Abstract
Chlorine (Cl2) gas exposure and toxicity remains a concern in military and industrial sectors. While post-Cl2 exposure damage to the lungs and other tissues has been documented and major underlying mechanisms elucidated, no targeted therapeutics that are effective when administered post-exposure, and which are amenable to mass-casualty scenarios have been developed. Our recent studies show nitrite administered by intramuscular (IM) injection post-Cl2 exposure is effective in preventing acute lung injury and improving survival in rodent models. Our goal in this study was to develop a rabbit model of Cl2 toxicity and test whether nitrite affords protection in a non-rodent model. Exposure of New Zealand White rabbits to Cl2 gas (600ppm, 45min) caused significant increases in protein and neutrophil accumulation in the airways and ~35% mortality over 18h. Nitrite administered 30min post Cl2 exposure by a single IM injection, at 1mg/Kg or 10mg/Kg, prevented indices of acute lung injury at 6h by up to 50%. Moreover, all rabbits that received nitrite survived over the study period. These data provide further rationale for developing nitrite as post-exposure therapeutic to mitigate against Cl2 gas exposure injury.
Keywords: halogen, nitric oxide, inflammation
Introduction
Chlorine gas (Cl2) is used widely in numerous industrial processes world-wide and in most cases requires transport, largely by rail, across long distances and through populated areas. There are several examples of train derailments and accidental exposure of humans to high levels of Cl2 (6, 9, 26, 36, 38). Moreover, Cl2 has as long history of use, including evidence in current day conflicts, as a chemical weapon. Recent research efforts have shown that Cl2 exposure results in extensive airway and systemic toxicity, that occurs both during and importantly post-exposure. Current therapies are limited to treating symptoms observed immediately post-exposure and largely entail respiratory supportive actions. A major limitation of current treatments is the lack of consideration of post-exposure toxicities, and the mechanisms involved. This is important since while little may be done to prevent during exposure injury, understanding post-exposure mechanisms may provide key information to develop novel and targeted therapeutics.
Recent studies using experimental models of Cl2 exposure have developed a comprehensive understanding of post-Cl2 exposure injury. This occurs over a time span ranging from hours-days and possibly longer, and afflicts the airways, pulmonary and systemic vasculatures (10, 17, 24, 29, 31, 35, 41). Injury is characterized by hypoxemia, oxidative and inflammatory stress, cellular dysfunction and death. Clinically, this presents initially as acute lung injury and development of acute respiratory distress syndrome and more chronically as reactive airway disease and increased sensitivity to pulmonary infections, as well as dermal injury (5, 10, 13, 18, 23, 33, 35, 40). In addition, extrapulmonary injury has also been documented to the pulmonary and systemic vasculature, and the heart (1, 15, 17, 40).
