Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of myeloid cells that are increased in the peripheral blood of cancer patients and limit productive immune responses against tumors. Immunosuppressive MDSCs are well characterized in murine splenic tissue and are found at higher frequencies in spleens of tumor-bearing mice. However, no studies have yet analyzed these cells in parallel human spleens. We hypothesized that MDSCs would be increased in the spleens of human cancer patients, similar to tumor-bearing mice. We compared the frequency and function of MDSC subsets in dissociated human spleen from 16 patients with benign pancreatic cysts and 26 patients with a variety of cancers. We found that total MDSCs (Linneg CD11bpos CD33pos HLA-DRneg), granulocytic MDSCs (additional markers CD14neg CD15pos), and monocytic MDSCs (CD14pos CD15neg) were identified in human spleen. The monocytic subset was the most prominent in both spleen and peripheral blood and the granulocytic subset was expanded in the spleen relative to matched peripheral blood samples. Importantly, the frequency of CD15pos MDSCs in the spleen was increased in patients with cancer compared to patients with benign pancreatic cysts and was associated with a significantly increased risk of death and decreased overall survival. Finally, MDSCs isolated from the spleen suppressed T cell responses, demonstrating for the first time the functional capacity of human splenic MDSCs. These data suggest that the human spleen is a potential source of large quantities of cells with immunosuppressive function for future characterization and in-depth studies of human MDSCs.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1953-z) contains supplementary material, which is available to authorized users.
Keywords: MDSCs, Human spleen, Immunosuppression, Cancer
Introduction
Myeloid-derived suppressor cells (MDSCs) are a diverse population of immunosuppressive myeloid cells characterized in humans by lack of lineage markers (B cell, T cell, and NK cell), low to negative expression of HLA-DR, and expression of the monocyte and myeloid markers CD11b and CD33 [1, 2]. Similar to mice, some human MDSC subsets express the granulocytic marker CD15, known as granulocytic or polymorphonuclear MDSCs, while others express the monocytic marker CD14, known as monocytic MDSCs [3–5]. MDSCs reduce inflammation following infection and their dysregulation has been implicated in diseases such as sepsis, autoimmunity, and cancer [6]. Largely characterized in mouse models of cancer and in cancer patients, MDSCs are expanded in number and/or show increased suppressive function when isolated from the spleens of tumor-bearing mice or the peripheral blood of cancer patients [7, 8]. They are also recruited to the tumor microenvironment where they prevent effective anti-tumor T cell responses [9].
The tissue distribution of MDSC subsets varies with tumor model, disease severity, and organ and is well characterized in mice [8]. However, the tissue distribution of MDSCs in human cancer patients has not been well established primarily due to the limited availability of various human tissues. Consequently, human MDSCs have largely been characterized in more easily attainable peripheral blood and tumor tissue samples. Despite these limitations, the clinical relevance of MDSCs has been clearly demonstrated in human peripheral blood, in which the frequency of MDSCs correlates with clinical outcomes and is an independent prognostic indicator of clinical disease progression in patients with pancreatic cancer, esophageal cancer, gastric cancer, and melanoma [10, 11]. Although there are significant biologic differences between the microarchitecture and function of mouse and human spleen [12], the functional capabilities of MDSC subsets isolated from the human spleen have not been described.
To determine if MDSCs isolated from human spleen tissue function similarly to those in historical mouse studies, we characterized MDSCs in patients undergoing splenectomy primarily in conjunction with distal pancreatectomy for the pathologic identification of pancreatic masses. Relevant for this patient population, increased frequencies of MDSCs have been detected in the peripheral blood and tumor tissue of mice bearing very early spontaneous pancreatic tumors [13, 14]. Pancreatic cancer patients also have increased frequencies of MDSCs in the peripheral blood and immunosuppressive MDSCs have been identified in pancreatic adenocarcinomas [3, 15–17]. More specifically, patients with both resectable pancreatic cancer, which make up only 15–20% of pancreatic cancer patients [18], and unresectable pancreatic cancer have increased levels of CD15pos MDSCs in the peripheral blood [19]. Furthermore, treatments associated with a reduction of MDSCs improve anti-tumor immune responses, reduce tumor volume, and increase survival in mice with pancreatic tumors [16, 20–22]. Although increased frequencies of MDSCs have been identified in the spleens of mice bearing pancreatic tumors [13], no studies have analyzed the frequency of these cells in the spleens of pancreatic cancer patients.
We found that the CD14pos MDSC subset was the most prominent subset in both spleen and peripheral blood. The CD15pos MDSC subset was increased in human spleens relative to matched peripheral blood samples, while the CD14pos subset was increased in peripheral blood samples relative to matched spleens. Importantly, frequencies of MDSCs in human spleen tissue did not correlate with frequencies in matched peripheral blood samples. Consistent with previous murine studies, the frequency of CD15pos MDSCs was increased in the spleens of cancer patients, which correlated with overall survival in cancer patients and predicted the risk of death in this cohort. Both subsets of splenic MDSCs suppressed T cell proliferation and activation after stimulation in mixed-lymphocyte reactions, but unlike studies in mice, splenic MDSCs isolated from patients with benign pancreatic cysts and advanced-stage cancer were both immunosuppressive with a high level of patient to patient variability. These data indicate that granulocytic MDSCs accumulate in the spleens of cancer patients similarly to tumor-bearing mice and that this accumulation may predict outcomes in cancer patients. However, the immunosuppressive function of human splenic MDSCs may potentially be broader than murine MDSCs and not exclusive to cancer patients.
