Abstract
Introduction
Depression and diabetes are highly prevalent worldwide and often co-exist, worsening outcomes for each condition. Barriers to diagnosis and treatment are exacerbated in low and middle-income countries with limited health infrastructure and access to mental health treatment. The INtegrating DEPrEssioN and Diabetes treatmENT (INDEPENDENT) study tests the sustained effectiveness and cost-effectiveness of a multi-component care model for individuals with poorly-controlled diabetes and depression in diabetes clinics in India.
Materials and Methods
Adults with diabetes, depressive symptoms (Patient Health Questionnaire-9 score ≥10), and ≥1 poorly-controlled cardiometabolic indicator (either HbA1c ≥8.0%, SBP ≥140mmHg, and/or LDL ≥130mg/dl) were enrolled and randomized to the intervention or usual care. The intervention combined collaborative care, decision-support, and population health management. The primary outcome is the between-arm difference in the proportion of participants achieving combined depression response (≥50% reduction in Symptom Checklist score from baseline) AND one or more of: ≥0.5% reduction in HbA1c, ≥5 mmHg reduction in SBP, or ≥10 mg/dl reduction in LDL-c at 24 months (12-month intervention; 12-month observational follow-up). Other outcomes include control of individual parameters, patient-centered measures (i.e. treatment satisfaction), and cost-effectiveness.
Results
The study trained seven care coordinators. Participant recruitment is complete – 940 adults were screened, with 483 eligible, and 404 randomized (196 to intervention; 208 to usual care). Randomization was balanced across clinic sites.
Conclusions
The INDEPENDENT model aims to increase access to mental health care and improve depression and cardiometabolic disease outcomes among complex patients with diabetes by leveraging the care provided in diabetes clinics in India (clinicaltrials.gov number: NCT02022111).
Keywords: depression, diabetes, collaborative care, decision support, healthcare delivery
INTRODUCTION
Depression and diabetes are highly prevalent conditions that often co-exist.1 These conditions interact with one another, with bidirectional negative impact on outcomes.2–7 Depression increases the risk of type 2 diabetes, adversely affects diabetes self-care, worsens glycemic control, and lowers quality of life, while diabetes is associated with increased risk of developing depression. 2,5,8–12 When cardio-metabolic and mental illnesses co-exist, and are uncontrolled, the risks of debilitating complications and mortality are compounded.8,9,13–20
Mental health conditions and cardio-metabolic diseases share common features: they are chronic, complex, progressive, and costly to care for.21 They require comprehensive care that includes risk factor management, lifestyle modification, and patient-empowered and self-guided care in collaboration with providers.22,23 However, major barriers to diagnosis and care exist at the patient (e.g. stigma, motivation, lack of awareness), provider (e.g., clinical inertia to intensify treatment), and system levels (e.g., fragmented care for different conditions, shortage of mental health professionals), all of which interact with each other.2,24,25 Barriers to care are of particular relevance for low- and middle-income countries (LMICs) like India where the healthcare infrastructure and workforce is limited. This is particularly relevant to depression, as there is a severe shortage of psychiatrists and mental health professionals in India.26–28 Solutions to overcome these challenges have been prominent contemporary global health topics of discussion.29
Due to the aforementioned barriers, depression and diabetes remain under-diagnosed and suboptimally treated as individual conditions and in combination.30–34 Furthermore, healthcare in India, like in many LMICs, is characterized by a mix of public and private providers and fragmentation across specialties. In India, approximately 85–95% of healthcare costs are borne by individuals and their families.35–37 As such, fee-paying patients commonly seek care from specialists that serve as their routine care provider for all health concerns. This linkage to care and existing patient-provider relationships present an opportunity to efficiently address co-existing mental health conditions.
We developed an integrated care model that brings depression care into the diabetes clinic to increase access to effective depression care and to improve outcomes for people with co-morbid depression and diabetes. Drawing on the strengths and experiences of collaborative care delivery from the US (TEAMCare) and India (CARRS Trial), as well as formative research to ensure cultural relevance, INtegrating DEPrEssioN and Diabetes treatmENT (INDEPENDENT) care is a multi-component care model that enhances the skills of diabetes clinic teams to reduce depressive symptoms and improve diabetes risk factor management, patient-reported quality of life, and treatment satisfaction.38–42 Here, we describe the components of INDEPENDENT care and the experimental design being used to test its effectiveness in diabetes care centers in India, as well as the results of recruitment.
