Abstract
Context
Heparin binding epidermal growth factor like growth factor (HB-EGF) is an emerging therapeutic for the regeneration of the tympanic membrane.
Objective
Our aim was to determine whether the doses of HB-EGF delivered in a sustained release hydrogel into a middle ear mouse model, would be measurable in the systemic circulation. We also aimed to observe, in the scenario that the intended dose was absorbed directly into the circulation, whether these levels could be measured above the background levels of HB-EGF in the circulation.
Methods
A total of 12 mice had trans tympanic injections of 5ug/ml of HB-EGF contained within a previously described novel hydrogel vehicle, while another 12 mice had intravenous delivery of 10ug/kg of HB-EGF. Intravenous blood samples were collected at 0, 3, 24, 168, 288 and 720 hours post injection. A double-antibody sandwich one-step process enzyme-linked immunosorbent assay (ELISA) was used to determine the level of HB-EGF in the serum.
Results
No mice in the trans tympanic administration group and no mice in the intravenous administration group were found to have blood level measured above that in the controls.
Discussion
The inability of the positive control to measure levels above background, suggest the total dose used in our studies, even if 100% absorbed into the system circulation is insignificant.
Conclusions
HB-EGF at the doses and delivery method proposed for treatment of chronic TM perforation in a mouse model are likely to have no measurable systemic effect.
Keywords: Tympanic Membrane, Regeneration, Tympanic perforation
Introduction
Heparin binding epidermal growth factor like growth factor (HB-EGF) has recently been identified as having a role in tympanic membrane (TM) wound healing and as a potential treatment for chronic TM perforations.(Santa Maria et al., Santa Maria et al., 2015) If successful, non-surgical treatments would be able to provide for a large area of unmet need in treating chronic TM perforation and chronic suppurative otitis media. So far such treatments have failed to demonstrate superiority to surgery or to be available for the developing world.(Santa Maria and Oghalai, 2014, Hong et al., 2013)
Before proceeding to human clinical trials, the safety of this treatment must be demonstrated. HB-EGF has been shown to be non-ototoxic in a mouse model with no long term negative effects on hearing. (Santa Maria et al., 2015) In the same model it was also shown that there were no observable irreversible histological changes in the middle ear following treatment. (Santa Maria et al., 2015)
Although growth factor treatment in the ear has never been tested for systemic exposure, this remains a risk of this therapy, especially in sustained release formulas. Our aim was to determine whether the doses delivered in a sustained release hydrogel used to treat chronic TM perforations, in a mouse model, would be measurable in the systemic circulation. We also aimed to observe, in the scenario that the intended dose was directly absorbed into the circulation, whether these levels were significant enough to be measured above the background levels of HB-EGF in the circulation.
Methods
Stanford's Administrative Panel on Laboratory Animal Care approved all animal work. Mice used for all experiments were 6-10 week old male CBA/CAJ (15-25g) mice purchased from Jackson Laboratories (Florida, USA). All otoscopic and surgical interventions were performed using inhaled isoflurane at 3-4% for induction and 1-2% for maintenance. All intravenous blood sampling was done to collect 0.05ml of blood. Blood sampling was divided amongst the cohort so that no more than 2ml of blood was sampled from an individual mouse in one week unless the mouse was sacrificed immediately after sampling.
Topical administration group
A total of 12 mice had trans tympanic injections of HB-EGF contained within a previously described novel hydrogel vehicle. (Santa Maria et al., 2015). Briefly, this polymer was developed in and provided by the Department of Orthopedic Surgery at Stanford University and contains a novel combination of chitosan, polylactide and fibrinogen with sodium metabisulfide as a crosslinking agent. (Santa Maria et al., 2015) Proheparin-Binding EGF-like Growth Factor (HB-EGF) was purchased from Prospec (New Jersey, USA, catalogue number CYT-068) at delivered via the hydrogel at 5ug/ml with a designed degradation time of 30 days. The hydrogel is designed to release the HB-EGF at a steady state concentration over these 30 days. A total volume of 0.4ml is delivered trans canal via a 27 gauge needle with the first amounts delivered trans tympanic and the excess allowed to fill into the external auditory canal. Intravenous blood samples were collected at 0 (immediately after trans canal injection), 3, 24, 168, 288 and 720 hours post injection. Each time point had a total of three animal samples with each sample being tested in duplicate. This provided six samples per time point for testing from three individual animals.