Materials and methods
Study population
Eligible patients over 18 years of age undergoing splenectomy as part of their routine medical care at the University of Colorado Hospital (Aurora, CO, USA) in conjunction with a distal pancreatectomy or gastrectomy. Indications for splenectomy were related to technical issues of the surgical approach or proximity to nearby tumors, including melanoma, ovarian cancer, and colon adenocarcinoma (Table 1). Tissue donated to this study was subjected to gross analysis and considered pathologically normal. After removal, spleen tissue was placed on ice and processed within 2 h as described below. Peripheral blood was also collected from a subset of tissue donors. Informed consent was obtained for all subjects through protocols approved by the Colorado Multiple Institutional Review Board.
Table 1.
Clinical characteristics of enrolled patients
| Clinical diagnosis | #of patients | Age (range) | Gender (M/F) | Stage (# of patients) |
|---|---|---|---|---|
| Benign pancreatic cyst | 14 | 56 (31–79) | 5/9 | n/a |
| Pancreatic adenocarcinoma | 9 | 63 (40–79) | 4/5 | I (2), IIa (2), IIb (3), IV (2) |
| Pancreatic neuroendocrine tumor | 10 | 57 (41–73) | 3/7 | I (4), Ib (1), IIb (2), IV (3) |
| Colon adenocarcinoma | 3 | 53 | 1/2 | Illb, IV (2) |
| Melanoma | 3 | 58 (30–76) | 0/3 | IV (3) |
| Ovarian cancer | 2 | 56, 65 | 0/2 | IIIc, IV |
| Total | 41 | 56 (31–79) | 13/28 |
Sample collection
Splenocytes were prepared by macerating the spleen tissue in a tissue sieve containing a wire mesh screen and phosphate-buffered saline (PBS). The cell suspension was filtered through a 100 micron filter and lymphocytes were isolated over a density gradient using Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA, USA). Peripheral blood was collected prior to surgery into tubes containing acid citrate dextrose anticoagulant (BD Biosciences, San Jose, CA, USA) and peripheral blood mononuclear cells (PBMC) were isolated over a density gradient as above. After washing with PBS, splenocytes and PBMC were cryopreserved in normal human serum (Gemini Bioproducts, Sacramento, CA, USA) containing 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen.
Flow cytometry
To define the MDSC subsets, frozen splenocytes and PBMC were thawed, washed, and 1 million cells were stained with fluorescent antibodies as previously described [10]. All antibodies were obtained from BioLegend (San Diego, CA, USA) except CD15 (BD Biosciences). All data were collected and analyzed as previously described [10].
H&E staining
Sorted splenocytes were fixed with 4% paraformaldehyde for 48 h. Cell pellets were resuspended in 0.9% agarose gel, dehydrated in ethanol, and imbedded in paraffin. Four micron sections were stained with hematoxylin and eosin (H&E) using a Tissue Tek® automated slide stainer and coverslipper (Sakura, Torrence, CA, USA). Images were taken on a Zeiss Axioskop light microscope equipped with an HBO 100 lamp and digital camera (SPOT RTke, Spot Diagnostics, Sterling Heights, MI, USA) using the 100× objective under oil immersion.
T cell suppression assays
T cells were separated from freshly isolated splenocytes using magnetic beads according to the manufacturer’s instructions (Pan T cell Isolation Kit, Miltenyi Biotec). To isolate MDSCs, CD11bpos cells were first enriched using CD11b microbeads (Miltenyi Biotec), stained with antibodies specific for lineage markers, CD11b, HLA-DR, CD14, and CD15. Linneg HLA-DRneg CD11bpos CD14neg CD15pos or Linneg HLA-DRneg CD11bpos CD14pos CD15neg MDSCs and HLA-DRpos controls were separated by FACS (greater than 95% pure). T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and 1 × 105 cells were stimulated in a mixed-lymphocyte reaction (MLR) with 2 × 104 monocyte-derived dendritic cells from a healthy donor (preparation as previously described [10]) in the presence of 1 × 105 HLA-DR + control cells, CD15pos MDSCs, or CD14pos MDSCs. After 4 days, the supernatants were removed and the cells were stained for T cell activation markers and analyzed by flow cytometry for the percentage of CD3pos CD8pos CFSElow CD25high cells. Percent suppression was calculated by dividing the frequency of CFSElow CD25high cells in the presence of MDSCs by the frequency of CFSElow CD25high cells in the presence of the control HLA-DRpos cells.