METHODS
Study design
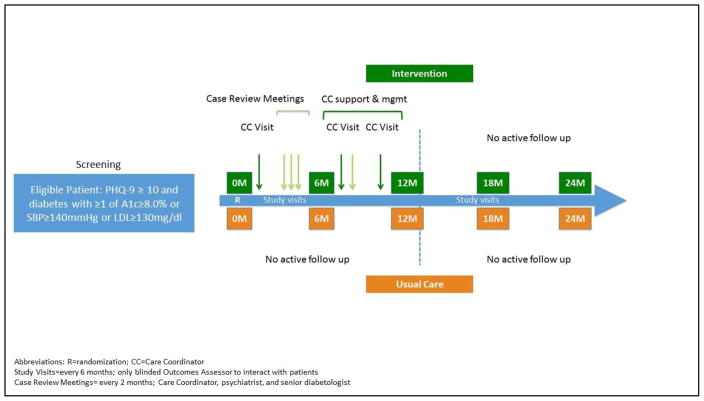
The INDEPENDENT study is a pragmatic randomized controlled trial comparing a multi-component depression and diabetes care program to usual care in four diverse diabetes clinics in India. The study has a 12-month active intervention phase followed by a 12-month observational follow-up period. During the active intervention phase, participants in the intervention arm engage in regular visits with a care coordinator. Throughout the study, a blinded outcomes assessor conducts research study visits every 6 months with participants in both arms to assess the effectiveness of the intervention and whether effects can be sustained up to 24 months post-randomization (Figure 1).
Figure 1.
INDEPENDENT Study Schematic
Clinical sites and ethics
The study is being conducted at four diabetes clinics in urban centers in India: a large public hospital outpatient clinic in Delhi and private diabetes clinics in Bangalore, Chennai, and Visakhapatnam. Clinic details and site teams are described in the Acknowledgements.
The study protocol was approved by the Indian and US coordinating centers (Institutional Ethics Committee of Madras Diabetes Research Foundation and Emory University Institutional Review Board, respectively), ethics committees at each clinic site, as well as the Health Minister Screening Committee of the International Health Division of the Indian Council of Medical Research. An external data safety and monitoring board (DSMB) comprised of a biostatistician, a senior endocrinologist, a mental health epidemiologist, and a pharmacologist has been established. The DSMB convened in the first and second year to review the study protocol and receives quarterly updates on recruitment and participant safety. As a safety precaution, all treating physicians were provided with continuing medical education from the study psychiatrists on the recognition and treatment of depressive symptoms and were notified of their patients’ depressive symptoms at the time of enrollment – regardless of intervention status. This means participants in the usual care arm are effectively receiving enhanced care.
Study population: eligibility criteria and recruitment
Participants are adults with diabetes and co-morbid depressive symptoms. Patients attending the participating clinics were eligible to participate if they met the following criteria: (1) age ≥35 years; (2) physician-confirmed diabetes; (3) Patient Health Questionnaire-9 (PHQ-9) score ≥10, indicating moderate-to-severe depressive symptoms; and (4) one or more poorly-controlled cardiometabolic indicators (HbA1c ≥8.0%, SBP ≥140 mmHg, or LDL-c ≥130 mg/dl) regardless of medications used.
Patients were ineligible if the individual: (1) reported serious suicidal ideation (reflected by a score of “3” for PHQ-9 item #9) or was deemed to have severe depression warranting specialized and/or inpatient psychiatric care; (2) was currently under a psychiatrist’s care, using antipsychotic medications or mood stabilizers, had diagnosed bipolar disorder or schizophrenia, or screened positive for cognitive impairment43; (3) had diabetes secondary to rare conditions (e.g., chronic pancreatitis or fibrocalculus pancreatic disease); (4) was pregnant or lactating; (5) had a documented CVD event (myocardial infarction, stroke) in previous 12 months; (6) had end-stage renal disease (on dialysis or requiring a transplant); (7) had malignancy or life-threatening disease with a life expectancy of less than 3 years; (8) reported current alcohol or drug use consistent with a substance use disorder (based on the AUDIT-10 and DAST-10 instruments); (9) physician-assessed history of chronic steroid use; or (10) had no fixed address or contact details. A self-harm risk reduction protocol was developed and adapted to each clinic site. The protocol outlined guidance on recognition of warning signs of self-harm and tiered steps for more intensive evaluation of risk by the usual diabetes care physician and/or study psychiatrist depending on severity of risk. The protocol was designed to be used by both research and clinical staff during the screening and follow-up research visits or during clinical visits. Individuals warranting more intensive psychiatric care were linked to services.
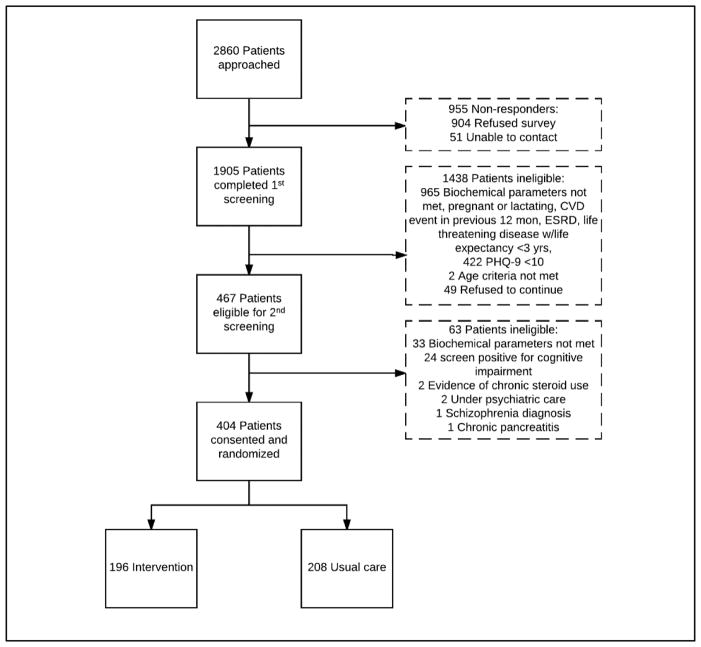
Recruitment began in March 2015 and was completed in May 2016. Potential participants were identified from medical records, recruited from outpatient clinics and diabetes awareness camps, and referred by current study participants. A total of 2860 individuals were approached, 1905 expressed interest and agreed to screening, and 404 were randomized (Figure 2). Individuals may have undergone up to two screening visits – a brief initial screening which was conducted in-person or by phone, and a subsequent in-person interview to determine eligibility. Individuals eligible to participate were given participant information sheets in their preferred language (Hindi, Kannada, Tamil, Telugu, or English) and enrolled after informed consent was obtained.