Nitric oxide (NO) is a key mediator of homeostasis in all organ systems and important in regulating inflammation. Our previous studies have shown that NO-formation mechanisms are disrupted by Cl2 exposure and that endothelial derived NO formation and signaling is inhibited (15, 17). We have suggested that decreased NO-bioavailability may underlie post-Cl2 exposure inflammation, and that therapeutic repletion of NO-signaling may prevent post-exposure ALI(32). Consistent with this idea, post-exposure administration of nitrite, an anion that is reduced to NO and other NO-containing species in vivo in hypoxic tissues, improved survival and prevented ALI in mice and rats exposed to Cl2 (15, 33, 39). While these data provide impetus for further development of nitrite as a post-exposure therapeutic, they are limited to demonstration of efficacy in small rodents. A key consideration for further development of nitrite as post-Cl2 exposure therapeutic is testing and demonstration of efficacy in larger animal models. Rabbits have been used extensively in studies aimed to identify the mechanisms of hyperoxic induced lung injury and develop severe hypoxemia and ALI (25, 30). Like Cl2, hyperoxia is known to upregulate oxidative stress and inflammation and cause extensive injury to the blood gas barrier. Thus, in this study, we exposed rabbits to Cl2 (in concentrations likely to be encountered in the vicinity of industrial accidents) and returned them to room air. We then tested whether post-Cl2 exposure administration of nitrite, by intramuscular injection, could improve survival and limit lung injury,
Materials and Methods
Materials
Unless stated otherwise all reagents were purchased from Sigma (St. Louis, MO, USA). Male (2.5 – 3 Kg) New Zealand White rabbits were purchased from Charles River (Indianapolis, IN, USA) and kept on 12h light-dark cycles with access to standard chow and water ad libitum prior to and post chlorine gas exposure. Rabbits were allowed to acclimate 2–4 days prior to initiating experiments
Methods
Rabbit exposure to chlorine gas
Whole body exposures of male rabbits to Cl2 were performed as previously described (22, 41). Exposures were performed with one rabbit in the chamber at any one time and all exposures were performed between 8am–12pm. Exposure conditions were 600ppm for 45min using Cl2 cylinders at this concentration in air. Cylinders were replaced when the pressure dropped to 500psi. In each case, immediately following exposure, rabbits were returned to room air. The rabbits were monitored hourly for 12h and every 6h thereafter for 24h. All experiments involving animals were conducted according to protocols approved by the UAB IACUC.
Previous studies have shown that post Cl2-exposure administration of the opioid analgesic buprenorphine improved locomotion and decreased immobility post exposure, presumably by decreasing pain (12). However, whether or not buprenorphine administration decreased lung injury and survival, and whether analgesics which may also affect inflammatory responses, affects the therapeutic efficacy of nitrite have not been assessed. Thus in some experiments as indicated, buprenorphine (0.05mg/kg) was administered via sub-cutaneous route in the fold of skin behind the neck 30min prior to chlorine gas exposure. The second group of animals did not receive any buprenorphine prior to Cl2 gas exposure before being returned to room air.
Intramuscular Nitrite administration
Rabbits received a single injection of PBS (vehicle) or sodium nitrite in PBS (1–10mg/kg final concentration) in the gluteus maximus region 30min post cessation of Cl2 exposure. Nitrite stocks were prepared daily in sterile PBS with injection volumes of 1ml.
Acute Lung injury
At indicated times, rabbits were sacrificed by lethal dose of ketamine/dexmedetomidine/acepromazine (100/0.5/2mg/Kg) administered by IM injection. An incision was made at the neck to expose the trachea, and an endotracheal cannula (OD 7mm, L 50mm) inserted. Lungs were lavaged with 3 × 30ml of PBS (similar to the total lung capacity (~90ml) of a 2Kg rabbit(14)); ~20ml was recovered. Bronchoalveolar lavage (BAL) protein and cell numbers reported have been adjusted for dilution accordingly. Rabbits were then exsanguinated by cardiac puncture for collection of blood. Lavage fluid recovered was gently mixed with rocking motion and 1ml aliquots of lavage fluid were kept on ice and centrifuged immediately at 300g for 10min to pellet cells. Supernatants were removed and stored on ice for protein analysis using the Bio-Rad Protein Assay Reagent Kit compared with BSA standards. Cells were resuspended in 100µl PBS and counted using a Neubauer hemocytometer. Cells were then placed on slides using a cellspin (Tharmac, Drosselweg, Germany) and stained using a two-stain set consisting of eosin Y and a solution of thiazine dyes (Quik-Stain; Siemens, Washington, DC). Differential counts (specifically monocytes, neutrophils, and lymphocytes) were then performed on slides via light microscopy.
Measurement of interleukin-8
BALF IL-8 levels were measured using ELISA (D800C) according to manufacturer’s instructions (R&D Systems, Inc, Minneapolis, MN). Optical densities were read using an synery H4 hybrid multimode microplate reader (Bio-TEK Instruments, Winooski, VT). IL-8 concentration was calculated by polynomial regression analysis. Samples were used undiluted and measured in duplicate per replicate.