Statistical analysis
All graphical and statistical analyses were performed using Prism Software (Version 6, GraphPad Software, San Diego, CA, USA). Group means were compared using an unpaired two-tailed Student’s t test for two independent groups, unpaired t tests with Welch’s corrections for two independent groups with significant variance differences by F tests, paired two-tailed Student’s t test for multiple measurements in the same patient, one-way ANOVA for three or more groups and two-way ANOVA for three or more groups with multiple comparisons using the Holm–Sidak method to adjust for multiple comparisons. Correlations were evaluated using Pearson correlation coefficients and log-rank tests were used to evaluate overall survival data plotted in Kaplan–Meir curves. Receiver operating characteristic curves based on logistic regression (yes or no event) were used to define cutoffs for high frequencies of CD15pos MDSCs for overall survival comparisons. Cox proportional hazard regression models were used to obtain hazard ratios. Error bars represent the standard error of the mean (S.E.) and p values less than 0.05 were considered significant throughout this study.
Results
Different MDSC subsets are prominent in human spleen and peripheral blood samples
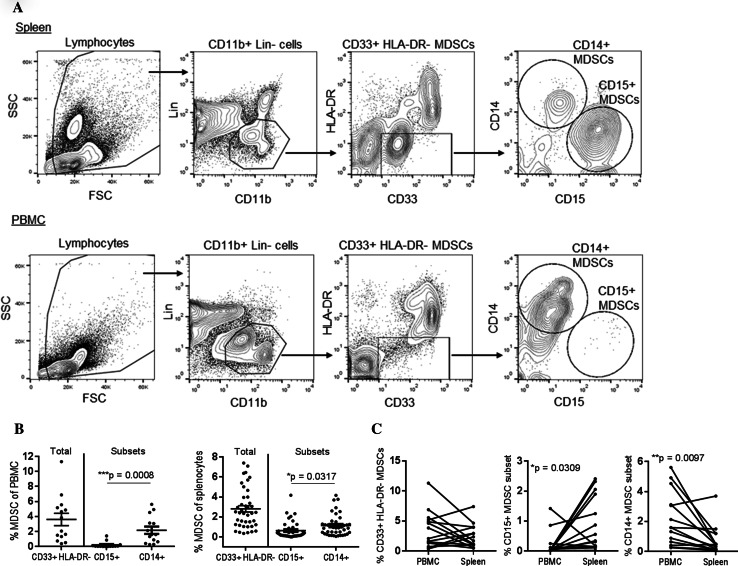
After compiling a repository of cryopreserved splenocytes and PBMC, we characterized the frequency of the following subsets of MDSCs in pathologically normal human spleen in all subjects in the study and in the peripheral blood of a subset of patients: total MDSCs [Linneg/low (CD3, CD19, CD56) CD11bpos CD33pos HLA-DRneg/low], granulocytic MDSCs [Linneg/low CD11bpos CD33pos HLA-DRneg/low CD14neg CD15pos], and monocytic MDSCs [Linneg/low CD11bpos CD33pos HLA-DRneg/low CD14pos CD15neg] (Fig. 1a). Both granulocytic and monocytic subsets of MDSCs were identified in human spleen tissue, with the monocytic CD14pos MDSC subset being the more abundant in both the spleen and peripheral blood (Fig. 1b). Compared to matched peripheral blood samples, the frequency of granulocytic CD15pos MDSCs was increased in the spleen (Fig. 1c, 0.21 ± 0.41 vs. 0.90 ± 0.24, p = 0.031), while the frequency of monocytic CD14pos MDSCs was increased in the peripheral blood (2.17 ± 0.48 v. 0.75 ± 0.26, p = 0.01). Most studies of human MDSCs have been conducted in the peripheral blood, however, we found no significant correlation between the frequencies of MDSCs in spleen tissue and peripheral blood (data not shown). Furthermore, frequencies of the MDSC subsets did not correlate with each other in either the peripheral blood or spleen (data not shown). Together, these data suggest there are differences in the distribution of granulocytic and monocytic MDSCs between the spleen and peripheral blood and that measured frequencies of MDSCs in the peripheral blood are likely not reflective of frequencies in the spleen in humans.
Fig. 1.
Myeloid-derived suppressor cell subsets identified in human spleen tissue. a Human splenocytes or PBMC from the same donor were stained with antibodies specific for Lineage markers (CD3, CD19, and CD56), CD11b, and CD33. The frequency of total MDSCs (Lin−, CD11b+, CD33+, HLA-DR−), CD15 + MDSCs (Lin−, CD11b+, CD33+, HLA-DR−, CD15+, CD14−), and CD14 + MDSCs (Lin−, CD11b+, CD33+, HLA-DR−, CD15−, CD14+) was determined by flow cytometry. b The frequency of MDSCs subsets in total PBMC or splenocytes was compared using ANOVA and compared between PBMC and spleen samples using a paired t test (c)
We next determined whether cryopreservation affected the frequency of MDSCs observed in the spleen in a subset of patient samples. Although the frequency of total MDSCs and CD14pos MDSCs did not significantly change after cryopreservation, the frequency of CD15pos MDSCs was significantly reduced (Supplemental Fig. 1). This result is consistent with previous literature showing that granulocytic MDSCs are more sensitive to cryopreservation than other MDSC subsets in the peripheral blood [23] and suggests that granulocytic MDSCs from the human spleen are similarly sensitive.