Figure 2.
INDEPENDENT study recruitment
Randomization
Randomization
A decision support and electronic health record (DS-EHR) system was developed for the trial. This stand-alone system (separate from electronic medical record systems used by some clinic sites) has clinical trial management, electronic health record, and decision support system functionalities. As part of its clinical trial management features, the DS-EHR randomized each participant to the intervention or usual care arm upon entering his/her baseline visit. Individuals were randomized within clinics in randomly generated blocks of 4, 6, 8, or 10. For participants assigned to the intervention arm, an electronic health record was automatically generated and loaded into the site-specific clinic dashboard where it was accessed by the care coordinator, thereby linking the clinical trial management and electronic health record functionalities of the DS-EHR. The DS-EHR is password protected and study staff have different levels of access to participant information according to their job description.
Intervention
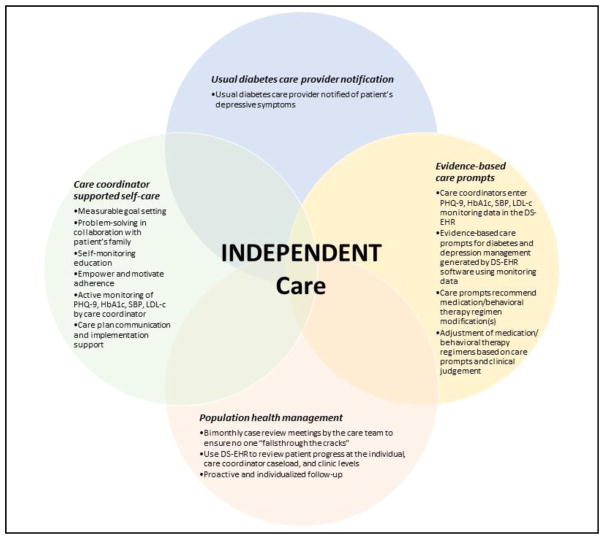
INDEPENDENT care is a multi-component care model that combines collaborative care with decision support technology to provide population health management for patients with co-morbid diabetes and depression (Figure 3). INDEPENDENT care is based on the four core principles of collaborative care: person-centered team care; population-based care; evidence-based care, and measurement-based treatment to target—tailored to the Indian cultural context. Cultural modifications were made based on formative research and included engaging families in the treatment process, provision of clear written information to participants, provision of non-jargon verbal information, and coaching to help participants cope with stigma.42,44 Population health is defined as the health outcomes of a group of individuals, including the distribution of such outcomes within the group45. This approach ensures that attention is paid to all individuals in the group, and no one “falls through the cracks” because they fail to engage in care. In the context of the current study, the population is the panel of intervention participants assigned to a care coordinator and tracked in the DS-EHR. Depression and diabetes outcomes are measured at care coordinator visits and recorded in the DS-EHR, and the decision support functionality generates evidence-based care prompts based on participant clinical values at a given point in time.
Figure 3.
INDEPENDENT Care
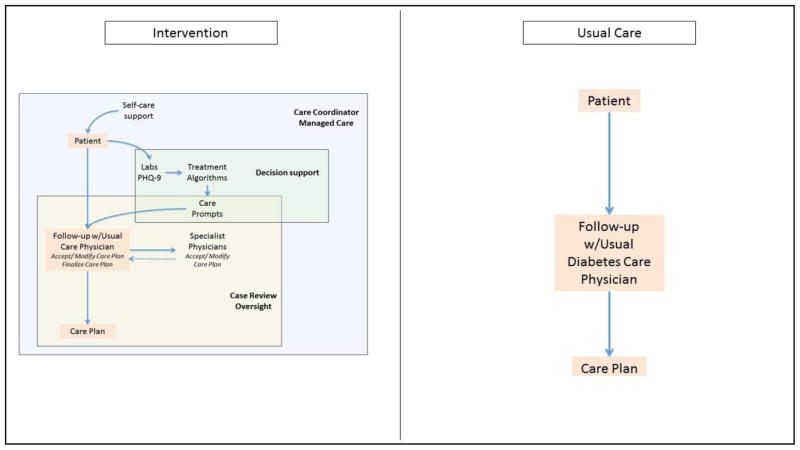
To implement this care model, the intervention adds a non-physician care coordinator and consulting psychiatrist to the patient-diabetologist dyad to form a care team. In addition to treatment from their usual diabetes care physician, participants in the intervention arm receive: (1) notification of their depressive symptoms to their usual diabetes care provider; (2) self-care support, proactive follow-up, and outcome monitoring by the care coordinator; (3) evidence-based care prompts to promote responsive pharmacotherapy modification(s) for diabetes and depression management and/or evidence-based behavioral interventions for depression, delivered by the care coordinator; and (4) bimonthly case review meetings by the care team to review the progress of all participants (at the individual, care coordinator caseload, and clinic levels), and to adjust treatment for participants who are not improving with respect to depression and cardiometabolic risk factors(Figure 4). Each of the components is described in greater detail below.