Survival Analysis
For each exposure, rabbits were randomly pre-assigned to either be exposed to Cl2 only, or Cl2 followed by nitrite therapy. Rabbits were euthanized based on any the following triggers alone or in combination a) body temperature below 90°F, b) >20% weight loss within 24h, c) inability of rabbit to support itself or laying on side.
Statistical analysis
The numbers of replicates are indicated in the figure legends. Survival was assessed using the Log rank (Mantel-Cox) test. Changes in BAL protein and cells were assessed by unpaired t-test or 1-way ANOVA with Tukey post-test as indicated. All analyses were performed using GraphPad Prism. Significance was set at 0.05.
Results
Nitrite therapy prevents Cl2 dependent ALI in rabbits
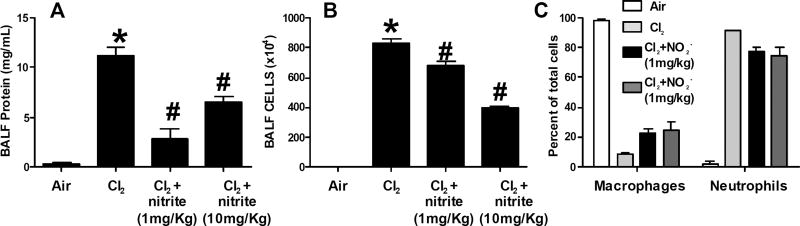
Rabbits were exposed to Cl2 (600ppm, 45min), and then treated with nitrite or vehicle 30min post-exposure. Rabbits were sacrificed at 6h and BAL fluid collected and analyzed for total protein concentration (Fig 1A) and inflammatory cell accumulation (Fig 1B–C). Cl2 exposure resulted in significant increases in BAL protein (Fig 1A) and cell content (Fig 1B), the increase in latter largely due to increased PMN (Fig 1C). Nitrite at 1 and 10mg/Kg significantly attenuated BAL protein and cell accumulation, with the lower dose being more effective in decreasing BAL protein and the higher dose more effective for decreasing the number of cells; these findings are similar to our previous observations using a rat model of Cl2 induced ALI(33). The highest dose of nitrite also significantly decreased the proportion of BAL cells that were neutrophils with a parallel increase in the percent of macrophages.
Figure 1. Effects of nitrite on Cl2 dependent acute lung injury.
Panel A–C: New Zealand white male rabbits were exposed to Cl2 (600ppm, 45min) and then returned to room air. Nitrite was administered by a single IM injection at the indicated doses 30min post-exposure. 6h post-exposure, rabbits were euthanized and BALF levels of protein (Panel A), inflammatory cells (Panel B) and cell differentials (Panel C) measured. For panels A-B, data shown mean ± SEM (n=3) *P<0.05 relative to air, #P<0.05 relative to Cl2 by 1-way ANOVA with Tukey post-test. For panel C, *P<0.05 relative to Cl2 by 1-way ANOVA with Tukey post-test.
