinate protein substrates, including some signaling proteins responsible for modulating AMPK-α phosphorylation and dephosphorylation at Thr-172, and targeting them for degradation via the ubiquitin-proteasome pathway, resulting in inhibition of AMPK signaling [56]. However, activation of AMPK by metformin (or another AMPK activator) increases BCA2 protein levels, inciting cancer cell survival and restarting the regulatory loop via PI3K/AKT. Therefore, the balance of AMPK and PI3K/AKT/BCA2 may control cancer cell sensitivity to metformin therapy and the tumor cell life-death switch [51,59].
AMPK Regulation by miRNAs
Mechanisms of AMPK regulation independent of genomic alterations may also affect its activity in cancer. One such possible mechanism is AMPK regulation by microRNAs (miRNAs) (Fig. 1) [20, 22, 60–62]. The miRNA miR-451 was recently linked to control of LKB1–AMPK signaling within the context of gliomas [31]. miR-451 suppresses the expression of CAB39 (MO25α), an LKB1-binding protein [63], through direct targeting of its 3′UTR [20, 21, 23, 31, 64–65]. Under normal glucose conditions, higher levels of miR-451 are induced, however changes in glucose levels may modulate the expression of this and other miRNAs resulting in differential regulation of up- and down-stream targets of AMPK, thus modulating the pathway, miR451 suppression of CAB39 leads to subsequent repression of LKB1 activity and, consequently reduced AMPK activation (Fig. 1). Reduced AMPK activity leads to increased activation of mTOR signaling, which then promotes cell growth. However, a reduction in glucose concentration results in a decline in miR-451 levels [31, 65–66]. Decreased miR-451 releases suppression on CAB39 and consequently stabilizes active LKB1 signaling complex and induces AMPK phosphorylation/activation, resulting in inhibition of the mTORC1 pathway, suppression of cell proliferation, initiation of metabolic stress responses, and increased cell polarity as well as activation of migration signal pathways via MARKs phosphorylation which also effects cytoskeletal regulation [66–68]. In this way miRNAs can regulate the AMPK pathway indirectly in response to changes in glucose availability.
It should also be noted that miR122 has been suggested to be a direct inhibitor of AMPK [65]. miR122 is constitutively expressed in the liver, and inhibition of miR122 is necessary for activation of enhanced AMPK signaling pathways [59, 62]. This happens during prolonged periods of starvation and due to several pharmacological interventions (such as by phenobarbital, or metformin). Treatment with these drugs reduces the negative feedback and allows for enhanced AMPK signaling once expression of this miRNA is decreased. Furthermore, miR122 is induced by a number of natural compounds, including resveratrol and indole-3-carbinol (I3C) [69–72], further expounding the complexity of the AMPK signaling system [73–74].
In silico analysis of the genome has revealed many additional miRNAs with putative sequences that could target the AMPK signaling pathway directly or indirectly [60–62]. However it is not clear what roles these miRNAs play in the regulation of the AMPK signaling. It is important to mention that many natural products, including “nutraceuticals”, play pivotal roles in modulation of miRNA expression, especially within the context of cancer [69]. Further complicating the system, a recent article suggests that the regulatory role of miRNAs is context-dependent, contingent on the status of the cell [64]. The authors suggest that miRNAs may differentially inhibit or induce the expression of their target transcripts depending on the context of the cell. Specifically, actively cycling cells have diminished expression of transcripts, while quiescent cells exhibit “enhanced” translation in response to miRNA binding and formation of the RNA-induced silencing complex (RISC complex) [69–72]. Many diverse cell-culture conditions can induce quiescence, including serum starvation, cell-cell contact inhibition and loss of adhesion, to name a few. These findings suggest an important context-dependent factor to consider when one assesses the effects of miRNA, possibly other RNAi technologies, and drugs designed to stimulate their expression.
AMPK DOWNSTREAM EVENTS
AMPK Signaling
AMPK promotes energy homeostasis through the direct regulation of metabolic enzymes via phosphorylation (Fig. 1). AMPK inhibits fatty acid synthesis and stimulates lipid oxidation via the phosphorylation/inactivation of acetyl-CoA carboxylase (ACC) 1 and ACC2, respectively [71, 73]. In muscle tissue, AMPK has been shown act on glycolysis via phosphorylation of phosphofructokinase under ischemia in heart tissue [9, 72]. AMPK has also been shown to regulate autophagy, a catabolic process important for the maintenance of cellular energy and cell survival in starved cells [74–76]. AMPK-dependent phosphorylation of the ULK kinases directly induces autophagy in starved cells, resulting in the removal of damaged mitochondria through specific activation of “mitophagy” [47, 77]. ULK kinases in turn can negatively regulate AMPK signaling through phosphorylation of AMPK subunits [47]. In situations of chronic nutrient starvation [77–79], AMPK can elicit changes in transcription through a number of mechanisms including phosphorylation of the transcriptional co-activator PGC1a, the transcription factor FOXO3, or the core histone H2B [80].
Although a cancer-specific role for AMPK in stimulating glycolysis has yet to be demonstrated, AMPK has also been shown to play a role in regulating tumor metabolism from studies using AMPKα-deficient cell lines and animal models [79]. Furthermore, as detailed below, AMPK appears to negatively regulate the expression of Hypoxia Inducible Factor 1α (HIF-1α), which plays an essential role in the Warburg effect [16, 75, 81–82].
Effects on Hypoxia and HIF1α
Metastatic transformation and tumor progression is associated with a myriad of changes in cellular behavior and physiology. Two key changes that occur during cancer progression are a shift in metabolic activity in the cells, known as the Warburg effect, and a decrease in extracellular oxygen and pH levels surrounding the tumor [16, 65, 75, 81–82]. These changes are the result of the high metabolic demands associated with rapidly dividing cells and the lack of sufficient vasculature to supply the tumors during the early stages of development. Hypoxia is a well-studied mechanism associated with increased metastatic potential in human cancer cells.
It is known that one of the pro-growth metabolic programs associated with the loss of LKB1–AMPK signaling is mechanistically mediated by the oxygen-sensitive transcription factor HIF-1α [40, 66]. Silencing AMPK promotes increased glucose uptake, glycolytic flux, and flow of carbon into the tricarboxylic acid (TCA) cycle to fuel pathways of both ATP production and biosynthesis. Therefore loss of AMPK expression and activity is associated with a HIF-1α-dependent transcriptional program that drives increased glycolysis under aerobic conditions, which resembles the Warburg effect. Consistently, HIF-1α protein levels are elevated in AMPKα-deficient cells under normoxia, leading to induction of HIF-1α-dependent transcriptional programs [36]. Lymphoma cells lacking AMPK activity display a reliance on HIF-1α to maintain heightened glycolytic metabolism, and the growth of AMPK-α1-deficient Myc lymphomas in vivo is diminished when HIF-1α stabilization is disrupted. In this regard HIF-1α acts as a regulator essential for the metabolic transformations associated with AMPK loss. While AMPK lies directly downstream of LKB1, the metabolic effects of LKB1 deficiency does not directly mirror those induced by AMPK loss [83]. In addition to displaying enhanced glycolysis, LKB1-null cells display enhanced glutamine metabolism, acting primarily as an anaplerotic substrate to support the TCA cycle and oxidative phosphorylation (OXPHOS) [83]. Likewise, LKB1 and AMPK have been shown to modulate expression of HIF-1α via various mechanisms. Silencing LKB1 thus increases both HIF-1α transcription and translation. These HIF-1a regulatory events are sensitive to inhibition of mTORC1 [68, 70–72].