Figure 4.
Intervention vs. Usual Care
Care Coordinator Support for Participants
In an effort to address the acute shortage of mental health professionals in India, we identified and trained locally-based, bilingual allied health professionals as care coordinators. Care coordinators had backgrounds in dietetics, diabetes education, psychology, and general medicine and had experience working with diabetes patients, but were not members of the clinic staff and were not mental health specialists.
An initial 5-day in-person training session and two-day refresher training at the coordinating site focused on identifying depressive symptoms, patient-centered depression and diabetes care, evidence-based brief behavioral interventions to support depression and diabetes self-care, patient outcome monitoring, and use of the DS-EHR system through a combination of didactic instruction, role play, and case studies. Care coordinators receive ongoing support through twice monthly coaching calls with investigators and clinicians experienced in collaborative care, individualized feedback on videotaped case review meetings, and annual refresher trainings. Additionally, the care coordinators formed and maintain a WhatsApp group that is used to pose questions to one another and the coaching team and problem-solve across sites.
Care coordinators are central figures in the INDEPENDENT care model – they encourage and support patient self-care; monitor patient outcomes on key indicators; proactively follow-up with participants who are not improving; manage case review meetings; and coordinate care between the patient and their care team. Participant care teams are comprised of the participant’s usual diabetes care physician, the care coordinator, and case review specialists – a psychiatrist and senior diabetologist.
To encourage and support sustained and effective depression and diabetes self-care, care coordinators engage participants in self-care education, motivational interviewing, behavioral activation, and problem solving treatment strategies. Patient education materials and behavioral activation techniques were adapted for the Indian cultural context during the formative research phase.42 Motivational interviewing is a therapeutic approach designed to help individuals explore and resolve ambivalence and foster commitment to behavior changes in a non-confrontational manner.46–48 In motivational interviewing, care coordinators reflectively listen to participants, help them recognize problems, monitor their readiness to change, help them set measurable goals, assess and overcome barriers, affirm their choices, and grow their self-efficacy and agency. Behavioral activation strategies are brief, structured psychological interventions based on extensive theoretical and clinical literature that reinforce behaviors to produce improvements in patient thoughts, mood, and quality of life.15,19 Problem solving treatment is a structured procedure for addressing problems systematically.49,50
In the initial visit, the care coordinator explained their role and obtained a detailed participant history of depression and diabetes and current and prior treatments for both conditions. During subsequent visits care coordinators: (a) assess barriers to care; (b) collaboratively set treatment goals; (c) provide verbal education regarding diabetes and depression self-care (self-monitoring; adherence to medication, diet, exercise; and smoking cessation); (d) use motivational interviewing and self-efficacy enhancement strategies (e.g., structured feedback) to promote monitoring of depressive symptoms, glucose, and blood pressure; and (e) proactively follow-up with participants to monitor depressive symptoms (regular PHQ-9’s in the participant’s preferred language) and cardiometabolic indicators (laboratory and home monitoring). The initial consultation was conducted in-person to build rapport while follow-up care coordinator visits are conducted in-person or over the phone. Care coordinator visits are conducted a minimum of once every month, but may be more frequent based on participant risk level and adherence.
Care coordinators support participants in depression and diabetes self-care and monitoring to help them reach PHQ-9, fasting blood glucose (FBG), HbA1c, SBP/DBP, and LDL-c targets. care coordinators track participants’ progress using the DS-EHR.