Nitrite therapy improves post-Cl2 gas exposure survival
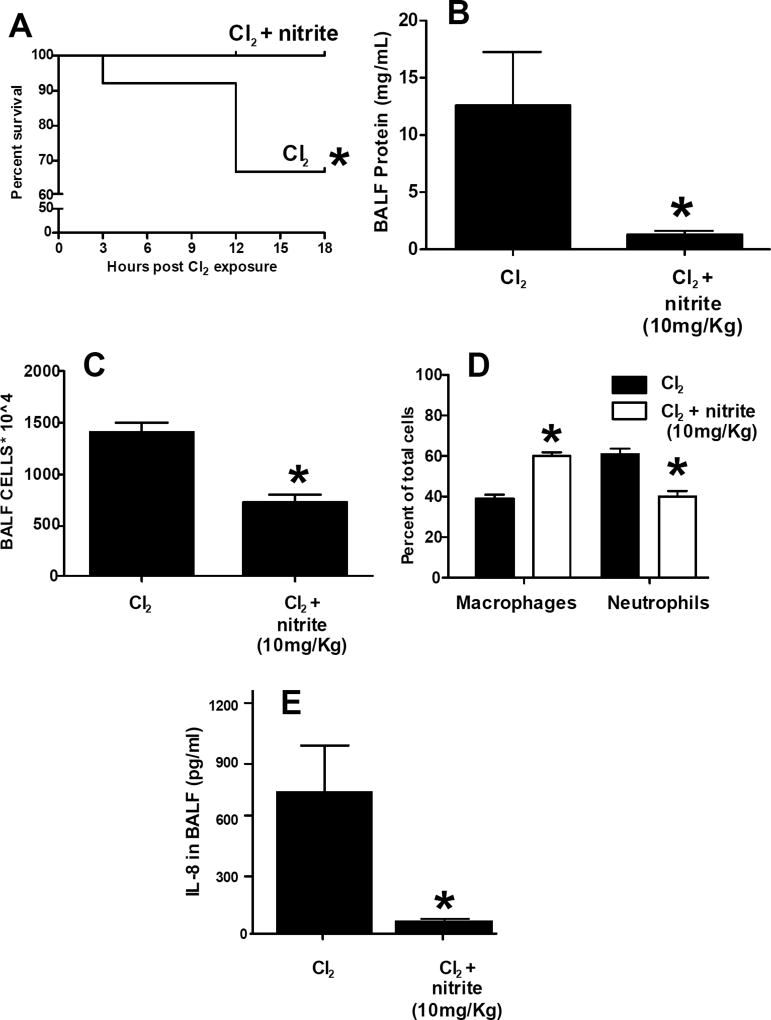
Rabbits were exposed to Cl2 at 400ppm (30min), 500ppm (30min) or 600ppm (45min) and 18h survival assessed. No mortality was observed with 400–500ppm Cl2 (not shown). Exposure to 600ppm Cl2 resulted in ~35% mortality over 18h, which was completely reversed with IM nitrite administered 30min post exposure (Fig 2A). For these studies, per IACUC recommendations, exposure protocol was modified to include administration of buprenorphine for pain management. Pilot studies (n=4 per group) showed no differences in Cl2 (600ppm, 45min) induced mortality in rabbits treated with either vehicle or buprenorphine. Moreover, Fig 2B–C shows BAL protein and inflammatory cell levels in rabbits still alive at 18h, and shows that nitrite treatment significantly decreased these indices by 95% and 50% respectively. For BAL cells, this level of protection afforded by nitrite was similar to that observed 6h after Cl2 exposure (Fig 1A). However, for BAL protein levels, the protection was greater 18h after Cl2 exposure. Nitrite elicited similar effects of the composition of BAL cells observed in Figure 1, with a shift towards more macrophages and fewer neutrophils (Figure 2D). Moreover, Figure 2E shows that nitrite treatment decreased BAL IL-8 levels by >90%.
Figure 2. Effects of nitrite on Cl2 gas dependent mortality.
New Zealand white male rabbits were administered buprenorphine (0.05mg/Kg) 30min prior to Cl2 exposure (600ppm, 45min) and then returned to room air. Nitrite (10mg/Kg) was administered by a single IM injection 30min post-exposure. Mortality was assessed over 18h. Data show Kaplan-Meier survival curves, *P<0.04 between groups determined by the Log-rank test (n=12 per group). Panel B-E: BAL protein, inflammatory cells, differential analyses and IL-8 respectively. Data shown mean ± SEM. *P<0.05 by unpaired t-test.