Effects on mTOR Pathway
In addition to its effects on glucose metabolism, AMPK loss can also promote unchecked activation of the mTORC1 pathway [44, 47–48]. The mTORC1 pathway is one of the most thoroughly investigated and understood mechanisms by which AMPK regulates cell growth. One such mechanism by which AMPK regulates mTORC1 is via direct phosphorylation of the tumor suppressor protein TSC2 at serine 1387 [33–34]. In addition to regulation of cell growth, mTORC1 also controls cell fate through induction of autophagy, a cellular process of “self engulfment” in which the cells break down their own organelles (macroautophagy) and cytosolic components (microautophagy) [65–66, 47–48, 73–75]. This process ensures sufficient metabolites when nutrient levels are low in the cell. Regulation of protein synthesis by mTORC1 is a well-known example of one such process/mechanism. In addition, mTORC1 is a key player in translation regulation via phosphorylating numerous translational regulators, including S6 kinase 1 (S6K1) [63–68, 71–76]. Due to the increased metabolic demand associated with cancer progression the synthesis of fatty acids, triglycerides, cholesterol, RNA, and proteins are all up-regulated in tumor cells. Because cellular energy is required for protein synthesis, metabolic stress-induced AMPK activation should cause great inhibition in protein synthesis in a normal cell. This halt in protein synthesis is carried out through a crosstalk between AMPK and mTORC1 while AMPK negatively regulates mTORC1 signaling via phosphorylating/activating the tumor suppressor TSC2 [19, 48, 74–76] that is an inhibitor of mTORC1. Activation of AMPK can also cause direct phosphorylation of Raptor that would lead to 14-3-3-mediated inhibition on mTORC1 [40, 77–82]. Therefore, a combination of AMPK inhibition and maintaining mTORC1 signaling would increase tumor growth via bypassing these cellular metabolic brakes. Thus AMPK functions as a cellular metabolic tumor suppressor protein, inhibiting cancer cell growth via regulating Warburg metabolism and essential biosynthetic pathways. These effects of AMPK could help explain the findings that metformin [84–87] and aspirin [14, 88–89] reduces the risk of cancer in some patients and that AMPK activators delay the onset of tumorigenesis in animal models [85, 90].
Effects on Cell Polarity, Migration and Cytoskeletal Dynamics
There is evidence that AMPK also controls cell polarity and cytoskeletal dynamics under some experimental conditions [2, 5, 8, 10, 11, 65, 85, 87, 91]. It is known that LKB1 plays an important role in regulating cell polarity in multiple eukaryotic model systems but recent evidence has directly implicated AMPK in this role [90]. In drosophila, AMPK loss leads to altered polarity. In mammalian Canine kidney epithelial cells, activation of AMPK is necessary for proper re-polarization [92]. In addition, LKB1 was localized to adherens junctions in these cells, and treatment with E-cadherin RNAi specific loss of the aforementioned localization associated with corresponding loss of AMPK activation at the same sites [30]. It has also been reported that AMPK regulates Afadin122, an adherens junction protein, and GBF1, a Golgi-specific nucleotide-exchange factor for Arf5, which may be involved in the control of cellular polarity. AMPK deficiencies have been shown to alter multiple cellular polarity markers [65]. Additional studies have found that CLIP170, the microtubule plus end protein is a direct substrate of AMPK [93]. A mutation of CLIP-170 at its AMPK site led to slower microtubule assembly. It has also been suggested that mTORC1 is a potential kinase acting on CLIP-170, indicating that CLIP-170 could be a point of cross-talk point between AMPK and mTOR signaling pathways. While the significance of the role of AMPK in cytoskeletal rearrangements in cancer is yet unclear, the evidence is compelling and warrants additional investigations into their potential mechanisms and therapeutic significance.
The Role of AMPK in Cancer
AMPK has also been shown to play a “context-dependent” role in the regulation of tumor cell metabolism and function. AMPK is situated at the center of multiple established tumor suppressor networks including LKB1, TSC2 and p53 [74–75, 79]. Loss of AMPK activity has been observed in several tumor types, and has been shown to reprogram tumor cell metabolism as well as enhance cell growth and proliferation [79]. For example, loss of AMPK has been shown to accelerate Myc-dependent lymphogenesis in mice [82], suggesting that loss of AMPK can cooperate with oncogenic drivers to promote tumorigenesis. Conversely, AMPK activation has also been shown to impart a growth advantage to tumor cells through dysregulation of cellular metabolism, thus allowing tumor cells to adapt to metabolic stressors. Silencing of AMPK has been shown to promote a metabolic shift characteristic of the Warburg effect both in transformed and non-transformed cells [75]. In addition, AMPK activation (via A-769662) has been shown to delay tumor onset in PTEN+/− mice [79], suggesting that AMPK activation can exert anti-tumor effects in vivo within the context of PTEN loss that results in increased AKT and mTORC1 signaling. Interestingly the majority of evidence supporting the use of AMPK activators has been derived from indirect AMPK-activating compounds through the application of a metabolic stress.
NATURAL COMPOUNDS THAT REGULATE AMPK ACTIVATION
Rationale for Targeting AMPK
AMPK-activating compounds have emerged as a viable method in targeting tumors as more evidence has emerged supporting an anti-tumorigenic role for the kinase. Several studies have proposed using agonists of AMPK for cancer treatment, and the number of patents describing AMPK activators has rapidly increased [84–85, 91, 94–95]. The most convincing data in support of the use of AMPK-activating compounds, as anti-cancer agents have been the epidemiological and clinical trials of biguanides compounds: Metformin and Phenformin [84, 86]. Growing interest in the LKB1-AMPK pathway in cancer prompted retrospective analysis of cancer incidence in patients with type II diabetes. Several studies found that metformin treatment was associated with a significantly lower cancer incidence in patients relative to those using other medications to manage their diabetes [85]. Experimental evidence has also supported an anti-neoplastic effect for metformin. Treatments of animals harboring tumor xenografts with metformin or Phenformin have been shown to delay tumor progression [85, 90]. Other AMPK agonists, such as AICAR and salicylate have also been shown to inhibit tumor cell proliferation both in vitro and in vivo and even in patients [86, 88, 92, 93, 96–102], providing further rationale for use of these agents for cancer therapy. Aspirin use lowers risk of developing some cancers (e.g., colorectal, esophagus, and stomach), but not others (e.g., breast cancer) [88–99]. The mechanism of action and regulation of aspirin remains to be further studied.
Natural Products that Activate the AMPK Signaling Pathway
Diverse assortments of natural products have been identified with the ability to activate AMPK either directly or indirectly through a variety of mechanisms (103) (Table 1).
Table 1.
Natural compounds acting on the AMPK pathway.
| Compound Name | Chemical Structure | Effects in Cancer | Mechanism of Action |
|---|---|---|---|
|
AICAR [103–110] (Adenosine mimic) |
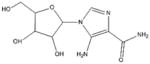
|
|
Direct
|
|
Salicylic acid [15, 57, 88–89, 111–114] Willow bark (Salix Alba) |
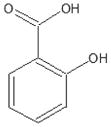
|
|
Direct
|
|
Metformin [44, 51, 56–57, 84–87, 115–116] French lilac (Galega officinalis) |
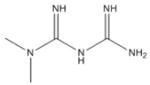
|
|
Indirect
|
|
Methotrexate [103, 118–121] (Antifolates) |
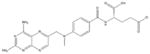
|
|
Indirect
|
|
Obovatol [122–124] Fruits of Magnolia (Obovata) |
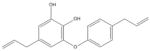
|
|
Indirect
|
|
Cucurbitane Triterpenoid [125–128] Bitter Gourd (Momordica Charantia) |
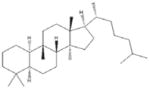
|
|
Indirect
|
|
Glabridin [129–132] Licorice (Glycyrrhiza Glabra) |
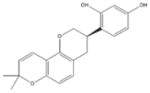
|
|
Indirect
|
|
Nootkatone [133–136] Grapefruit, and Pummelo (Nootka Cypress Tree) |
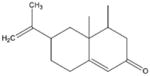
|
|
Indirect
|
|
2,5-Bis-aryl-3,4- Di-methyl-tetra- hydrofuran (Nectandrin B) [137–139] Nutmeg (Myristica Fragrans) |
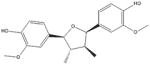
|
|
Indirect
|
|
Δ9- Tetrahydrocannabinol [23, 41–43, 103, 140–143] (Cannabis Sativa) |
|
|
Indirect
|
|
Indole-3-Carbinol [29, 144–146] (Cruciferous Vegetables) |
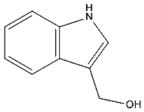
|
|
Indirect
|
|
DIM [29, 144–146] (Cruciferous Vegetables) |
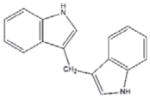
|
|
Indirect
|
|
Epigallocatechin Gallate (EGCG) [147–154] Green Tea (Camellia Sinensis) |
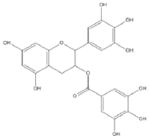
|
|
Indirect
|
|
Resveratrol [22, 31, 60, 155–156] Grapes, lilies, peanuts, wine, soy and berries |
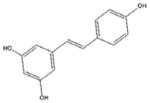
|
|
Indirect
|
|
Ginsenoside Rg3 [103, 157–159] Ginseng (Panax Root) |
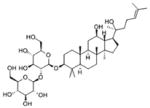
|
|
Indirect
|
Direct AMPK Activators
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) and ZMP
AICAR (Table 1) is an AMPK agonist that acts as an AMP mimetic. AICAR has been used in the past to reduce the severity of heart attacks, and to treat metabolic disorders. AICAR also plays a prominent role in cancer biology and therapy as it has been shown to inhibit proliferation, promote senescence, and induce apoptosis via activation of AMPK [104]. AICAR may be phosphorylated to form ZMP, which closely imitates AMP, and directly binds to the AMPK-γ subunit to achieve the previously described AMPK activation and its dependent effects in cancer cells [105]. AICAR treatment in cancer cell lines has been shown to inhibit the mTOR pathway and activate c-Jun N-terminal kinases (JNKs) and caspase-3 activation, resulting in induction of apoptosis [106]. Treatment of cancer cells with AICAR also leads to cell cycle arrest in S-phase, and is associated with strong anti-proliferative effects. Numerous studies have demonstrated that AICAR can be utilized, as an effective compound to inhibit cancer cell growth, and have illustrated the efficacy of targeting AMPK in cancer therapy [103,104–110].