Evidence-based Care Prompts and Decision Support
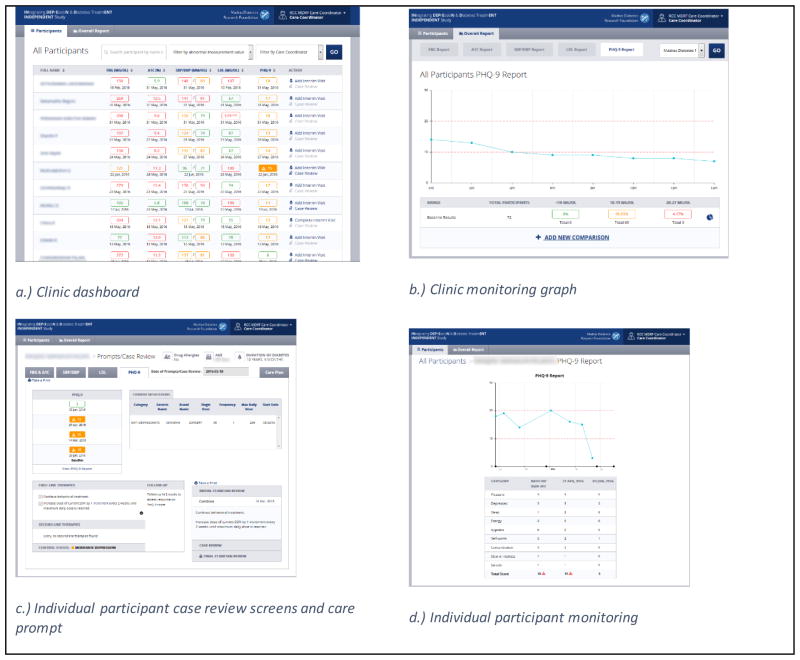
The DS-EHR system is tool to support population health management within a given clinic. The DS-EHR: (1) displays consultation and laboratory data in a single platform; (2) has a clinic-level dashboard to assist in prioritizing participants for follow-up; (3) provides visualization tools at the clinic and individual participant levels to monitor trends in clinical indicators over time; (4) provides individualized clinical care prompts for glucose, blood pressure, and lipid management; as well as (5) care prompts for the medical management of depression. Data entry into the system is managed by the care coordinator, while the display can be shared with the usual diabetes care physician and case review specialists.
Participant responses to depression and diabetes treatment are monitored through repeat measures of clinical indicators collected at interim visits. Upon entry into the DS-EHR, the most recent indicator value is displayed in the clinic dashboard using a traffic light color scheme (green, adequate control; yellow, moderate control; red, poor control) allowing for participant monitoring and prioritization (Figure 5a). Adequate indicator values were defined as: FBG, <110 mg/dl; HbA1c, <7%; SBP, <120 mmHg; DBP, <80 mmHg; lipids, <100 mg/dl (<70 mg/dl if history of CVD); PHQ-9, <10. Indicator values can also be displayed graphically (Figure 5b and 5d).
Figure 5.
Screenshots from the decision support-electronic health record (DS-EHR) software system
The DS-EHR recommends guideline-based care prompts for glucose, blood pressure, lipid, and depression management. Care prompts are generated by programmed algorithms for each indicator that take a treat-to-target approach by considering the participant’s most recent indicator values and current therapies with clinical targets. Care prompts may recommend maintenance, initiation, increases, or simplification of medication regimens, or higher or lower frequency of follow-up or intensity of behavioral therapy (Figure 5c).
Guideline-based algorithms for glucose, blood pressure, and lipid management were used successfully in the CARRS Trial,29 and these were updated to align with the 2015 clinical guidelines and to include newer therapies, with a preference given towards generics. A guideline-based algorithm for depression management was developed based on prescription practices, comfort, ease, and experience which was mutually agreed upon between investigators from India and the US. The depression algorithm was piloted in clinics prior to the start of the trial.
care coordinators record all participant interactions, clinical indicator values, therapies, and clinical care decisions in the DS-EHR. The system then generates care prompts that the care coordinator shares with the participant’s usual diabetes care physician. Care prompts are intended to encourage responsive treatment modification, however, physicians are given discretion to accept the care prompt or modify the care plan based on their clinical judgment. If modified, changes to the care plan and their justification are documented in the system to promote accountability. Once the participant is adequately controlled on all disease parameters, the care coordinator and participant develop a relapse prevention plan together that includes maintenance medications, behavioral goals, and symptoms associated with poor disease control.
Case Review Oversight
Case review oversight is a systematic process that operationalizes depression and diabetes population health management within a given clinic. Case reviews involve the care coordinator, a specialist psychiatrist, and a specialist diabetologist/endocrinologist at each clinic and occur twice monthly. Case review meetings occur “offline” – i.e. the participant is not present – and involve discussion of all participants (new participants, those with no or low improvement, and those on maintenance plans); the time spent on each participant is proportional to their needs. Care coordinators provide key contextual information on participants that is not captured in the DS-EHR (i.e. participant mood, medication adherence, supportive environment, etc.), while the DS-EHR is used to review the participant’s health record, most recent care plan, and trends in indicator management over time.
The case review team reviews each participant’s current care plan and recommends continuation or modification of the plan. Modifications and their justification are documented in the DS-EHR and communicated to the usual diabetes care physician. As the usual diabetes care physician is given full discretion over their patient’s care, he/she may accept or further modify the revised care plan put forth by the case review team, provided a justification is documented. The care coordinator communicates the final care plan to the participant and helps them implement the recommendations. In addition to supporting participants’ care, case review meetings also give the specialist physicians an opportunity to educate and support the care coordinators.
Usual Care
Participants randomized to the usual care arm continued to see their usual diabetes care physician for management of their diabetes and received whatever depression care or referrals clinics typically provided (and did not receive any treatment for depression through the research study). As described previously, all treating physicians received continuing medical education on the recognition and treatment of depressive symptoms and were notified of their patients’ depressive symptoms at the time of enrollment, therefore, participants in the usual care arm are effectively receiving enhanced care.