Discussion
Exposure to high levels of Cl2 has occurred in the military arena and after accidental release. Unfortunately, the possibility of Cl2 exposure to civilian populations remains in both settings and at least with accidental exposure, likely to remain given the widespread use of Cl2 in various industrial processes that require transport of this halogen across populated areas. For example, modelling studies using data from the release of Cl2 gas after a train derailment in South Carolina, USA, in 2005 indicate that humans 0.5km downwind of the accident were exposed to >500ppm Cl2 gas for 30–60min(19). The last 10 years has seen many advances in our understanding of the effects and underlying mechanisms by which post-Cl2 exposure injures the cardiorespiratory system. Acute lung injury is an early feature that readily develops into reactive airway syndrome, characterized by increased permeability, inflammation, fibrosis and airway plugging. Paralleling damage to the lung, are injury to the pulmonary and systemic vasculatures and cardiac system (1, 15, 17, 40). In addition to morbidities, mortality is also evident both during and post-exposure. Thus any therapeutic that could be administered early post-exposure by first responders, which improves acute post-exposure survival, would be of great benefit allowing subsequent transport of exposed individuals to primary care facilities.
Increased oxidative stress, inflammation and dysfunction in endogenous repair processes and are all underlying mechanisms for post Cl2 toxicity and this information is being used to develop and test a host of targeted therapies (4, 7, 8, 10, 13, 17, 20, 22, 24, 27–29, 31, 34, 39, 41). Indeed post exposure administration of Vitamin C (an antioxidant) and desferal (an iron chelator) have been shown to improve survival and enhance lung epithelial repair(11). Our recent focus has been on NO-repletion therapeutics. The rationale for this being that Cl2 exposure results in endothelial dysfunction characterized by a loss of NO-bioavailability (15, 17) which would also predispose to inflammation and oxidative stress. NO-repletion therapies exist; the two most appreciated being inhaled NO administration or use of PDE-5 inhibitors (21, 37). However, the efficacy of these towards Cl2 toxicity is unclear and with inhaled NO certainly, logistic and price constraints would likely preclude its administration in the field, in a mass casualty situation. We have tested nitrite therapy on the basis that circulating nitrite levels are decreased post-Cl2 exposure (17) and that nitrite is a relatively inexpensive therapeutic amenable to storage for long periods and administration by IM injection. Also, our previous studies have shown nitrite administered within 30–60min post exposure to mice or rats post-Cl2 exposure, prevents leak and inflammatory injury to the lungs, prevents development of reactive airways and improves post-exposure survival, especially in the early phases post exposure (15, 33, 39). Moreover, nitrite is an active agent in cyanide antidote kits and has an established safety profile for use in humans.
This therapeutic profile provided rationale for further testing of nitrite as a counter-measure for Cl2 toxicity. In this context, a key and required element of developing nitrite-therapeutics is to test efficacy in a different non-rodent animal model. We utilized a rabbit model and first established Cl2 exposure conditions and then tested whether nitrite could prevent ALI improve 18h survival, a time over which significant protection is observed in mice. At both 1mg/Kg and 10mg/Kg, doses shown to protect in mouse and rat modes, nitrite administered by IM injection 30min post Cl2 exposure, prevented accumulation of protein and inflammatory cells in the airways. Consistent with our previous data with mice, lower doses of nitrite were more effective and preventing BAL protein, whereas the higher doses are more effective at inhibiting BAL neutrophil accumulation. We do not have an explanation for this dose-selectivity of nitrite towards permeability and inflammation component of ALI and underscore the important point that both these measures were decreased supporting protective effects of nitrite. Notable also was that nitrite therapy not only decreased total inflammatory cell accumulation but at longer times post-Cl2 exposure altered the cell composition with less neutrophils and more macrophages. This is consistent with our previous data with mice showing that nitrite-dependent protection was largely mediated by limiting neutrophil-dependent ALI(16). We also show that a likely mechanism for this effect is reduction in IL-8 levels, a pro-neutrophilic chemokine and consistent with our prior studies suggesting limiting neutrophil trafficking to the lungs is an important mechanism underlying nitrite therapy. Exposure of humans and animals to Cl2 causes pain presumably because of the stimulation of transient receptor potential ankyrin 1 (TRPA1) channels in airway sensory neurons and tissue damage(2, 3). Pain in turn may cause inflammation which may aggravate lung injury. However, in our studies, nitrite was protective even in rabbits treated with buprenorphine. This is important and suggests future experimental studies evaluating nitrite will not be affected by possible requirement to use pain-relieving medication. We also show that nitrite acutely improves survival after Cl2 exposure in rabbits. This is an important result which in addition to documenting efficacy in a non-rodent model, further supports the proposal that nitrite will be an effective therapeutic to be administered by first responders to improve immediate / short-term survival after Cl2 exposure that will allow for administration of other targeted therapies that can be administered in a more controlled primary care setting type environment.