Salicylates and A-769662
Salicylic acid (Table 1) has been isolated from willow bark (Salix Alba) since ancient times [15, 57, 88]. It is believed that plants produce salicylic acid as a defensive mechanism against infectious pathogens. The salts and esters of salicylic acid are known as salicylates. In modern society, most salicylates are replaced by its synthetic acetylated derivative acetylsalicylate, more commonly known as aspirin, for medicinal use [111]. It also can be administered as salsalate, the self-condensed dimer of salicylic acid. Upon administration to patients, both aspirin and salsalate are rapidly converted into salicylic acid. It has been demonstrated that salicylate can activate AMPK at physiological concentrations that are observed in the plasma of patients after administration of higher doses of either aspirin or salsalate [89].
Salicylate has been shown to directly bind to AMPK. This direct binding to AMPK leads to allosteric activation by inhibiting the dephosphorylation of the activation site threonine 172. Salicylate’s mode of action is similar to the synthetic compound A-769662, a direct AMPK activator, and recent data indicates that they both bind to the β subunit of AMPK [112]. Salicylate’s ability to enhance fat utilization and to decrease plasma fatty acid levels was diminished in AMPK-knockout mice. Some of the therapeutic properties of aspirin and salicylate could be attributed to inhibition of cyclooxygenase as well as IKKβ [113]. However, it should be noted that some of these effects were still observed in mice deficient in these proteins, suggesting that salicylate may have additional, yet undetermined, targets [114]. Clinical trials with patients taking daily aspirin for 5 or more years have revealed a significantly reduced risk of colorectal cancer and other adenocarcinomas. It has also been found that, men who take aspirin daily have a lower risk for prostate cancer. In addition to these clinical studies numerous other controlled investigations utilizing aspirin in cancer cells have shown that its chemopreventitive effects are at least in part attributed to its AMPK-activating ability [89, 103, 111–114].
Indirect AMPK Activators
The majority of compounds that activate AMPK do so indirectly via induction of upstream or downstream regulators on AMPK signaling (Table 1).
Biguanides: Metformin and Phenformin
Metformin (Table 1) is the Type II diabetes drug prescribed most widely that has been found to be able to activate AMPK an LKB1-dependent manner [84–86, 103]. Several clinical trials are currently underway to investigate the potential of metformin for preventing cancer of breast, prostate and colorectal [44, 51, 59, 84–87, 94, 115, 116]. Biguanides such as metformin and phenformin apparently activate AMPK by inhibiting complex I of the mitochondria, leading to decreased ATP production and levels and a subsequent increases in intracellular ADP/AMP and thus are indirect activators of AMPK [116]. Research from our lab has demonstrated that activation of AMPK by metformin triggers a growth inhibitory signal that is associated with a transcription-independent induction of BCA2 protein levels, and a corresponding increase in AKT activation that is decreased in response to concurrent AMPK inhibition [51]. Furthermore, BCA2 siRNA and inhibition of AKT also increased metformin-mediated growth inhibition in several human breast cancer cell lines [51]. These data suggest that metformin plus a BCA2 inhibitor could serve as a potential strategy for breast cancer treatment. However, metformin has been associated with negative side effects conceivably resulting from the inhibition of mitochondrial respiration and consequent lactic acidosis caused by increased glycolysis [115].
Folic Acid
Folic acid (Table 1), a component of the vitamin B complex (Vitamin B9) is discovered in yeast, spinach and cod liver oil, and plays a crucial role in de novo pyrimidine nucleotide synthesis. As a result, folic acid is critical for the synthesis of nucleic acids and proteins [103, 117–119]. In the past, antifolates were used to treat anemia, inflammatory diseases, rheumatoid arthritis, psoriasis, bacterial infections, spina bifida, and opportunistic infections associated with HIV/AIDS [119]. The development of antifolate drugs was accelerated due to results from a leukemia study, which concluded that folic acid increases the proliferation of cancer cells [82, 105]. As a result, folic acid analogs (Anti-folates) were predicted to be a viable method for impeding the spread of cancer [118].
Advances in antifolate research have given rise to a whole new class of antifolates, for which the prototype is methotrexate (Table 1). Methotrexate has been found to play a role in treating tumors with high specificity, sparing the normal tissues. Antifolates act by reversibly inhibiting dihydrofolate reductase (DHFR), which is critical for tetra-hydrofolate synthesis [120]. By inhibiting DHFR, antifolates are able to inhibit nucleic acid synthesis and proliferation. This drug works especially well in many types of cancer cells because the alpha folate receptor, which plays an important role in folate transport, is overexpressed in cancer cells [117–121,]. Recently, antifolates have been shown to activate p53 and AMPK leading to the induction of signaling pathways, resulting in cancer cell senescence. Antifolate agents have succeeded as a form of antimetabolite chemotherapy, which has led these drugs to be used for other diseases that are the result of rapidly dividing cells, including bacterial and parasite infections [103, 119,].
Obovatol
Obovatol (Table 1) is a phenolic compound isolated from the Magnolia Obovata plant that has been utilized in the past as an anti-oxidant and anti-inflammatory agent [122]. Obovatol has also been used to treat neurodegeneration (Alzheimer’s disease), anxiety, depression, and metabolic disorders such as diabetes [103, 123]. Obovatol has been identified as a potent AMPK-activating agent. One study showed that Obovatol and its derivatives strongly activated AMPK in L6 myotube cells in a concentration-dependent manner [17]. Recently Obovatol has been linked with anti-tumor-like activity and is slowly emerging as a novel drug to treat cancers that are resistant to chemotherapy. Nuclear transcription factor-kappa B (NF-κB) activation is associated with chemoresistance in prostate, colon and other cancers and Obovatol has been reported to be able to inhibit cancer cell via inhibition of NF-κB activity in both prostate and colon cancer cells [17, 123]. Combination experiments with other drugs such as paclitaxel, cisplatin and doxorubicin have shown synergistic effect in inhibiting NF-κB and a 60–80% increase in apoptosis inhibition. However, it is unclear whether the inhibition of NF-κB is associated with AMPK inhibition..