Data Collection and Management
Participants in both arms attended a baseline (prior to randomization: 0 month) visit and will attend 6-monthly study-related data collection visits over the duration of the study (6, 12, 18, 24 months). All study visits are completed by an outcomes assessor blinded to participants’ intervention status. As one of its clinical trial management features, the DS-EHR notifies the outcomes assessor when participants are eligible for study visits. Study visits include questionnaires, clinical examination, and biochemical measures collected at the clinic laboratory (Table 1). The questionnaires collect information on medical history, self-care activities, depressive symptoms, quality of life, satisfaction, and costs of care. Costs of care questions capture direct non-medical (participant time spent traveling to and attending appointments) and indirect costs (lost productivity associated with illness or premature mortality). Direct costs of care will be assessed based on utilization using clinic records. The clinical examination and biochemical tests collect data on HbA1c, SBP/DBP, and LDL-c. Blood samples are analyzed for HbA1c and LDL-c by local laboratories which are enrolled in an external quality assurance scheme. Blood pressure is collected using electronic devices (Omron T9P). Participants are also asked open-ended questions about diabetes complications and any adverse (e.g., hypoglycemia) or serious adverse events (e.g., myocardial infarction) or hospitalizations. The severity (mild, moderate, and severe) of all reported adverse events is assessed and site investigators assess the likelihood of whether serious adverse events are causally related to the study intervention. Study visit data is entered by the outcomes assessor and the DS-EHR includes automated plausibility checks to reduce data entry errors.
Table 1.
Schedule of Evaluations
| Study Measure | Method | Baseline | Study Visits | Endline | Interim Visits1 |
|---|---|---|---|---|---|
|
| |||||
| 0 mo | 6, 12, 18 mo | 24 mo | as needed | ||
| Demographics | Q | X | |||
| Depressive symptoms | |||||
| PHQ-9 | Q | X | X | X | X |
| SCL-20 | Q | X | X | X | |
| Other psychiatric conditions | |||||
| MoCA; AUDIT-10; DAST-10; | Q | X | X | ||
| GAD-7; Bipolar disease/Psychosis | Q | X | X | X | |
| Medical history | |||||
| Patient history | Q | X | |||
| Family history | Q | X | |||
| Medication use | Q | X | X | X | X |
| Medical complications | Q | X | X | X | X |
| Risk factor control | |||||
| SBP/DBP, HR | E | X | X | X | X |
| FBG | L | X | X | X | X |
| HbA1c | L | X | X | X | X |
| Lipids (TC, HDL, LDL, TG) | L | X | X | X | X |
| Lifestyle habits: PA, alcohol and tobacco use | Q | X | X | X | X |
| Anthropometry: height, weight, BMI, WC | E | X | X | X | X |
| Clinical examinations: | |||||
| Physical, eye, and foot inspections | E | X | X | X | X |
| Electrocardiogram | E | X | X | X | X |
| Microalbuminuria (UACR) | L | X | X | ||
| Serum creatinine, Na+; K+; ALT | L | X | X | X | |
| Patient self-efficacy: SDSCA | Q | X | X | X | |
| Quality of life, health utility, satisfaction | |||||
| HUI-3 | Q | X | X | ||
| DTSQ | Q | X | X | ||
| Costs of care: direct, indirect | Q, R | X | X | ||
| SAEs/AEs | Q | X | X | X | |
Abbreviations: PHQ-9, Patient Health Questionnaire-9; SCL-20, Symptom Checklist-20; MoCA, Montreal Cognitive Assessment; AUDIT-10, Alcohol Use Identification Test-10; DAST-10, Drug Abuse Screening Test-10; GAD-7, Generalized Anxiety Disorder-7; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; HR, Heart Rate; FBG, Fasting Blood Glucose; HbA1C, Hemoglobin A1C; TC, Total Cholesterol; HDL, High Density Lipoprotein; LDL, Low Density Lipoprotein; TG, Triglycerides; BMI, Body Mass Index; WC, waist circumference; UACR, Urine Albumin Creatinine Ratio; Na+, Sodium; K+, Potassium; ALT, Alanine Aminotransferase; HUI-3, Health Utilities Index-3; DTSQ, Diabetes Treatment Satisfaction Questionnaire; SDSCA, Summary of Diabetes Self-Care Activities; SAEs, Severe Adverse Events; AEs, Adverse Events; Q, Questionnaire; E, Examination; L, Laboratory test; R, Records.
Interim visits measures are collected from participants in the intervention arm, as needed, at the discretion of the care coordinator. Incidence of medical complications and severe and adverse events were asked at each interim visit.
Since this is a pragmatic trial, costs associated with the study visits are paid for by the study, but no additional compensation is offered to participants in the intervention arm and any further interim visits at the discretion of the participant’s care team – either related to more intensive follow-up as part of the intervention or usual care – are borne by participants themselves.
All study personnel have been trained in procedures to minimize the potential for breaches of confidentiality. Computer files are password-protected and hard copies of interview questionnaires are stored in locked file cabinets in restricted-access buildings. All data files will be de-identified prior to analysis.