It was not our goal to use the rabbit model to discern long-term effects of Cl2 gas exposure and test effects of nitrite-therapy in this regard, but to focus on toxicity occurring acutely after exposure. Over this small time period, it is unlikely that major improvements in lung histology will be observed; data from rodent studies demonstrate that the majority of the airway injury characterized by de-epithelialization occurs during Cl2 exposure(10). Our previous studies did demonstrate a modest improvement in airway injury and cell death in rats exposed to Cl2 and nitrite however (39). Further studies evaluating the therapeutic effects of nitrite that focus on longer-term assessment of airway histology in rodent and rabbit models is warranted. The goal of this study was to test if nitrite therapy administered by IM injection post exposure could protect against primary morbidity and mortality observed after Cl2 exposure in a rabbit model. Our data suggest that nitrite is protective in this model and that this is similar to prior studies using mice and rats. Collectively, these data provide rationale for further development of nitrite as counter-measure therapy that can be administered in mass-casualty situations to improve immediate survival and limit ALI that occurs after Cl2 exposure
Acknowledgments
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number U01ES023759 (RPP), and 5U01ES026458 02 and 1 U01 ES027697 01 (SM)
Footnotes
Conflict of Interest: RPP is a co-inventor on a patent for use of nitrite salts for the treatment of cardiovascular conditions
References
- 1.Ahmad S, Ahmad A, Hendry-Hofer TB, Loader JE, Claycomb WC, Mozziconacci O, Schoneich C, Reisdorph N, Powell RL, Chandler JD, Day BJ, Veress LA, White CW. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol. 2015;52:492–502. doi: 10.1165/rcmb.2014-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. American journal of physiology Lung cellular and molecular physiology. 2014;307:L158–172. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proceedings of the American Thoracic Society. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlisle M, Lam A, Svendsen ER, Aggarwal S, Matalon S. Chlorine-induced cardiopulmonary injury. Ann N Y Acad Sci. 2016;1374:159–167. doi: 10.1111/nyas.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cevik Y, Onay M, Akmaz I, Sezigen S. Mass casualties from acute inhalation of chlorine gas. South Med J. 2009;102:1209–1213. doi: 10.1097/SMJ.0b013e3181bfdc67. [DOI] [PubMed] [Google Scholar]
- 7.Chang W, Chen J, Schlueter CF, Rando RJ, Pathak YV, Hoyle GW. Inhibition of chlorine-induced lung injury by the type 4 phosphodiesterase inhibitor rolipram. Toxicol Appl Pharmacol. 2012;263:251–258. doi: 10.1016/j.taap.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Mo Y, Schlueter CF, Hoyle GW. Inhibition of chlorine-induced pulmonary inflammation and edema by mometasone and budesonide. Toxicol Appl Pharmacol. 2013 doi: 10.1016/j.taap.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RB. Chlorine: state of the art. Lung. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- 10.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol. 2012;46:599–606. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanucchi MV, Bracher A, Doran SF, Squadrito GL, Fernandez S, Postlethwait EM, Bowen L, Matalon S. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol. 2012;46:599–606. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filippidis AS, Zarogiannis SG, Randich A, Ness TJ, Matalon S. Assessment of locomotion in chlorine exposed mice by computer vision and neural networks. J Appl Physiol (1985) 2012;112:1064–1072. doi: 10.1152/japplphysiol.01023.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gessner MA, Doran SF, Yu Z, Dunaway CW, Matalon S, Steele C. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol. 2013;304:L765–773. doi: 10.1152/ajplung.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holm BA, Notter RH, Siegle J, Matalon S. Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol (1985) 1985;59:1402–1409. doi: 10.1152/jappl.1985.59.5.1402. [DOI] [PubMed] [Google Scholar]