Cucurbitane Triterpenoids (CTs)
Cucurbitacins (CT) (Table 1) are a group of triterpenoid compounds that were originally isolated from Momordica Charantia L, a vegetable common in China and India [126]. CTs are found in members of the family Cucurbitaceae that includes the common pumpkins and gourds [124]. There are numerous structural variations of cucurbitacins, which are isolated from multiple sources including plants, mushrooms, and mollusks. Ye et al., found that CTs may induce glucose uptake, associated with the translocation of GLUT4 to the cell membrane and activation of AMPK [67, 108]. However, the exact mechanism by which CTs activate AMPK is unclear. CTs increase AMPK activity with or without AMP, and do so in an additive (but not synergistic) fashion. This suggests that AMPK activation by CTs could be due to a mechanism different from that of AMP association or regulation. Therefore, because CTs do not activate AMPK via inhibiting mitochondrial or whole cell respiration, they may be better tolerated as anti-diabetic or cancer chemotherapeutic agents in the context of AMPK activation [103, 124–128].
Glabridin
First isolated in 1976, Glabridin (Table 1) is an isoflavane that is found in the root extract of the leguminous plant, Licorice (Glycyrrhiza glabra L.) [103]. It is widely used in the food and dietary supplement market as well as in the cosmetics industry. Glabridin is localized in the cork layer and in decayed roots of G. glabra and represents about 0.1–0.4% of the roots’ dry weight. Glabridin participates in the regulation of cellular energy expenditure through AMPK stimulation and the up-regulation of PPAR-γ [129]. Glabridin has also been identified as an indirect AMPK activator in myoblast C2C12 cells. However, glabridin treatment resulted in a significant decrease in total body weight in C57BL/6JL Lepob/Lepob mice, although it also played a role in regulating blood glucose levels in ZDF rats132. The regulation of blood glucose and body weight was linked to AMPK activation [103, 129–132].
Nootkatone
(+)-Nootkatone (Table 1) is a sesquiterpenoid that was isolated originally from Alaska yellow cedar in 1962 [103]. It has also been found in grapefruit oil, vetiver grass oil, pummelo fruit, and alpinia oxyphylla miquel fruit. (+)-Nootkatone can also be synthesized from (+)-valencene, readily available from Valencia oranges, by chemical transformations [133]. Further, Nootkatone could be produced efficiently through biotransformation from (+)-valencene by the green algae Chlorella species as well as from fungi [134]. Nootkatone has been reported to be a potent AMPK activator (135) in myoblast C2C12 cells associated with a strong promotion of glucose and lipid metabolism. In this study, Nootkatone exhibited isoform selectivity, activating AMPK-α by about 11-fold as compared to AMPK-β (by about 3.5-fold). Isoform specificity is a significant pharmacologic factor, and may be beneficial in the selective treatment of disease, should isoform specificity be identified in any of these diseases. Administration of Nootkatone has also been shown to exhibited similar beneficial physiological effects as metformin and other AMPK activating compounds. The beneficial effects include a reduction in body weight, triglycerides, cholesterol, and body fat accumulation, as well as enhanced endurance and reduced fatigue. These physiological effects are consistent with other AMPK activators. However, the precise mechanism of Nootkatone has yet to be elucidated [103, 133–136].
Nectandrin B: 2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignans
2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignan (Table 1) compounds, isolated from Myristica fragrans [103], is a potent AMPK activator that might be useful in the prevention or treatment of AMPK-related disorders including diabetes and metabolic syndrome. It was shown that these compounds could activate AMPK and ACC significantly. An in vivo study using high fat diet-induced obese mice revealed that Nectandrin B decreased body weight (8.89 g) as compared with control (13.4 g) via AMPK activation [137–139]. The role of this compound in cancer is currently underexplored.
Cannabinoids
Cannabinoids are derived from the genus of plants known as cannabis, which include three primary species (Cannabis –sativa, -Indica, and –Ruderalis). Cannabis plants produce a vast variety cannabinoid compounds that possess diverse physiological, pharmacological and psychological activities. Δ9 -tetrahydrocannabinol (Δ9-THC) (Table 1) constitutes the main psychoactive ingredient of Cannabis Sativa [23, 41, 43, 103, 140–143]. In addition, Cannabinoids compose a diverse class of compounds with similar structures that possess a multitude of unique molecular targets and biochemical mechanisms of actions [41]. In hepatocellular carcinoma cells, Δ9-THC and other cannabinoids have been shown to trigger an ER stress response, associated with a potent and abrupt activation of AMPK. This subsequent activation of AMPK has been shown to cooperate with the TRIB3-mediated (a negative regulator of NF-κB) inhibition of the AKT-mTORC1 signaling axis, as well as induction of autophagy-mediated cell death [23, 142]. A one-hour treatment with several different cannabinoids (Including: Natural compounds and synthetic derivatives lacking the psychoactive side-effects) is sufficient to induce a robust increase AMPK phosphorylation, with a sustained effect for up to 24 h. Total AMPK protein levels were decreased after 12 or 24 h treatment. Further, Compound C, a semi-selective AMPK inhibitor inhibited the induced AMPK phosphorylation and autophagy [140]. Cannabinoids are a critically underexplored class of compounds that possess potent and diverse pharmacological properties. Compounds that retain the pharmacological properties of cannabinoids without the psychological (psychoactive) side effects may be beneficial for therapies of multiple diseases including cancer [23, 41, 43, 103, 140–143].
Indole-3-carbinol (I3C) and Diindolylmethane (DIM)
I3C, as well as its in vivo dimeric product DIM (Table 1), is produced from glucosinolates present in Cruciferae vegetables (including broccoli and cabbage) (144). It has been well documented that I3C and DIM have cancer-inhibitory effects via regulating genes involved in cell growth, apoptosis, signal transduction, transcription and oncogenesis (144–146). Recently, we reported that AMPK is a molecular target of DIM in human prostate cancer cells. We found that DIM activates AMPK pathway, in association with inhibition of the mTOR and androgen receptor (AR) as well as induction of apoptosis in both androgen-sensitive (LNCaP) and –resistant (C4-2B) prostate cancer cells and xenografts [29]. Our finding demonstrates that DIM could be used as anti-cancer agent for prevention and treatment regardless of androgen status.
Epigallocatechin gallate (EGCG) and novel EGCG analogs
Catechins including epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG) and EGCG (Table 1) are the main constituents of green tea (Camelia sinensis) and account for 30–40% of its dry extract [147–148]. Among them, EGCG is the most abundant catechin in green tea and has also been used as an anti-obesity agent in animal models and in humans [147–149]. EGCG is also well known for its antioxidant and anticancer properties; EGCG inhibits cancer cell growth, associated with activation of AMPK [147–150]. But its disadvantages such as less potency and low bioavailability have limited its development and use in the clinic. Therefore, efforts were taken to develop the synthetic analogs of EGCG [147–148, 151]. We reported that some novel EGCG analogs 4 and 6 were more potent AMPK activators than metformin and EGCG and were capable of inhibiting cancer cell growth, up-regulating p21 (a cdk inhibitor), down-regulating the mTOR signaling, and suppressing stem cell population in human breast cancer cells [152]. EGCG also has been shown to regulate miRNAs (mir-221), which negatively regulates PTEN expression, resulting in enhancement of the effects of AMPK activation and induction of apoptosis [153, 154].
Resveratrol (trans-3,5,4′-trihydroxystilbene)
Resveratrol (Table 1), a dietary polyphenol compound found in red wines and a large number of plants including grapes berries, and peanuts, has been reported to activate AMPK and possess similar beneficial effects in metabolic disease as the known AMPK activators, AICAR and metformin [155]. Resveratrol has been shown to be able to cause a rapid activation of AMPK via inhibiting the F1F0 mitochondrial ATPase. Resveratrol, as a potent AMPK activator could be useful in the treatment of diabetes and metabolic syndromes [156]. It has been reported that resveratrol may also act as an exercise substitutive agent, which, through AMPK activation, induces energy metabolism. Several studies have come to question the suggestion about the direct binding of resveratrol to sirtuins for activation. The activation of SIRT1 (NAD-dependent deacetylase sirtuin-1) by resveratrol in cells and animals appears to require increased NAD+ levels by AMPK activity. The detailed mechanism about how resveratrol induces AMPK activation requires further investigation [156]. It should also be mentioned that Resveratrol has been shown to be a regulator of miRNAs [22, 31, 60]. It is unclear the exact role of these miRNAs in AMPK signaling regulation, however, resveratrol and many other natural compounds described in this review have been shown to regulate miRNAs with reputed targets in the AMPK signaling and regulatory pathway [61]. For a detailed description of the known mechanism of these phenomena, please see the section titled “AMPK regulation by miRNAs”.