The frequency of MDSCs in the spleen is increased in cancer patients
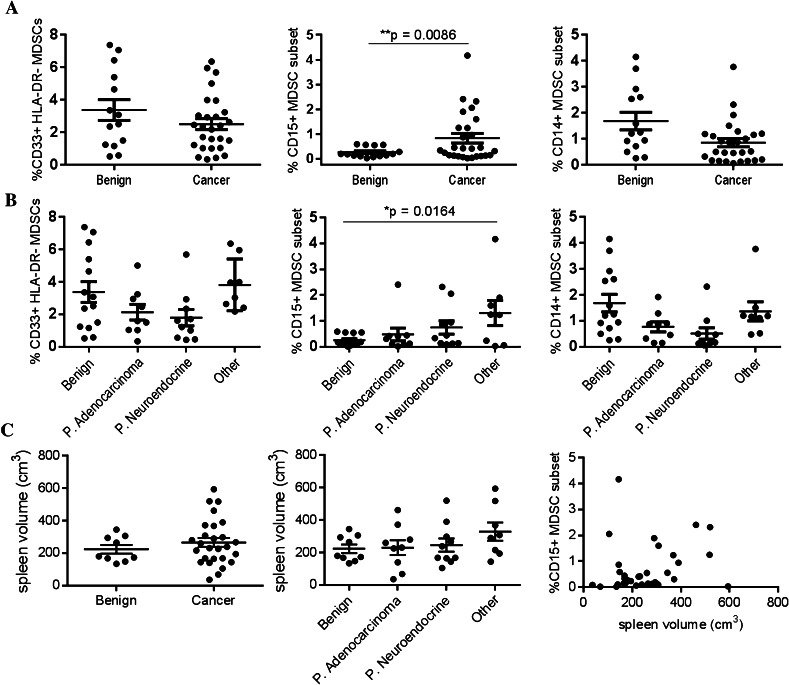
The frequency of MDSCs is increased in both the blood and spleen of tumor-bearing mice in several different tumor models [7]. Several groups have shown that MDSCs are also increased in the peripheral blood of human cancer patients [10, 11], but no studies have determined whether they are also increased in the spleens of cancer patients. Therefore, we compared the frequency of MDSCs in patients with various forms and stages of cancer. The frequency of total and CD14pos MDSCs was similar in patients with benign pancreatic cysts and those with various cancers (Fig. 2a). However, the frequency of CD15pos MDSCs was significantly increased in cancer patients compared to those with benign cysts (0.83 ± 0.19 vs. 0.26 ± 0.06, p = 0.009) and in patients diagnosed with advanced-stage cancer (Stage III and IV) compared to those with benign cysts (Supplemental Fig. 2, 1.02 ± 0.31 vs. 0.26 ± 0.06, p = 0.034). Interestingly, the frequency of CD15pos MDSCs was not increased in patients with pancreatic adenocarcinoma or neuroendocrine tumors, but was increased in patients with other types of cancer (colon adenocarcinoma, melanoma, ovarian cancer), potentially due to the known poor prognosis and the prevalence of advanced-stage disease in this group of patients (Fig. 2b). Unlike tumor-bearing mice, splenomegaly is not typically observed in patients with solid tumors and the increased frequency of CD15pos MDSCs was not associated with increased spleen volume (Fig. 2c). We also found that the frequency of MDSCs in human spleens was not associated with other clinical characteristics such as age at the time of surgery, body mass index, or gender (Supplemental Fig. 3).
Fig. 2.