- 15.Honavar J, Bradley E, Bradley K, Oh JY, Vallejo MO, Kelley EE, Cantu-Medellin N, Doran S, Dell'italia LJ, Matalon S, Patel RP. Chlorine gas exposure disrupts nitric oxide homeostasis in the pulmonary vasculature. Toxicology. 2014;321:96–102. doi: 10.1016/j.tox.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honavar J, Doran S, Oh JY, Steele C, Matalon S, Patel RP. Nitrite therapy improves survival postexposure to chlorine gas. Am J Physiol Lung Cell Mol Physiol. 2014;307:L888–894. doi: 10.1152/ajplung.00079.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, Mohankumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine Gas Exposure Causes Systemic Endothelial Dysfunction by Inhibiting eNOS-dependent Signaling. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyle GW. Mitigation of chlorine lung injury by increasing cyclic AMP levels. Proceedings of the American Thoracic Society. 2010;7:284–289. doi: 10.1513/pats.201001-002SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jani DD, Reed D, Feigley CE, Svendsen ER. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res. 2016;26:58–74. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koohsari H, Tamaoka M, Campbell HR, Martin JG. The role of gamma delta T cells in airway epithelial injury and bronchial responsiveness after chlorine gas exposure in mice. Respir Res. 2007;8:21. doi: 10.1186/1465-9921-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang JD, Jr, Smith AB, Brandon A, Bradley KM, Liu Y, Li W, Crowe DR, Jhala NC, Cross RC, Frenette L, Martay K, Vater YL, Vitin AA, Dembo GA, Dubay DA, Bynon JS, Szychowski JM, Reyes JD, Halldorson JB, Rayhill SC, Dick AA, Bakthavatsalam R, Brandenberger J, Broeckel-Elrod JA, Sissons-Ross L, Jordan T, Chen LY, Siriussawakul A, Eckhoff DE, Patel RP. A randomized clinical trial testing the anti-inflammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One. 2014;9:e86053. doi: 10.1371/journal.pone.0086053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008;295:L733–743. doi: 10.1152/ajplung.90240.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Weng Z, Doran SF, Srivastava RK, Afaq F, Matalon S, Athar M. Chlorine Induces the Unfolded Protein Response in Murine Lungs and Skin. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2012-0488RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med. 2003;168:568–574. doi: 10.1164/rccm.200201-021OC. [DOI] [PubMed] [Google Scholar]
- 25.Matalon S, Egan EA. Effects of 100% O2 breathing on permeability of alveolar epithelium to solute. J Appl Physiol Respir Environ Exerc Physiol. 1981;50:859–863. doi: 10.1152/jappl.1981.50.4.859. [DOI] [PubMed] [Google Scholar]
- 26.Matalon S, Maull EA. Understanding and treating chlorine-induced lung injury. Proc Am Thorac Soc. 2010;7:253. doi: 10.1513/pats.7.4.253. [DOI] [PubMed] [Google Scholar]