Intravenous administration group
A total of 12 mice had intravenous delivery of 10ug/kg of HB-EGF (Proheparin-Binding EGF-like Growth Factor (HB-EGF) was purchased from Prospec (New Jersey, USA, catalogue number CYT-068)) Intravenous blood samples were collected at 0 (immediately after intravenous injection), 3, 24, 168, 288 and 720 hours post injection. Each time point had a total of three animal samples with each sample being tested in duplicate. This provided six samples per time point for testing from three individual animals. Five mice had intravenous sampling of blood prior to injection of HB-EGF and served as negative controls. Each was repeated in duplicate to give a total of 10 samples. Another three mice had intravenous sampling of blood with the HB-EGF directly added to the vial after collection to act as a positive control. Each was repeated in duplicate to give a total of six samples.
Enzyme linked immunosorbent assay
A commercially available double-antibody sandwich enzyme-linked immunosorbent one-step process assay was used to determine the level of HB-EGF in the serum samples according to manufacturer's instructions (MyBioSource, San Diego, USA, catalogue number MBS811617). From each animal approximately 0.05 ml of blood was collected into a non-pyrogenic and endotoxin tube and centrifuged at 3000 rpm for 20 min. All samples were assayed in duplicate. The optical density of each sample was determined using a microplate reader at 450 nm (TECAN Infinite F50). The concentration of HB-EGF was expressed as pg/ml.
Results
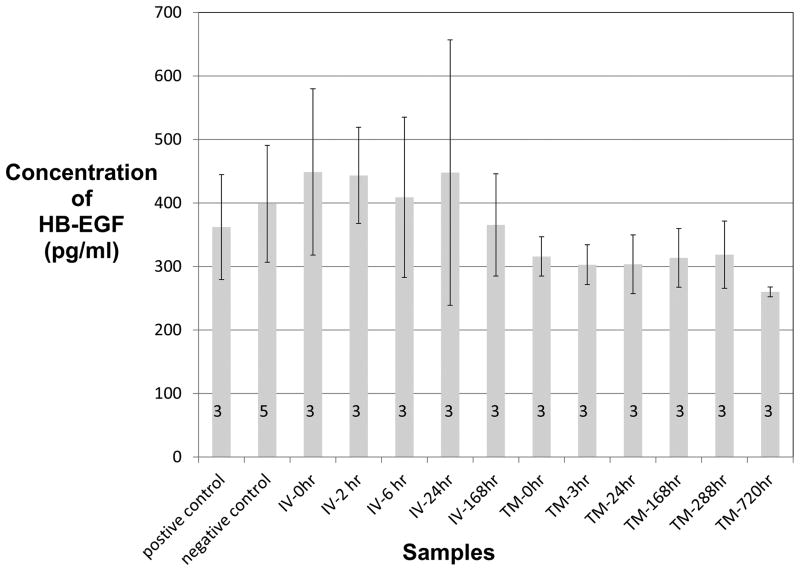
The systemic blood levels of HB-EGF, as measured by ELISA, are summarized in Figure 1. The positive control (362.1±82.5 pg/ml) did not measure blood levels above that of the negative control (398.8±92.1 pg/ml).
Figure 1.
Figure 1 demonstrates the blood levels of HB-EGF as detected by ELISA following 5ug/ml trans tympanic delivery and 10ug/kg IV delivery. The negative control represents blood levels measured without any additional HB-EGF exposure. The positive control represents directly injecting 10ug/kg to a blood sample after collection from a non-HB-EGF treated mouse. The number of animal samples used per time point is indicated on each column. HB-EGF (heparin binding epidermal growth factor like growth factor), IV (intravenous), ELISA (enzyme-linked immunosorbent assay)
Trans tympanic administration group
Mean HB-EGF levels of 3 mice in the trans tympanic administration group were lower than those of 5 mice in the negative control and those in the positive control. The trans tympanic administration group measured mean levels of 315.71±31 at 0 hrs, 302.8±31.4 at 3hrs, 303.5±46.4 at 24hrs, 313.5±46.3 at 168hrs, 318.6±52.8 at 288hrs and 260±7.8 at 720 hrs, respectively. No mice in the trans tympanic administration groups were found to have blood level measured above that in the control groups.