Ginseng (Ginsenosides)
Ginseng is one of the most popular herbal medicines worldwide. Its presumed therapeutic properties include improving physical performance and sexual function, treating cancer, diabetes and hypertension. Ginsenosides, a class of plant steroid glycosides and triterpene saponins, are found mainly in the plant genus Panax (ginseng). They exert antidiabetic and anticancer effects. It was reported that Ginsenoside Rg3 was able to inhibiting adipocyte differentiation, associated with inhibition of PPAR-γ in the treated cells. Rg3 was also able to activate AMPK in a time-dependent fashion, demonstrating that the anti-obesity effect of Ginsenoside Rg3 is related to activation of the AMPK signaling pathway and inhibition of PPAR-γ [157]. A Recent study reported that Rg3 induces apoptosis via the CaMKK-AMPK pathway in HT-29 colon cancer cells. The AMPK activity was confirmed using either compound C and AMPK-specific siRNA or STO-609 (a chemical inhibitor of CaMKKβ) [158]. More recently, it was reported that 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol (20-GPPD), a metabolite of ginseng saponin, caused colon cancer cell death through the induction of cytoplasmic Ca2+. While 20-GPPD-induced activation of AMPK appeared to play a key role in the apoptotic death, it did not involve LKB1, the upstream AMPK kinase [103, 157, 159].
STRATEGIES FOR THE DEVELOPMENT OF NOVEL AMPK-ACTIVATING COMPOUNDS
Natural compounds have been modified over the millennia by evolution to perform specific roles in an organism’s biology. So far, we have detailed a number of natural compounds that have been shown to exhibit potent AMPK-activating qualities with significant implications in cancer and other metabolic diseases. Specialized approaches to identify and predict these properties may aide scientists in the identification of new classes of AMPK activators that may serve as prototypes for whole new categories of drugs with improved efficacies, selectivity and specificity.
Many of the described AMPK activators have come from plants and other nutritional sources. However, expanding the scope of the investigation to include extremophilic microbes (Extremophiles) or microbes that can survive in extreme environments which would place tremendous evolutionary selective pressure and force fine tuning of their molecular machinery, to stabilize their energy states under these extremes of physiological states, could lead to the identification of additional novel, specific AMPK activators with diverse biological activities and unique pharmacologic properties [96, 100, 160–162]. The extreme nature from which these organisms originate, forces unique evolutionarily responses and characteristics that could potentially be utilized within the context of human disease and cancer. In addition to mining the environment and microbiome for natural compounds, novel AMPK activators could be developed through the application of synthetic combinatorial chemistry [160–162]. Modification of candidate compounds may improve on what nature has provided and allow for the development of AMPK-activating drugs with improved pharmacodynamics and/or pharmacokinetics that could be utilized in diverse scientific arenas including cancer therapy.
CONCLUSIONS
The intention of this article was to review the significance of the AMPK signaling pathway in normal and cancer cells, in order to briefly summarize what is known about natural compounds that activate this pathway. These studies have shown that a variety of natural compounds can either activate AMPK directly and/or indirectly. In addition, this review intended to highlight potential strategies for the identification and development of novel targets and compounds within the AMPK pathway in cancer chemotherapeutics. AMPK is a master regulator of cellular-energy and metabolic homeostasis and is a re-invigorated and critical target in cancer biology and chemotherapeutics. These natural compounds could be used as prototypical compounds for the development of “Novel” next generation AMPK-activating drugs. Studying these natural compounds may reveal novel compounds with unique structural, chemical (pharmacokinetic) and mechanistic (pharmacodynamic) properties and could indicate new strategies, which may contribute to the intelligent design and development of next generation of AMPK activators. Furthermore, the repurposing of old drugs; based on their known applications, to a new disease model is a promising strategy for drug development as it bypasses much of the complications and difficulty associated with bringing a new drug to the clinic. Many of the natural compounds discussed in this review have alternative uses and could potentially be repurposed to treat cancer and other disorders associated with aberrant AMPK signaling. It is our hope that by absorbing the lessons learned from natural AMPK-activating compounds we might develop novel compounds with enhanced selectivity, improved efficacy and decreased toxicity for treatment of human diseases including cancer.
Acknowledgments
This work was partially supported by the DeRoy Testamentary Foundation (PhD fellowship to R.T.A), POPII-Canadian Institutes of Health Research (to T.H.C. and Q.P.D.), NIH/NCI 1R01CA20009, 5R01CA127258-05 and R21CA184788 (to Q.P. Dou), and NIH P30 CA22453 (to Karmanos Cancer Institute).
LIST OF ABBREVIATIONS
- AMPK
Adenosine Monophosphate-Activated Protein Kinase
- mTORC1
Mammalian target of rapamycin complex 1
- LKB1
Liver kinase B1
- CAMKKβ
Calcium-activated calmodulin-dependent kinase kinase β
- Thr172
Threonine residue 172
- named α1 and α2
AMPK α-catalytic subunit
- β1 and β2
β-subunit genes
- γ1
γ2 and γ3, γ-subunit genes
- TSC2
Tuberous Sclerosis 2
- CB2
Cannabinoid receptor type 2
- BCA2
Breast Cancer Associated Gene 2
- siRNA
Small interfering RNA
- pAMPKα1
Phosphorylated AMPKα1
- miRNAs
microRNAs
- RISC complex
RNA-induced silencing complex
- ACC
Acetyl-CoA carboxylase
- TCA
Tricarboxylic acid
- OXPHOS
Oxidative phosphorylation
- S6K1
S6 kinase 1
- MLC
Myosin light chain
- KLC2
Kinesin Light Chain 2
- AICAR
5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- JNKs
c-Jun N-terminal kinases
- NF-κB
Nuclear transcription factor-kappaβ
- CTs
Cucurbitane Triterpenoids
- Δ9-THC
Δ9-tetrahydrocannabinol
- I3C
Indole-3-carbinol
- DIM
Diindolylmethane
- EC
Epicatechin
- EGC
Epigallocatechin
- ECG
Epicatechin-3-gallate
- EGCG
Epigallocatechin gallate
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article’s content has no conflicts of interests.