- 27.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGovern TK, Powell WS, Day BJ, White CW, Govindaraju K, Karmouty-Quintana H, Lavoie N, Tan JJ, Martin JG. Dimethylthiourea protects against chlorine induced changes in airway function in a murine model of irritant induced asthma. Respir Res. 2010;11:138. doi: 10.1186/1465-9921-11-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res. 2012;13:107. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nickerson PA, Matalon S, Farhi LE. An ultrastructural study of alveolar permeability to cytochrome C in the rabbit lung: effect of exposure to 100% oxygen at one atmosphere. Am J Pathol. 1981;102:1–9. [PMC free article] [PubMed] [Google Scholar]
- 31.O'Koren EG, Hogan BL, Gunn MD. Loss of Basal Cells Precedes Bronchiolitis Obliterans-Like Pathological Changes in a Murine Model of Chlorine Gas Inhalation. Am J Respir Cell Mol Biol. 2013 doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samal A, Honovar J, White CR, Patel RP. Potential for chlorine gas-induced injury in the extrapulmonary vasculature. Proc Am Thorac Soc. 2010;7:290–293. doi: 10.1513/pats.201001-006SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samal AA, Honavar J, Brandon A, Bradley KM, Doran S, Liu Y, Dunaway C, Steele C, Postlethwait EM, Squadrito GL, Fanucchi MV, Matalon S, Patel RP. Administration of nitrite after chlorine gas exposure prevents lung injury: effect of administration modality. Free Radic Biol Med. 2012;53:1431–1439. doi: 10.1016/j.freeradbiomed.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song W, Wei S, Liu G, Yu Z, Estell K, Yadav AK, Schwiebert LM, Matalon S. Postexposure administration of a {beta}2-agonist decreases chlorine-induced airway hyperreactivity in mice. Am J Respir Cell Mol Biol. 2011;45:88–94. doi: 10.1165/rcmb.2010-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuck SA, Ramos-Barbon D, Campbell H, McGovern T, Karmouty-Quintana H, Martin JG. Time course of airway remodelling after an acute chlorine gas exposure in mice. Respir Res. 2008;9:61. doi: 10.1186/1465-9921-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Sickle D, Wenck MA, Belflower A, Drociuk D, Ferdinands J, Holguin F, Svendsen E, Bretous L, Jankelevich S, Gibson JJ, Garbe P, Moolenaar RL. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasquez EC, Gava AL, Graceli JB, Balarini CM, Campagnaro BP, Pereira TM, Meyrelles SS. Novel Therapeutic Targets for Phosphodiesterase 5 Inhibitors: current state-of-the-art on systemic arterial hypertension and atherosclerosis. Curr Pharm Biotechnol. 2016;17:347–364. doi: 10.2174/1389201017666151223123904. [DOI] [PubMed] [Google Scholar]
- 38.Wenck MA, Van Sickle D, Drociuk D, Belflower A, Youngblood C, Whisnant MD, Taylor R, Rudnick V, Gibson JJ. Rapid assessment of exposure to chlorine released from a train derailment and resulting health impact. Public Health Rep. 2007;122:784–792. doi: 10.1177/003335490712200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadav AK, Doran SF, Samal AA, Sharma R, Vedagiri K, Postlethwait EM, Squadrito GL, Fanucchi MV, Roberts LJ, 2nd, Patel RP, Matalon S. Mitigation of chlorine gas lung injury in rats by postexposure administration of sodium nitrite. Am J Physiol Lung Cell Mol Physiol. 2011;300:L362–369. doi: 10.1152/ajplung.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaky A, Bradley WE, Lazrak A, Zafar I, Doran S, Ahmad A, White CW, Dell'Italia LJ, Matalon S, Ahmad S. Chlorine inhalation-induced myocardial depression and failure. Physiol Rep. 2015;3 doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarogiannis SG, Jurkuvenaite A, Fernandez S, Doran SF, Yadav AK, Squadrito GL, Postlethwait EM, Bowen L, Matalon S. Ascorbate and deferoxamine administration after chlorine exposure decrease mortality and lung injury in mice. Am J Respir Cell Mol Biol. 2011;45:386–392. doi: 10.1165/rcmb.2010-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]