Intravenous administration group
The intravenous administration group measured mean levels of 448.8±130.9 at 0 hrs, 443.3±75.7 at 2hrs, 409±126.2 at 6hrs, 447.9±208.9 at 24hrs, and 365.3±80.6 at 168hrs, respectively. No mice in the intravenous administration group were found to have blood level measured above that in the negative and positive controls.
Discussion
The recent discovery of HB-EGF as a potential treatment for chronic TM perforations requires demonstration of its safety before progressing to further investigation. HB-EGF has not previously been studied as a therapy in the ear. HB-EGF has shown a lack of detectable effects on hearing as measured by auditory brain response and distortion production otoacoutic emissions measured at different time points after transtympanic exposure. (Santa Maria et al., 2015) It has been suggested that HB-EGF may be a possible mediator of inflammation in otitis media. (Suzukawa et al., 2014) HB-EGF however is more likely to be a consequence of inflammation within the middle ear with its key role identified in tympanic membrane wound healing. (Santa Maria et al.) When middle ears are exposed to very high doses of HB-EGF there are no measurable histological after effects. (Santa Maria et al., Santa Maria et al., 2015) The other concern for growth factor treatments for the tympanic membrane is the potential to induce cholesteatoma or malignancy formation, but this is yet to be observed in any animal or human studies of tympanic membrane growth factor treatment. (Acharya et al., 2015, Hakuba et al., 2010, Hakuba et al., 2014, Hakuba et al., 2003, Kanemaru et al., 2011, Ramalho and Bento, 2006, Ma et al., 2002, Dvorak et al., 1995, Santa Maria et al., 2015) Based on all available research to date, the application of trans tympanic HB-EGF is safe to the local tissues. A phase one clinical trial will be needed to definitively answer this question. Conversely HB-EGF TM regeneration has not shown to have any protective effects on hearing outside of the wound healing mechanism previously described. (Santa Maria et al., 2015). HB-EGF in inner ear hair cell regeneration remains undefined. (Ronaghi et al., 2012)
The next step in the determination of the safety of HB-EGF treatment for chronic TM perforations is to investigate for systemic absorption. HB-EGF is an endogenous growth factor produced within many tissues including the gastrointestinal tract, reproductive system, urinary tract, lung, muscle, brain and heart and as such would not expect to have any systemic toxicity at normal physiological levels. (Nakagawa et al., 1997, Murayama et al., 1995, Abraham et al., 1993, Ross, 1986) HB-EGF is synthesized in the body as a transmembrane, active precursor protein which is converted by matrix metalloproteinases to form an active soluble growth factor.(Higashiyama et al., 1992) Its tissue distribution and plasma clearance in neonatal and adult rats has been studied. After intravenous administration, the plasma clearance of HB-EGF is biphasic. It has a distribution half-life of 0.8 min and an elimination half-life of 26.67 min. Most of its distribution shortly after administration is within the plasma, liver, kidneys, bile, and urine.(Feng et al., 2006, Yang et al., 2014) The rapid decline of HB-EGF in plasma is dues to a rapid irreversible uptake process. (Burwen et al., 1985, Dunn and Hubbard, 1984, Kim et al., 1988, St Hilaire et al., 1983)Given its rapid distribution and elimination, it is not surprising that the small doses used in our study, even if delivered intravenously, cannot be detected above background levels. The therapeutic time window of this delivery is four weeks duration and there were no measured levels above that of the background levels at any time point during the therapeutic window in our study. Low concentrations following sustained delivery via TM administration support our hypothesis that this is a safe dose and method of administration in terms of potential systemic effects. The inability of the positive control to measure levels above background, suggest the total dose used in our studies, even if 100% absorbed into the system circulation is insignificant. Histological tissue examination of various organs had previously shown no detectable systemic toxicity following intravenous HB-EGF administration of 30ug/kg in a rat model. (Coowanitwong et al., 2008) HB-EGF delivered trans tympanic is likely safe in terms of systemic effects.