The frequency of CD15 + MDSCs in human spleen tissue is increased in patients with cancer. a Human splenocytes were stained as in Fig. 1 and the frequency of MDSCs was compared between patients with cancer (see Table 1) and those with benign pancreatic lesions using Student’s t test. b The frequency of MDSCs was compared between different types of cancer by one-way ANOVA. c Three dimensional spleen volumes were calculated using length, width, and thickness measurements recorded after splenectomy and compared between patients with cancer, across cancer types, and across stages of disease
The frequency of granulocytic MDSCs in the spleens of cancer patients correlates with clinical outcomes
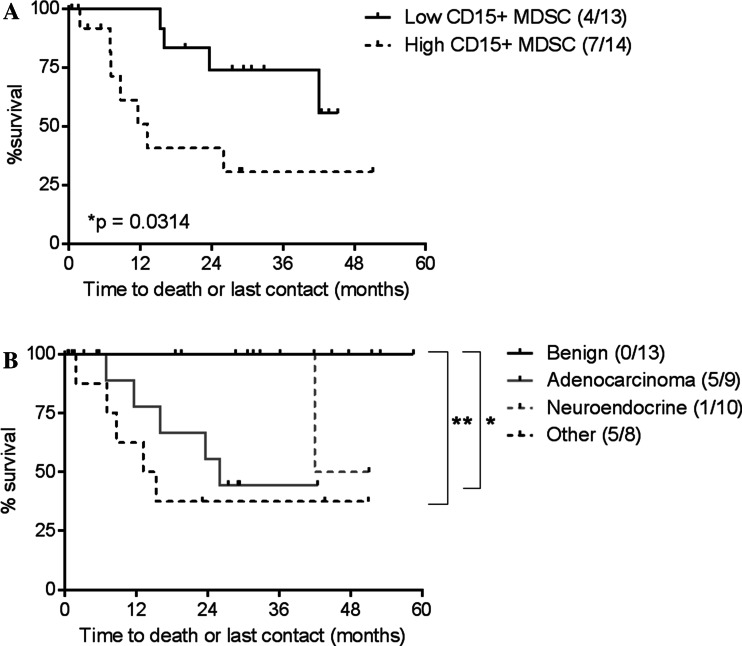
We next determined whether the increased frequency of granulocytic MDSCs in the spleens of cancer patients compared to those with benign cysts correlated with clinical outcomes. Using univariate analysis, we found that cancer patients with a low frequency of CD15pos MDSCs (<0.41%) had a significantly longer survival following surgical intervention compared to patients with a high frequency of MDSCs (p = 0.031, Fig. 3a). Patients with melanoma, ovarian cancer, and colon cancer (“other”) had the poorest survival compared to patients with benign cysts, consistent with their increased frequency of granulocytic MDSCs (Fig. 3b). Using a Cox regression model, we next determined that patients with a high frequency of CD15pos MDSCs in the spleen (≥0.41%) had a significantly increased risk of death (hazard ratio of 3.63, p = 0.043). The small number of patients in this study prevented reliable multivariate comparisons that consider other clinical factors.
Fig. 3.
A low frequency of CD15+ MDSCs correlates with increased overall survival. a Overall survival of cancer patients with a high (≥0.41%) or low (<0.41%) frequency of CD15+ MDSCs was plotted on a Kaplan–Meir survival curve and compared using a log-rank test. Outcomes of each group are shown in the legend (death events/total patients). b Overall survival of cancer patients diagnosed with benign cysts, pancreatic adenocarcinoma, pancreatic neuroendocrine, or other cancers (melanoma, ovarian, and colon cancer) were compared using a log-rank test. Outcomes of each group are shown in the legend (death events/total patients)
MDSCs isolated from human spleens are immunosuppressive
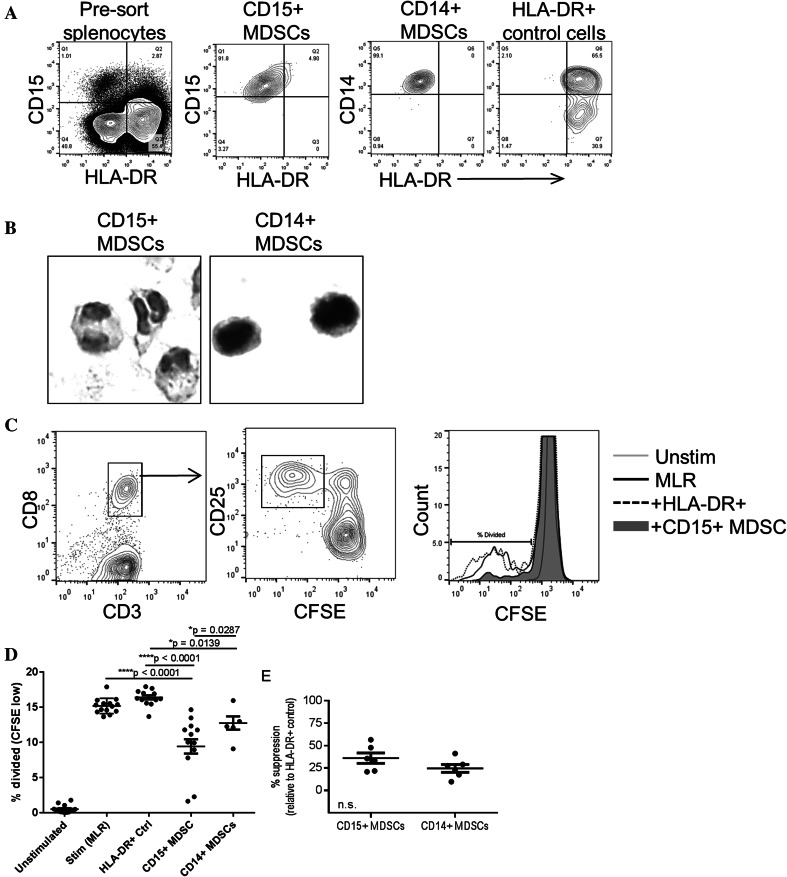
Although several phenotypic markers are used to define MDSCs by flow cytometry, ex vivo assays demonstrating the immunosuppressive capacity of these cells better define their function. Previous reports have shown that cryopreserved MDSCs lose suppressive function [23]. Therefore, we performed T cell suppression assays using freshly isolated splenocytes and analyzed the suppressive capacity of the CD15pos and CD14pos MDSC subsets. To obtain a large number of cells for T cell suppression assays, CD11bpos cells were first enriched by magnetic separation then stained with lineage markers (CD3, CD19, and CD56) and the phenotypic markers CD11b, CD15, CD14, and HLA-DR and separated by FACS. The purity of each MDSC subset was greater than 90% after enrichment by FACS (Fig. 4a). As a negative control for T cell suppression using the same number of myeloid cells in the assay, Linneg CD11bpos HLA-DRpos cells were also separated by FACS. The nuclear morphology of CD15pos and CD14pos MDSC subsets isolated from human spleen was then determined by H&E staining of paraffin-imbedded cells (Fig. 4b). The CD15pos cells were largely granulocytic while the CD14pos cells were largely monocytic, consistent with previous studies of human MDSCs isolated from peripheral blood samples [24, 25].