Outcome definitions
The primary outcome is the between-group difference in the proportion of trial participants in each arm achieving combined improvements in depressive symptoms and CVD risk factors at 24 months. Improvements are defined as ≥50% reduction in the Symptoms Checklist (SCL-20) score from baseline AND one or more of the following: ≥0.5% reduction in HbA1c, ≥5 mmHg reduction in SBP, or ≥10 mg/dl reduction in LDL-c. Though the PHQ-9 is a widely used screening tool with high sensitivity and specificity for depression symptoms and is used for eligibility and monitoring in our study, regular use leads to test-retest bias.51–53 As a result, we will use the SCL-20 depression scale as our main depression outcome measure.54 This tool had been used successfully in other studies and is very sensitive to changes in depression among people with diabetes.39,55
We will also examine between-group differences in secondary outcomes including: mean changes in SCL-20, HbA1c, SBP, and LDL-c at 12 and 24 months, as well as participant-reported outcomes such as treatment satisfaction, health-related quality of life, health expenditures, and within-trial cost-utility.
Analytical Plan
A priori sample size calculations estimated 360 participants across the four clinic sites (180 intervention, 180 usual care) would provide 80% power (α=0.05) to detect a clinically relevant 15% absolute difference (30% of intervention arm participants vs. 15% of usual care participants) in achieving the primary outcome, accounting for an anticipated 20% loss to follow-up. The sample size estimation was guided by: (1) studies in India and elsewhere showing that <50% of all people with diagnosed diabetes routinely achieve single or multiple CVD risk factor targets; and (2) results from the TEAMCare study which demonstrated a 30% absolute difference between arms (60% TEAMCare vs. 30% usual care) in the proportions of subjects achieving ≥50% reduction in SCL-20 score at 12 months and 15% absolute difference (37% vs. 22%) in the proportion of subjects achieving significant reductions or target control for all three CVD risk factors (HbA1c, SBP, LDL-c). 25,31,33,40,56 All analyses will be based on intention-to-treat principles and two-sided P<0.05 will be used to indicate statistical significance.
We describe participant characteristics in the intervention and usual care groups to assess adequacy of randomization at baseline. Continuous variables were compared using Student’s t-tests or Wilcoxon-rank sum tests and categorical variables were compared using chi-square or Fishers’ exact tests. To estimate the between-group relative risk of achieving combined improvements in depressive symptoms and CVD risk factors, we will use log-binomial models with generalized estimating equations. Models will be adjusted for baseline SCL-20 and CVD indicator values, treatment group, time, treatment by-time interaction, and site. Where there are significant differences in characteristics between treatment groups at baseline we will adjust models for these characteristics.
If and where data are missing, we will assess likely mechanism of missingness and consider multiple imputation or inverse probability weighting to determine how sensitive the primary outcome results are to the missing data.57–59 We will examine the results for heterogeneity of effect across sites, age, sex, education, income, body mass index, duration of diabetes, prior history of CVD, prior history of microvascular complications, insulin use, and baseline values of SCL-20, HbA1c, SBP, and LDL-c.
RESULTS
Seven care coordinators were hired and trained over a five-day training in September 2014 and two-day refresher training in February 2015. Care coordinators have backgrounds in dietetics, diabetes education, psychology, and general medicine. Six of the care coordinators are women and one is a man, with experience ranging from early-to mid-career professionals. Each clinic site has two care coordinators, except Vishakhapatnam which has one. Care coordinator caseloads range from 14–53 participants.
Recruitment was completed over 14-months from March 2015–May 2016. One thousand nine hundred and five patients were screened and 404 were randomized, with 155, 90, 84, and 75 enrolled at clinics in Chennai, Delhi, Vishakhapatnam, and Bangalore, respectively. The most common reasons for ineligibility were cardiometabolic risk factors outside the inclusion criteria and no depressive symptoms.
Recruitment was not without challenges. It was logistically challenging to administer the screening questionnaires and maintain patient privacy in crowded clinic settings. Eligibility screening was designed as a two-step process with a brief initial screener that could be administered by phone and longer eligibility questionnaire that needed to be administered in person. However, if screening was done in person, it either required patients to stay at the clinic longer to complete both parts at once or return a second day. Many individuals did not return for the second day screening despite multiple phone calls from the study staff. Stigma towards mental health conditions may have also been a barrier to enrollment. Additionally, in the Chennai site, recruitment was delayed for one month due to the severe flooding that took place in south India in 2015.60–63
Recruitment was completed on schedule largely due to implementation of a well-coordinated recruitment plan. The coordinating center had weekly phone calls with sites to track progress on weekly recruitment goals, problem-solve recruitment challenges, and maintain motivation among study staff. At AIIMS, EDC, and DIACON participants were primarily recruited from reviewing outpatient charts. At MDRF, referrals from enrolled participants were a major source of identifying new participants.
Overall, baseline characteristics were similar between the intervention and usual care groups (Table 2). Participants were in their late 40s to early 60s, over half were female, most were married, and the majority had at least a primary school education. Only 13% had private insurance. The mean duration of diabetes was 8.9±7.0 years, with over 90% reporting taking oral hypoglycemic agents, and 36% reporting taking insulin. Over 90% of participants had retinopathy and approximately 5% had a history of cardiovascular disease. On average, participants had HbA1c ≥9%, SBP >130 mmHg, DBP ≥80 mmHg, and LDL-c ≥100 mg/dl. The mean SCL-20 and PHQ-9 scores were 25.8±9.6 and 13.0±2.5 respectively, indicating mild-moderate depression. The majority of participants met the cardiometabolic risk factor inclusion criteria on the basis of elevated HbA1c.