Conclusion
HB-EGF at the doses and delivery method proposed for treatment of chronic TM perforation, trans tympanic administration does not raise above background levels and is likely to have no measureable systemic effect.
Acknowledgments
Garnett Passe and Rodney Williams Memorial Foundation
Stanford's SPARK
Stanford Child Health Research Institute
Wallace H. Coulter Foundation
NIH R01AR057837 (NIAMS)
NIH R01DE021468 (NIDCR)
DOD W81XWH-10-1-0966 (PRORP)
Footnotes
Declaration of Interest: The authors disclose the potential conflict of interest: Filed patents: U.S. Non Provisional No. 61/823,749, April 2014, P Santa Maria, S Kim, Y Yang, U.S. Non Provisional No. 61/810,101, April 2014, S Kim, Y Yang, Founding stock in Auration biotech: P Santa Maria
References
- Abraham JA, Damm D, Bajardi A, Miller J, Klagsbrun M, Ezekowitz RA. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun. 1993;190:125–33. doi: 10.1006/bbrc.1993.1020. [DOI] [PubMed] [Google Scholar]
- Acharya AN, Coates H, Tavora-Vieira D, Rajan GP. A pilot study investigating basic fibroblast growth factor for the repair of chronic tympanic membrane perforations in pediatric patients. Int J Pediatr Otorhinolaryngol. 2015;79:332–5. doi: 10.1016/j.ijporl.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Burwen SJ, Barker ME, Goldman IS, Jones AL. The effect of concentration on hepatic transport of exogenous epidermal growth factor. Hepatology. 1985;5:211–4. doi: 10.1002/hep.1840050209. [DOI] [PubMed] [Google Scholar]
- Coowanitwong I, Keay SK, Natarajan K, Garimella TS, Mason CW, Grkovic D, Bauer KS. Toxicokinetic study of recombinant human heparin-binding epidermal growth factor-like growth factor (rhHB-EGF) in female Sprague Dawley rats. Pharm Res. 2008;25:542–50. doi: 10.1007/s11095-007-9392-3. [DOI] [PubMed] [Google Scholar]
- Dunn WA, Hubbard AL. Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: ligand and receptor dynamics. J Cell Biol. 1984;98:2148–59. doi: 10.1083/jcb.98.6.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak DW, Abbas G, Ali T, Stevenson S, Welling DB. Repair of chronic tympanic membrane perforations with long-term epidermal growth factor. Laryngoscope. 1995;105:1300–4. doi: 10.1288/00005537-199512000-00007. [DOI] [PubMed] [Google Scholar]
- Feng J, Mehta VB, El-Assal ON, Wu D, Besner GE. Tissue distribution and plasma clearance of heparin-binding EGF-like growth factor (HB-EGF) in adult and newborn rats. Peptides. 2006;27:1589–96. doi: 10.1016/j.peptides.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Hakuba N, Iwanaga M, Tanaka S, Hiratsuka Y, Kumabe Y, Konishi M, Okanoue Y, Hiwatashi N, Wada T. Basic fibroblast growth factor combined with atelocollagen for closing chronic tympanic membrane perforations in 87 patients. Otol Neurotol. 2010;31:118–21. doi: 10.1097/MAO.0b013e3181c34f01. [DOI] [PubMed] [Google Scholar]
- Hakuba N, Tabata Y, Hato N, Fujiwara T, Gyo K. Gelatin hydrogel with basic fibroblast growth factor for tympanic membrane regeneration. Otol Neurotol. 2014;35:540–4. doi: 10.1097/MAO.0000000000000200. [DOI] [PubMed] [Google Scholar]
- Hakuba N, Taniguchi M, Shimizu Y, Sugimoto A, Shinomori Y, Gyo K. A new method for closing tympanic membrane perforations using basic fibroblast growth factor. Laryngoscope. 2003;113:1352–5. doi: 10.1097/00005537-200308000-00016. [DOI] [PubMed] [Google Scholar]
- Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–12. [PubMed] [Google Scholar]