References
- 1.Rehman G, Shehzad A, Khan AL, Hamayun M. Role of AMP-activated protein kinase in cancer therapy. Arch Pharm (Weinheim) 2014;347(7):457–468. doi: 10.1002/ardp.201300402. [DOI] [PubMed] [Google Scholar]
- 2.Hardie DG. Minireview: the AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 3.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase-development of the energy sensor concept. J Physiol. 2006;574(1):7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93(4):891S–89. S6. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 5.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 6.Sanz P, Rubio T, Garcia-Gimeno MA. AMPKbeta subunits: More than just a scaffold in the formation of AMPK complex. FEBS J. 2013;280(16):3723–3733. doi: 10.1111/febs.12364. [DOI] [PubMed] [Google Scholar]
- 7.Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S, Towler MC, Brown LJ, Ogunbayo OA, Evans AM, Hardie DG. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong KJ, Kim GW, Chung SH. AMP-activated protein kinase: An emerging target for ginseng. J Ginseng Res. 2014;38(2):83–88. doi: 10.1016/j.jgr.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iseli TJ, Turner N, Zeng XY, Cooney GJ, Kraegen EW, Yao S, Ye Y, James DE, Ye JM. Activation of AMPK by bitter melon triterpenoids involves CaMKKbeta. PLoS One. 2013;8(4):e62309. doi: 10.1371/journal.pone.0062309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271(44):27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 11.Fogarty S, Hawley SA, Green KA, Saner N, Mustard KJ, Hardie DG. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010;426(1):109–118. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phoenix KN, Devarakonda CV, Fox MM, Stevens LE, Claffey KP. AMPKalpha2 Suppresses Murine Embryonic Fibroblast Transformation and Tumorigenesis. Genes Chromos Cancer. 2012;3(1):51–62. doi: 10.1177/1947601912452883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie DG. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62(7):2164–2172. doi: 10.2337/db13-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie DG, Ross FA, Hawley SA. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem Biol. 2012;19(10):1222–1236. doi: 10.1016/j.chembiol.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, Mamer OA, Avizonis D, DeBerardinis RJ, Siegel PM, Jones RG. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–124. doi: 10.1016/j.cmet.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SY, Cho JS, Yuk DY, Moon DC, Jung JK, Yoo HS, Lee YM, Han SB, Oh KW, Hong JT. Obovatol enhances docetaxel-induced prostate and colon cancer cell death through inactivation of nuclear transcription factor-kappaB. J Pharmacol Sci. 2009;111(2):124–136. doi: 10.1254/jphs.09048fp. [DOI] [PubMed] [Google Scholar]
- 18.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004;29(1):18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11(3):213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826(2):272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci. 2011;36(9):470–477. doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Kong D, Wang Z, Sarkar FH. Regulation of microRNAs by natural agents: an emerging field in chemoprevention and chemotherapy research. Pharm Res. 2010;27(6):1027–1041. doi: 10.1007/s11095-010-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D’Alessandro A, Zolla L, Palmieri M. Cannabinoids inhibit energetic metabolism and induce AMPK-dependent autophagy in pancreatic cancer cells. Cell Death Dis. 2013;4:e664. doi: 10.1038/cddis.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang JT, Lee MS, Kim HJ, Sung MJ, Kim HY, Kim MS, Kwon DY. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother Res. 2009;23(2):262–266. doi: 10.1002/ptr.2606. [DOI] [PubMed] [Google Scholar]
- 25.Greiner AK, Papineni RV, Umar S. Chemoprevention in gastrointestinal physiology and disease. Natural products and microbiome. Am J Physiol Gastrointest Liver Physiol. 2014;307(1):G1–G15. doi: 10.1152/ajpgi.00044.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574(Pt 1):85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu YQ, Cheng X, Guo LX, Mao C, Chen YJ, Liu HX, Xiao QC, Jiang S, Yao ZJ, Zhou GB. Identification of an annonaceous acetogenin mimetic, AA005, as an AMPK activator and autophagy inducer in colon cancer cells. PLoS One. 2012;7(10):e47049. doi: 10.1371/journal.pone.0047049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Banerjee S, Cui QC, Kong D, Sarkar FH, Dou QP. Activation of AMP-activated protein kinase by 3,3′-Diindolylmethane (DIM) is associated with human prostate cancer cell death in vitro and in vivo. PLoS One. 2012;7(10):e47186. doi: 10.1371/journal.pone.0047186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baas AF, Kuipers J, Van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116(3):457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 31.Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37(5):620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Mol Cell Biol Res Commun. 2006;340(1):43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKalpha2 but not AMPKalpha1. Am J Physiol Endocrinol Metab. 2006;290(5):E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Nat Acad Sci USA. 2002;99(25):15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 36.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109(7):3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 38.Ki SH, Lee JW, Lim SC, Hien TT, Im JH, Oh WK, Lee MY, Ji YH, Kim YG, Kang KW. Protective effect of nectandrin B, a potent AMPK activator on neointima formation: Inhibition of Pin1 expression through AMPK activation. Br J Pharmacol. 2013;168(4):932–945. doi: 10.1111/j.1476-5381.2012.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, Shaw RJ. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;23(2):143–158. doi: 10.1016/j.ccr.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Guindon J, Hohmann AG. The endocannabinoid system and cancer: Therapeutic implication. Br J Pharmacol. 2011;163(7):1447–1463. doi: 10.1111/j.1476-5381.2011.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kola B, Boscaro M, Rutter GA, Grossman AB, Korbonits M. Expanding role of AMPK in endocrinology. Trends Endocrinol Metab. 2006;17(5):205–215. doi: 10.1016/j.tem.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Velasco G, Sanchez C, Guzman M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12(6):436–444. doi: 10.1038/nrc3247. [DOI] [PubMed] [Google Scholar]
- 44.Wu N, Gu C, Gu H, Hu H, Han Y, Li Q. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma. 2011;58(6):482–490. doi: 10.4149/neo_2011_06_482. [DOI] [PubMed] [Google Scholar]
- 45.Pearce LR, Atanassova N, Banton MC, Bottomley B, Van der Klaauw AA, Revelli JP, Hendricks A, Keogh JM, Henning E, Doree D, Jeter-Jones S, Garg S, Bochukova EG, Bounds R, Ashford S, Gayton E, Hindmarsh PC, Shield JP, Crowne E, Barford D, Wareham NJ, O’Rahilly S, Murphy MP, Powell DR, Barroso I, Farooqi IS UK consortium. KSR2 mutations are associated with obesity, insulin resistance, and impaired cellular fuel oxidation. Cell. 2013;155(4):765–777. doi: 10.1016/j.cell.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carling D, Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012(1):81–6. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tao R, Gong J, Luo X, Zang M, Guo W, Wen R, Luo Z. AMPK exerts dual regulatory effects on the PI3K pathway. J Mol Signal. 2010;5(1):1. doi: 10.1186/1750-2187-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burger A, Amemiya Y, Kitching R, Seth AK. Novel RING E3 ubiquitin ligases in breast cancer. Neoplasia. 2006;8(8):689–695. doi: 10.1593/neo.06469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burger AM, Gao Y, Amemiya Y, Kahn HJ, Kitching R, Yang Y, Sun P, Narod SA, Hanna WM, Seth AK. A novel RING-type ubiquitin ligase breast cancer-associated gene 2 correlates with outcome in invasive breast cancer. Cancer Res. 2005;65(22):10401–10412. doi: 10.1158/0008-5472.CAN-05-2103. [DOI] [PubMed] [Google Scholar]
- 51.Buac D, Kona FR, Seth AK, Dou QP. Regulation of metformin response by breast cancer associated gene 2. Neoplasia. 2013;15(12):1379–1390. doi: 10.1593/neo.131434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li CM, Narayanan R, Lu Y, Hurh E, Coss CC, Barrett CM, Miller DD, Dalton JT. 2-Arylthiazolidine-4-carboxylic acid amides (ATCAA) target dual pathways in cancer cells: 5′-AMP-activated protein kinase (AMPK)/mTOR and PI3K/Akt/mTOR pathways. Int J Oncol. 2010;37(4):1023–1030. [PubMed] [Google Scholar]
- 53.Kuznetsov JN, Leclerc GJ, Leclerc GM, Barredo JC. AMPK and Akt determine apoptotic cell death following perturbations of one-carbon metabolism by regulating ER stress in acute lymphoblastic leukemia. Mol Cancer Ther. 2011;10(3):437–447. doi: 10.1158/1535-7163.MCT-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, Junger WG, Robson SC, Wu Y. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS One. 2013;8(4):e60184. doi: 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amemiya Y, Azmi P, Seth A. Autoubiquitination of BCA2 RING E3 ligase regulates its own stability and affects cell migration. Mol Cancer Res. 2008;6(9):1385–1396. doi: 10.1158/1541-7786.MCR-08-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zungu M, Schisler JC, Essop MF, McCudden C, Patterson C, Willis MS. Regulation of AMPK by the ubiquitin proteasome system. Am J Pathol. 2011;178(1):4–11. doi: 10.1016/j.ajpath.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1(1):15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Chalfant CE, Spiegel S. Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci. 2005;118(Pt 20):4605–4612. doi: 10.1242/jcs.02637. [DOI] [PubMed] [Google Scholar]