Fig. 4.
MDSCs isolated from human spleen tissue suppress T cell proliferation and activation. a The purity of CD15+, CD14+ MDSCs, and HLA-DR + control cells was determined after separation by FACS. b H&E stains of sorted CD15+ and CD14+ MDSCs imbedded in paraffin. c CFSE-labeled T cells were stimulated with allogenic dendritic cells in the presence or absence of HLA-DR+ control cells and MDSCs. After 4 days, cells were stained with antibodies specific for CD3, CD8, and CD25 and the frequency of divided (CD25+ CFSElow) CD8 T cells was determined by flow cytometry. The histogram depicts CD3+ CD8+ cells. d The percentage of divided T cells in the presence of HLA-DR+ control cells and MDSCs was determined as in c and compared using one-way ANOVA (***p < 0.0001), p values shown are adjusted for multiple comparisons across groups. e The percent suppression of proliferation by CD15+ or CD14+ MDSCs was determined for each sample relative to HLA-DR+ cells and compared using a t test (no significant difference) in patient samples with CD14 functional analysis
To determine the functional capacity of splenic MDSCs, CFSE-labeled T cells isolated from spleens of the same patients as the MDSCs were stimulated in an MLR using monocyte-derived allogeneic dendritic cells from a healthy donor. The frequency of T cells that proliferated after stimulation (CD25high CFSElow) was determined in the presence and absence of each MDSC subset or the control HLA-DRpos cells (Fig. 4c, d). The average amount of T cell proliferation was decreased in the presence of both MDSC subsets relative to stimulation alone and with added HLA-DRpos control cells (Fig. 4d). Although there was significantly less T cell division in the presence of CD15pos MDSCs compared to CD14pos MDSCs (Fig. 4d), the amount of immunosuppression was not significantly different between these subsets when the data was normalized to the amount of proliferation in control wells in each patient (Fig. 4e). Although this study was too small to perform statistical analysis on the association of MDSC function and cancer status, one or more subsets of MDSCs isolated from each spleen suppressed T cell responses (Table 2). Surprisingly, the variable amount of immunosuppression was not associated with cancer diagnosis, stage of disease, or tumor type (Table 2). These data demonstrate that the phenotypic markers used to identify MDSCs in human peripheral blood samples also identify suppressive MDSCs in human spleen.
Table 2.
MDSCs isolated from the spleens of both cancer patients and those with benign cysts suppress T cell activation and proliferation
| Patient tumor type | Stage | % Suppressiona | |
|---|---|---|---|
| CD15+ | CD14+ | ||
| Benign p. cyst | n/a | 55.0 | n/a |
| Benign p. cyst | n/a | 89.7 | n/a |
| Benign p. cyst | n/a | 47.8 | 17.5 |
| P. adenocarcinoma | I | 40.6 | n/a |
| P. adenocarcinoma | I | 13.9 | n/a |
| P. neuroendocrine tumor | I | 21.8 | 28.9 |
| P. neuroendocrine tumor | I | 33.3 | 9.75 |
| P. neuroendocrine tumor | Ib | 40.6 | n/a |
| P. neuroendocrine tumor | IIb | 8.63 | n/a |
| P. neuroendocrine tumor | IV | 33.3 | 9.75 |
| P. neuroendocrine tumor | IV | 85.9 | n/a |
| Ovarian cancer | IV | 21.8 | 28.9 |
| Melanoma | IV | 35.6 | 41.1 |
| Colon adenocarcinoma | IIIb | 47.3 | n/a |
aCFSE-labeled T cells were stimulated with allogenic dendritic cells in the presence of HLA-DR+ control cells or MDSCs. After 4 days, cells were stained with antibodies specific for CD3, CD8, and CD25 and the frequency of divided (CFSE low) CDS T cells was determined by flow cytometry. The percent suppression of proliferation was determined for each sample relative to HLA-DR+ cells
Discussion
Using the unique resources available at the University of Colorado Hospital, we compared, for the first time, the frequency and function of MDSC subsets isolated from the human spleen. In comparing human peripheral blood and spleen, we found that the most abundant MDSC population in both the spleen and peripheral blood was the CD14pos monocytic MDSC subset, which was 2–3 fold enriched in the peripheral blood compared to the spleen. In contrast, the frequency of CD15pos granulocytic MDSCs was twofold increased in the spleen relative to the peripheral blood. These data are consistent with previous studies demonstrating that the spleen acts as a reservoir for granulocytic MDSCs in tumor-bearing mice [26, 27] and indicates that the spleen may be a site of their accumulation in humans. In further support of this hypothesis, the frequency of CD15pos granulocytic MDSCs in the spleen did not correlate with the frequencies observed in the peripheral blood. Together, our study demonstrates that the previously described subsets of MDSCs can be identified in the human spleen and that these subsets may vary in trafficking and accumulation patterns.