- Hong P, Bance M, Gratzer PF. Repair of tympanic membrane perforation using novel adjuvant therapies: a contemporary review of experimental and tissue engineering studies. Int J Pediatr Otorhinolaryngol. 2013;77:3–12. doi: 10.1016/j.ijporl.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Kanemaru S, Umeda H, Kitani Y, Nakamura T, Hirano S, Ito J. Regenerative treatment for tympanic membrane perforation. Otol Neurotol. 2011;32:1218–23. doi: 10.1097/MAO.0b013e31822e0e53. [DOI] [PubMed] [Google Scholar]
- Kim DC, Sugiyama Y, Satoh H, Fuwa T, Iga T, Hanano M. Kinetic analysis of in vivo receptor-dependent binding of human epidermal growth factor by rat tissues. J Pharm Sci. 1988;77:200–7. doi: 10.1002/jps.2600770304. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhao H, Zhou X. Topical treatment with growth factors for tympanic membrane perforations: progress towards clinical application. Acta Otolaryngol. 2002;122:586–99. doi: 10.1080/000164802320396259. [DOI] [PubMed] [Google Scholar]
- Murayama Y, Miyagawa J, Higashiyama S, Kondo S, Yabu M, Isozaki K, Kayanoki Y, Kanayama S, Shinomura Y, Taniguchi N, et al. Localization of heparin-binding epidermal growth factor-like growth factor in human gastric mucosa. Gastroenterology. 1995;109:1051–9. doi: 10.1016/0016-5085(95)90562-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Hayase Y, Sasahara M, Haneda M, Kikkawa R, Higashiyama S, Taniguchi N, Hazama F. Distribution of heparin-binding EGF-like growth factor protein and mRNA in the normal rat kidneys. Kidney Int. 1997;51:1774–9. doi: 10.1038/ki.1997.244. [DOI] [PubMed] [Google Scholar]
- Ramalho JR, Bento RF. Healing of subacute tympanic membrane perforations in chinchillas treated with epidermal growth factor and pentoxifylline. Otol Neurotol. 2006;27:720–7. doi: 10.1097/01.mao.0000226316.04940.f9. [DOI] [PubMed] [Google Scholar]
- Ronaghi M, Nasr M, Heller S. Concise review: Inner ear stem cells--an oxymoron, but why? Stem Cells. 2012;30:69–74. doi: 10.1002/stem.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986;314:488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Santa Maria PL, Oghalai JS. Is office-based myringoplasty a suitable alternative to surgical tympanoplasty? Laryngoscope. 2014;124:1053–4. doi: 10.1002/lary.24221. [DOI] [PubMed] [Google Scholar]
- Santa Maria PL, Redmond SL, Atlas MD, Ghassemifar R. The role of epidermal growth factor in the healing tympanic membrane following perforation in rats. J Mol Histol. 2010;41:309–14. doi: 10.1007/s10735-010-9287-1. [DOI] [PubMed] [Google Scholar]
- Santa Maria PL, Varsak KY, Kim S, Yang YP. Heparin Binding – Epidermal Growth Factor Like Growth Factor for Regeneration of Chronic Tympanic Membrane Perforations in Mice. Tissue Eng Part A. 2015;21:1483–94. doi: 10.1089/ten.tea.2014.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Hilaire RJ, Hradek GT, Jones AL. Hepatic sequestration and biliary secretion of epidermal growth factor: evidence for a high-capacity uptake system. Proc Natl Acad Sci U S A. 1983;80:3797–801. doi: 10.1073/pnas.80.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K, Tomlin J, Pak K, Chavez E, Kurabi A, Baird A, Wasserman SI, Ryan AF. A mouse model of otitis media identifies HB-EGF as a mediator of inflammation-induced mucosal proliferation. PLoS ONE. 2014;9:e102739. doi: 10.1371/journal.pone.0102739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Su Y, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) therapy for intestinal injury: Application and future prospects. Pathophysiology. 2014;21:95–104. doi: 10.1016/j.pathophys.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]