- 59.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F, Viollet B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen MB, Wei MX, Han JY, Wu XY, Li C, Wang J, Shen W, Lu PH. MicroRNA-451 regulates AMPK/mTORC1 signaling and fascin1 expression in HT-29 colorectal cancer. Cell Signal. 2014;26(1):102–109. doi: 10.1016/j.cellsig.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 61.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6(6):590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howard K. Unlocking the money-making potential of RNAi. Nat Biotechnol. 2003;21(12):1441–1446. doi: 10.1038/nbt1203-1441. [DOI] [PubMed] [Google Scholar]
- 63.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 65.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, Subramaniam A, Propp S, Lollo BA, Freier S, Bennett CF, Bhanot S, Monia BP. MiR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Lee JH, Koh H, Kim M, Kim Y, Lee SY, Karess RE, Lee SH, Shong M, Kim JM, Kim J, Chung J. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nat Rev. 2007;447(7147):1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 67.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7(5):1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 68.Zoncu R, Efeyan A, Sabatini DM. MTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Boil. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarkar FH, Li Y, Wang Z, Padhye S. Lesson learned from nature for the development of novel anti-cancer agents: Implication of isoflavone, curcumin, and their synthetic analogs. Curr Pharm Des. 2010;16(16):1801–1812. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68(18):7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henin N, Vincent MF, Gruber HE, Van den Berghe G. Inhibition of fatty acid and cholesterol synthesis by stimulation of AMP-activated protein kinase. FASEB J. 1995;9(7):541–546. doi: 10.1096/fasebj.9.7.7737463. [DOI] [PubMed] [Google Scholar]
- 72.Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008;2(4):323–326. doi: 10.1007/s12263-007-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20(18):6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab. 2005;288(6):E1062–E1066. doi: 10.1152/ajpendo.00561.2004. [DOI] [PubMed] [Google Scholar]
- 77.Shanware NP, Bray K, Abraham RT. The PI3K, metabolic, and autophagy networks: Interactive partners in cellular health and disease. Ann Rev Pharmacol Toxicol. 2013;53:89–106. doi: 10.1146/annurev-pharmtox-010611-134717. [DOI] [PubMed] [Google Scholar]
- 78.Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Mol Cell. 2013;49(3):379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, McBurnie W, Fleming S, Alessi DR. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 80.Jung TW, Choi HY, Lee SY, Hong HC, Yang SJ, Yoo HJ, Youn BS, Baik SH, Choi KM. Salsalate and adiponectin improve palmitate-induced insulin resistance via inhibition of selenoprotein p through the ampk-foxo1alpha pathway. PLoS One. 2013;8(6):e66529. doi: 10.1371/journal.pone.0066529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kitajima Y, Miyazaki K. The critical impact of hif-1a on gastric cancer biology. Cancer. 2013;5(1):15–26. doi: 10.3390/cancers5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aryal P, Kim K, Park PH, Ham S, Cho J, Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and downregulation of mTORC1 complex components in human cancer cells. FEBS J. 2014;281(20):4644–4658. doi: 10.1111/febs.12969. [DOI] [PubMed] [Google Scholar]
- 83.Hardie DG, Alessi DR. LKB1 and AMPK and the cancer-metabolism link - ten years after. BMC Biol. 2013;11:36. doi: 10.1186/1741-7007-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev Res. 2010;3(11):1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 85.Appleyard MV, Murray KE, Coates PJ, Wullschleger S, Bray SE, Kernohan NM, Fleming S, Alessi DR, Thompson AM. Phenformin as prophylaxis and therapy in breast cancer xenografts. Br J Cancer. 2012;106(6):1117–1122. doi: 10.1038/bjc.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pollak MN. Investigating metformin for cancer prevention and treatment: The end of the beginning. Cancer Discov. 2012;2(9):778–790. doi: 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 87.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, Peggie MW, Zibrova D, Green KA, Mustard KJ, Kemp BE, Sakamoto K, Steinberg GR, Hardie DG. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109(10):1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, Viollet B, Thompson CB. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;6(14):6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 91.Yuan HD, Quan HY, Zhang Y, Kim SH, Chung SH. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep. 2010;3(5):825–831. doi: 10.3892/mmr.2010.328. [DOI] [PubMed] [Google Scholar]
- 92.Nakano A, Takashima S. LKB1 and AMP-activated protein kinase: Regulators of cell polarity. Genes Cells. 2012;17(9):737–747. doi: 10.1111/j.1365-2443.2012.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y, Seguchi O, Higo S, Shintani Y, Asanuma H, Asakura M, Minamino T, Kaibuchi K, Mochizuki N, Kitakaze M, Takashima S. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12(6):583–590. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 94.Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 96.Lanzotti V, Xiao J. Natural products in cancer prevention and therapy, a selection of topics presented in the 2nd edition pse symposium (naples, italy, 25th to 28th of june 2013) Curr Med Chem Anticancer Agents. 2014;14(10):1313–1314. doi: 10.2174/187152061410141111101706. [DOI] [PubMed] [Google Scholar]
- 97.Gullett NP, Ruhul Amin AR, Bayraktar S, Pezzuto JM, Shin DM, Khuri FR, Aggarwal BB, Surh YJ, Kucuk O. Cancer prevention with natural compounds. Semin Oncol. 2010;37(3):258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 98.Supakul R, Liangpunsakul S. Alcoholic-induced hepatic steatosis--role of ceramide and protein phosphatase 2A. J Lab Clin Med. 2011;158(2):77–81. doi: 10.1016/j.trsl.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saunders FR, Wallace HM. On the natural chemoprevention of cancer. Plant Physiol Biochem. 2010;48(7):621–626. doi: 10.1016/j.plaphy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Clardy J, Walsh C. Lessons from natural molecules. Nat Rev. 2004;432(7019):829–837. doi: 10.1038/nature03194. [DOI] [PubMed] [Google Scholar]
- 101.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2010;62(1):1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 103.Uddin MN, Sharma G, Choi HS, Seong-IL Lim, Won Keun Oh. AMPK Activators from Natural Products: A Patent Review. Nat Prod Sci. 2013;19(1):1–7. [Google Scholar]
- 104.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J Biol Chem. 1995;270(45):27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 105.Leclerc GM, Leclerc GJ, Fu G, Barredo JC. AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia. J Mol Sig. 2010;5:15. doi: 10.1186/1750-2187-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49(6):896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- 107.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353(1):33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 108.Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J Appl Physiol. 1999;87(5):1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- 109.Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Mol Cell Biol Res Commun. 2001;287(2):562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 110.Song XM, Fiedler M, Galuska D, Ryder JW, Fernstrom M, Chibalin AV, Wallberg-Henriksson H, Zierath JR. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45(1):56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- 111.Steinberg GR, Dandapani M, Hardie DG. AMPK: mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol Metab. 2013;24(10):481–487. doi: 10.1016/j.tem.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282(45):32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293(5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 114.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282(45):32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 115.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, Rattan R, Cliby W, Shridhar V. Metformin intake is associated with better survival in ovarian cancer: A case-control study. Cancer. 2013;119(3):555–562. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glynn SA, Albanes D. Folate and cancer: a review of the literature. Nutr Cancer. 1994;22(2):101–119. doi: 10.1080/01635589409514336. [DOI] [PubMed] [Google Scholar]
- 118.Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biol Ther. 2012;13(14):1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Picciano MF, Stokstad R, Gregory JF. Folic acid metabolism in health and disease: Historical perspective on key advances in the biochemistry and physiology of folates. american chemical society. food and nutritional biochemistry subdivision contemporary issues in clinical nutrition. Vol. 13. New York: Wiley-Liss; 1990. pp. 1–21. [Google Scholar]