The diverse patient population in this study afforded us the opportunity to analyze the frequency of MDSCs in spleen tissue from patients with various types of cancer and stages of disease. A caveat to this and many other studies of human MDSCs is that we analyzed the frequency of MDSCs in frozen splenocyte preparations. Similar to the results in studies of human MDSCs isolated from peripheral blood [23], we found that cryopreservation greatly reduced the frequency of splenic CD15pos granulocytic MDSCs. Whether granulocytic MDSCs undergo cell death or have reduced CD15 expression after cryopreservation is not well established and not directly addressed in this study. However, the frequency of total MDSCs was not decreased following cryopreservation, suggesting that the granulocytic MDSCs may have lost expression of surface markers. Despite the highly selected patient population and the sensitivity of these cells to cryopreservation, we found a statistically significant increase in the frequency of granulocytic MDSCs in the spleens of patients with cancer. However, this increase was modest compared to previous studies in tumor-bearing mice [7, 8]. Highlighting the biologic differences between human and murine spleen, hematopoiesis is limited in human spleen and patients with solid tumors rarely exhibit splenomegaly [12, 28]. These factors may contribute to the lower frequency of MDSCs observed in human spleens compared to tumor-bearing mice.
A recent study demonstrated increased frequencies of CD15pos MDSCs in the peripheral blood of patients with resectable pancreatic adenocarcinoma [19]. We did not find an increased frequency of MDSCs in the spleen tissue of patients with either pancreatic adenocarcinoma or pancreatic neuroendocrine tumors. However, increased frequencies of MDSCs are first observed in the blood and mesenteric lymph nodes of mice bearing spontaneous pancreatic cancer and do not accumulate in the spleens until later in tumor development [13]. Therefore, changes in the frequency of splenic MDSCs may not be apparent in patients with resectable pancreatic cancer at earlier stages of disease and the small number of patients with advanced pancreatic cancer in this study may have limited the observed frequencies of MDSCs in the pancreatic cancer cases presented here (2/9 and 3/10 in the adenocarcinoma and neuroendocrine groups, respectively). In support of this hypothesis, we found that CD15pos MDSCs were increased in the spleens of patients with advanced cancer compared to those with benign cysts, and in patients with colon adenocarcinoma, melanoma, and ovarian cancer, all of which had advanced disease at the time of surgery. Furthermore, we found that an increased frequency of splenic CD15pos MDSCs was associated with decreased overall survival. Although this finding would need to be corroborated in a larger cohort in which multiple clinical variables could be considered, these results suggests that accumulation of CD15pos MDSCs in the spleen may be a prognostic indicator in cancer patients undergoing splenectomy.
Other studies have shown an association of peripheral MDSCs with age [29] and obesity [30, 31], risk factors associated with poorer outcomes in pancreatic cancer patients [32]. We were unable to correlate the frequency of MDSC in the spleen with these clinical characteristics. These results indicate that age and obesity-associated increases in peripheral MDSCs may be independent of spleen tissue, possibly a result of increased hematopoiesis in the bone marrow or increased mobilization to the peripheral blood rather than increased extramedullary hematopoiesis or accumulation in the spleen [8, 26].
Our functional experiments demonstrated that both subsets of MDSCs suppressed T cell proliferation and activation. However, this immunosuppressive function was not associated with cancer diagnosis, type, or stage (Table 2). These results contrast with those in mice, in which MDSCs home to the spleen and acquire suppressive function only after exposure to the environment of a tumor-bearing host [33, 34]. These results also contrast with studies of MDSCs isolated from human peripheral blood, in which MDSCs isolated from cancer patients have more immunosuppressive activity than those isolated from healthy donors [10, 35, 36]. These data suggest that some immunosuppressive MDSC subsets may be found in the human spleen regardless of the cancer status of the patient and that there may be differences in the suppressive function of MDSCs isolated from human spleen and peripheral blood. Alternatively, physiologic or genetic alterations associated with benign cysts may alter the function of splenic MDSCs on the local level, making MDSCs isolated from these patients uncharacteristic of true healthy donors.
Studies of human MDSCs are often limited by their rarity in the peripheral blood, making large scale studies challenging. The human spleen may provide a plentiful source of MDSC’s for functional assays and in-depth characterization. Despite the large number of cells available in the human spleen, there are several limitations to this study. Although we processed the spleen samples immediately upon arrival in the laboratory, the amount of exposure to general anesthetic, related to the length of each operation, and the ischemic time, related to when the splenic blood supply was severed prior to tissue removal, were variable between patients and not controlled for in this study. The variability of stages represented in our patient population and their overall “health” as a highly selected patient population deemed fit for surgical resection may have limited our ability to detect a concomitant increase in the frequency of monocytic MDSCs in this study, as has also been reported in tumor-bearing mice [27]. In addition, the size of this study limited our ability to perform multivariate survival analysis and to statistically analyze the differences in immunosuppressive function between MDSC subsets and groups of patients with benign disease or cancer.
In summary, this study demonstrates that two commonly described subsets of immunosuppressive MDSCs can be identified in the human spleen, providing a comparison to murine spleen tissue and human peripheral blood samples for future reference.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by funding provided by the University of Colorado Cancer Center Support Grant (P30CA046934), American Cancer Society 2012 Roaring Fork Valley Postdoctoral Research Award, and the Conner Family Foundation Grant.
Abbreviations
- CFSE
Carboxyfluorescein succinimidyl ester
- DMSO
Dimethyl sulfoxide
- H&E
Hematoxylin and Eosin
- MDSC
Myeloid-derived suppressor cell
- MLR
Mixed-lymphocyte reaction
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 2.Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van der Burg SH, Welters MJ, Walter S. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65(2):161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 4.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, Rodriguez PC, Sica A, Umansky V, Vonderheide RH, Gabrilovich DI. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. doi: 10.1038/ncomms12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serafini P. Myeloid derived suppressor cells in physiological and pathological conditions: the good, the bad, and the ugly. Immunol Res. 2013;57(1–3):172–184. doi: 10.1007/s12026-013-8455-2. [DOI] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 10.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother. 2013;62(11):1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60(10):1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiniger BS. Human spleen microanatomy: why mice do not suffice. Immunology. 2015;145(3):334–346. doi: 10.1111/imm.12469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao F, Obermann S, von Wasielewski R, Haile L, Manns MP, Korangy F, Greten TF. Increase in frequency of myeloid-derived suppressor cells in mice with spontaneous pancreatic carcinoma. Immunology. 2009;128(1):141–149. doi: 10.1111/j.1365-2567.2009.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67(19):9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 15.Goedegebuure P, Mitchem JB, Porembka MR, Tan MC, Belt BA, Wang-Gillam A, Gillanders WE, Hawkins WG, Linehan DC. Myeloid-derived suppressor cells: general characteristics and relevance to clinical management of pancreatic cancer. Curr Cancer Drug Targets. 2011;11(6):734–751. doi: 10.2174/156800911796191024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghansah T, Vohra N, Kinney K, Weber A, Kodumudi K, Springett G, Sarnaik AA, Pilon-Thomas S. Dendritic cell immunotherapy combined with gemcitabine chemotherapy enhances survival in a murine model of pancreatic carcinoma. Cancer Immunol Immunother. 2013;62(6):1083–1091. doi: 10.1007/s00262-013-1407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61(12):4756–4760. [PubMed] [Google Scholar]
- 18.Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States) Cancer Causes Control. 2006;17(4):403–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 19.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, Gillanders WE, Linehan DC, Goedegebuure P. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother. 2012;61(9):1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 21.Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. 2009;9(7–8):900–909. doi: 10.1016/j.intimp.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2013;62(2):225–236. doi: 10.1007/s00262-012-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. 2012;381(1–2):14–22. doi: 10.1016/j.jim.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res. 2012;18(19):5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11(7):816–826. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman MH, Saunders EF. Hematopoiesis in the human spleen. Am J Hematol. 1981;11(3):271–275. doi: 10.1002/ajh.2830110307. [DOI] [PubMed] [Google Scholar]
- 29.Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, Wang J, Mountz JD, Zhang HG. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev. 2007;128(11–12):672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Bao Y, Mo J, Ruan L, Li G. Increased monocytic CD14(+)HLADRlow/- myeloid-derived suppressor cells in obesity. Mol Med Rep. 2015;11(3):2322–8. doi: 10.3892/mmr.2014.2927. [DOI] [PubMed] [Google Scholar]
- 31.Xia S, Sha H, Yang L, Ji Y, Ostrand-Rosenberg S, Qi L. Gr-1 + CD11b + myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J Biol Chem. 2011;286(26):23591–23599. doi: 10.1074/jbc.M111.237123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasenda B, Bass A, Koeberle D, Pestalozzi B, Borner M, Herrmann R, Jost L, Lohri A, Hess V. Survival in overweight patients with advanced pancreatic carcinoma: a multicentre cohort study. BMC Cancer. 2014;14:728. doi: 10.1186/1471-2407-14-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol. 2003;74(2):186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b + Gr-1 + myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181(5):3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 35.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14(24):8270–8278. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 36.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, Zanovello P. IL4Ralpha + myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182(10):6562–6568. doi: 10.4049/jimmunol.0803831. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.