- 120.Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69(13):5467–5474. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Beckers A, Organe S, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, Brusselmans K, Verhoeven G, Swinnen JV. Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther. 2006;5(9):2211–2217. doi: 10.1158/1535-7163.MCT-06-0001. [DOI] [PubMed] [Google Scholar]
- 122.Ma H, Jo YJ, Ma Y, Hong JT, Kwon BM, Oh KW. Obovatol isolated from Magnolia obovata enhances pentobarbital-induced sleeping time: Possible involvement of GABAA receptors/chloride channel activation. Phytomedicine. 2009;16(4):308–313. doi: 10.1016/j.phymed.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 123.Huh TL, Song H, Kim JE, Kwon BM, Han DC, Kim HN. Composition for the treatment of diabetes and metabolic syndrome containing Obovatol and its synthesized derivatives. US20100125103. US Patent. 2010 May 20;
- 124.Ye Y, James DE, Tan MCooney GJ, Ke C, Kraegen EW, Chen T, Ye J, Li X. Use of compounds extracted from Momordica charantia L. in the manufacture of medicaments for prevention and treatment of diabetes and obesity. EP2255816. European Patent. 2010 Dec 1;
- 125.Zhao GT, Liu JQ, Deng YY, Li HZ, Chen JC, Zhang ZR, Zhou L, Qiu MH. Cucurbitane-type triterpenoids from the stems and leaves of Momordica charantia. Fitoterapia. 2014;95:75–82. doi: 10.1016/j.fitote.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 126.Chen J, Tian R, Qiu M, Lu L, Zheng Y, Zhang Z. Trinorcucurbitane and cucurbitane triterpenoids from the roots of Momordica charantia. Phytochemistry. 2008;69(4):1043–1048. doi: 10.1016/j.phytochem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 127.Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat Prod Rep. 2005;22(3):386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 128.Ramalhete C, Mansoor TA, Mulhovo S, Molnar J, Ferreira MJ. Cucurbitane-type triterpenoids from the African plant Momordica balsamina. J Nat Prod. 2009;72(11):2009–2013. doi: 10.1021/np900457u. [DOI] [PubMed] [Google Scholar]
- 129.Hsieh MJ, Lin CW, Yang SF, Chen MK, Chiou HL. Glabridin inhibits migration and invasion by transcriptional inhibition of matrix metalloproteinase 9 through modulation of NF-kappaB and AP-1 activity in human liver cancer cells. Br J Pharmacol. 2014;171(12):3037–3050. doi: 10.1111/bph.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee JW, Choe SS, Jang H, Kim J, Jeong HW, Jo H, Jeong KH, Tadi S, Park MG, Kwak TH, Man Kim J, Hyun DH, Kim JB. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J Lipid Res. 2012;53(7):1277–1286. doi: 10.1194/jlr.M022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park MG, Yoo SK. WO2007058480. Composition having effect on treatment and prevention of diseases syndrome treatment with glabridin. 2007 May 24;
- 132.Simmler C, Pauli GF, Chen SN. Phytochemistry and biological properties of glabridin. Fitoterapia. 2013;90:160–184. doi: 10.1016/j.fitote.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xie J, Wang S, Sun B, Ito Y. Isolation and purification of nootkatone from the essential oil of fruits of Alpinia oxyphylla Miquel by high-speed counter-current chromatography. Food Chem. 2009;117(2):375–380. doi: 10.1016/j.foodchem.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fraatz MA, Berger RG, Zorn H. Nootkatone--a biotechnological challenge. Appl Microbiol Biotechnol. 2009;83(1):35–41. doi: 10.1007/s00253-009-1968-x. [DOI] [PubMed] [Google Scholar]
- 135.Choi HJ, Lee JH, Jung YS. (+)-Nootkatone inhibits tumor necrosis factor alpha/interferon gamma-induced production of chemokines in HaCaT cells. Mol Cell Biol Res Commun. 2014;447(2):278–284. doi: 10.1016/j.bbrc.2014.03.121. [DOI] [PubMed] [Google Scholar]
- 136.Murase T, Misawa K, Haramizu S, Minegishi Y, Hase T. Nootkatone, a characteristic constituent of grapefruit, stimulates energy metabolism and prevents diet-induced obesity by activating AMPK. Am J Physiol Endocrinol Metab. 2010;299(2):E266–75. doi: 10.1152/ajpendo.00774.2009. [DOI] [PubMed] [Google Scholar]
- 137.Oh WK, Nguyen PH, Le TVT, Kang HW, Shin ES, Chio JK, Seo DB, Lee SJ. Composition for preventing or treating obesity related diseases mediated by the activation of AMPK and including 2,5-bis-aryl-3,4-dimethyltetrahydrofuran lignans as active ingredients. US20120083525. US Patent. 2012 Apr 5;
- 138.Hien TT, Oh WK, Nguyen PH, Oh SJ, Lee MY, Kang KW. Nectandrin B activates endothelial nitric-oxide synthase phosphorylation in endothelial cells: Role of the AMP-activated protein kinase/estrogen receptor alpha/phosphatidylinositol 3-kinase/Akt pathway. Mol Pharmacol. 2011;80(6):1166–1178. doi: 10.1124/mol.111.073502. [DOI] [PubMed] [Google Scholar]
- 139.Ramamurthy S, Ronnett G. AMP-Activated protein kinase (ampk) and energy-sensing in the brain. Exp Neuro Biol. 2012;21(2):52–60. doi: 10.5607/en.2012.21.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vara D, Salazar M, Olea-Herrero N, Guzman M, Velasco G, Diaz-Laviada I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Diff. 2011;18(7):1099–111. doi: 10.1038/cdd.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152(5):576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, Gonzalez-Feria L, Iovanna JL, Guzman M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119(5):1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21(5):744–757. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- 144.Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21(5):656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: Pathogenesis, prevention and treatment. Toxicol Appl Pharmacol. 2007;224(3):326–336. doi: 10.1016/j.taap.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang MS, Gupta RC. Determination of Energy Charge Potential in the C6 Glioma and the HepG-2 Cell Culture. Toxicol Mech Meth. 2003;13(2):97–101. doi: 10.1080/15376510309843. [DOI] [PubMed] [Google Scholar]
- 147.Landis-Piwowar K, Chen D, Foldes R, Chan TH, Dou QP. Novel epigallocatechin gallate analogs as potential anticancer agents: A patent review (2009 - present) Exp Opin Ther Pat. 2013;23(2):189–202. doi: 10.1517/13543776.2013.743993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kanwar J, Taskeen M, Mohammad I, Huo C, Chan TH, Dou QP. Recent advances on tea polyphenols. Front Biosci (Elite Ed) 2012;4:111–131. doi: 10.2741/363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Byun EB, Choi HG, Sung NY, Byun EH. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor on lipopolysaccharide-stimulated dendritic cells. Mol Cell Biol Res Commun. 2012;426(4):480–485. doi: 10.1016/j.bbrc.2012.08.096. [DOI] [PubMed] [Google Scholar]
- 150.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247(1):115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 151.Kim JW, Amin AR, Shin DM. Chemoprevention of head and neck cancer with green tea polyphenols. Cancer Prev Res. 2010;3(8):900–909. doi: 10.1158/1940-6207.CAPR-09-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen D, Pamu S, Cui Q, Chan TH, Dou QP. Novel epigallocatechin gallate (EGCG) analogs activate AMP-activated protein kinase pathway and target cancer stem cells. Bioorg Med Chem. 2012;20(9):3031–3037. doi: 10.1016/j.bmc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Landis-Piwowar KR, Huo C, Chen D, Milacic V, Shi G, Chan TH, Dou QP. A novel prodrug of the green tea polyphenol (−)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67(9):4303–4310. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 154.Jochmann N, Baumann G, Stangl V. Green tea and cardiovascular disease: From molecular targets towards human health. Curr Opin Clin Nutr Metab Care. 2008;11(6):758–765. doi: 10.1097/MCO.0b013e328314b68b. [DOI] [PubMed] [Google Scholar]
- 155.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635(1–3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 156.Hsu MH, Savas U, Lasker JM, Johnson EF. Genistein, resveratrol, and 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside induce cytochrome P450 4F2 expression through an AMP-activated protein kinase-dependent pathway. J Pharmacol Exp Ther. 2011;337(1):125–136. doi: 10.1124/jpet.110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hwang JA, Hwang MK, Jang Y, Lee EJ, Kim JE, Oh MH, Shin DJ, Lim S, Ji G, Oh U, Bode AM, Dong Z, Lee KW, Lee HJ. 20-O-beta-d-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng, inhibits colon cancer growth by targeting TRPC channel-mediated calcium influx. J Nutr Biochem. 2013;24(6):1096–1104. doi: 10.1016/j.jnutbio.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 158.Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62(5):453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 159.Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ, Kim MJ, Kim HS, Ha J, Kim MS, Kwon DY. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Mol Cell Biol Res Commun. 2007;364(4):1002–1008. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- 160.Canganella F, Wiegel J. Extremophiles: From abyssal to terrestrial ecosystems and possibly beyond. Naturwissenschaften. 2011;98(4):253–279. doi: 10.1007/s00114-011-0775-2. [DOI] [PubMed] [Google Scholar]
- 161.Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharm Res. 2010;27(6):950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 162.Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109(7):3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]