Table 2.
Sociodemographic and clinical characteristics of diabetes patients with co-morbid depression by intervention status presented as mean ± SD or n (%).
| Overall | Clinic 1 (MDRF) |
Clinic 2 (AIIMS) |
Clinic 3 (EDC) |
Clinic 4 (DIACON) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I n=1 96 |
UC n=2 08 |
p | I n=7 8 |
UC n=7 7 |
p | I n=4 1 |
UC n= 49 |
p | I n=3 9 |
UC n=4 5 |
p | I n= 38 |
UC n=37 |
p | |
| Demographics | |||||||||||||||
| Age | 52.1 ± 8.2 | 53.3 ± 8.9 | 0.2 | 52.3 ± 7.6 | 53.4 ± 9.4 | 0.46 | 50.2 ± 8.1 | 51.5 ± 8.3 | 0.5 | 50.8 ± 8.8 | 53.0 ± 9.1 | 0.29 | 55.1 ± 8.2 | 55.9 ± 8.1 | 0.7 |
| Female | 107 (54.6) | 132 (63.5) | 0.06 | 44 (56.4) | 52 (67.5) | 0.15 | 25 (61.0) | 32 (65.3) | 0.67 | 12 (30.8) | 19 (42.2) | 0.27 | 26 (68.4) | 29 (78.4) | 0.32 |
| Married | 166 (84.7) | 179 (86.1) | 0.73 | 62 (79.5) | 64 (83.1) | 0.92 | 40 (97.6) | 45 (91.8) | 0.23 | 37 (94.9) | 43 (95.6) | 0.5 | 27 (71.1) | 27 (73.0) | 0.36 |
| Private insurance | 24 (12.2) | 28 (13.4) | 0.71 | 12 (15.4) | 11 (14.3) | 0.84 | 2 (4.9) | 9 (18.4) | 0.05 | 2 (5.1) | 2 (4.4) | 1 | 8 (21.1) | 6 (16.2) | 0.59 |
| Education | |||||||||||||||
| Post- secondary | 43 (21.9) | 48 (23.1) | 16 (20.5) | 19 (24.7) | 8 (19.5) | 10 (20.4) | 12 (30.8) | 15 (33.3) | 7 (18.4) | 4 (10.8) | |||||
| Primary/secondary | 134 (68.4) | 138 (66.3) | 0.9 | 58 (74.4) | 54 (70.1) | 0.82 | 28 (68.3) | 32 (65.3) | 0.94 | 24 (61.5) | 24 (53.3) | 0.65 | 24 (63.2) | 28 (75.7) | 0.48 |
| No formal education/unsure | 19 (9.7) | 22 (10.6) | 4 (5.1) | 4 (5.2) | 5 (12.2) | 7 (14.3) | 3 (7.7) | 6 (13.3) | 7 (18.4) | 5 (13.5) | |||||
| Income ≤10,000 INR (≤200 USD) | 55 (28.1) | 66 (31.7) | 23 (29.5) | 29 (37.7) | 5 (12.2) | 15 (30.6) | 11 (28.2) | 6 (13.3) | 16 (42.1) | 16 (43.2) | |||||
| 10,001–30,000 INR (200–450 USD) | 93 (47.4) | 99 (47.6) | 0.57 | 37 (47.4) | 33 (42.9) | 0.55 | 25 (61.0) | 18 (36.7) | 0.04 * | 15 (38.5) | 31 (68.9) | 0.01* | 16 (42.1) | 17 (45.9) | 0.85 |
| >30,000 INR (>450 USD) | 48 (24.5) | 43 (20.7) | 18 (23.1) | 15 (19.5) | 11 (26.8) | 16 (32.7) | 13 (33.3) | 8 (17.8) | 6 (15.8) | 4 (10.8) | |||||
| Depressive symptoms | |||||||||||||||
| SCL-20 | 25.8 ± 9.6 | 26.8 ± 9.6 | 0.3 | 31.3 ± 8.5 | 31.7 ± 8.9 | 0.85 | 23.3 ± 8.5 | 27.0 ± 9.6 | 0.06 | 21.4 ± 9.0 | 21.9 ± 7.4 | 0.6 | 21.4 ± 8.1 | 22.3 ± 7.9 | 0.66 |
| PHQ-9 | 13.0 ± 2.5 | 13.4 ± 2.5 | 0.05 | 14.3 ± 2.6 | 14.6 ± 2.7 | 0.47 | 12.4 ± 2.9 | 13.1 ± 2.4 | 0.06 | 12.4 ± 1.2 | 12.7 ± 1.7 | 0.4 | 11.8 ± 1.5 | 12.3 ± 2.1 | 0.28 |
| Diabetes characteristics | |||||||||||||||
| Years with diabetes | 8.9± 7.0 | 10.0 ± 7.3 | 0.11 | 9.7± 6.7 | 10.4 ± 7.1 | 0.63 | 7.1± 6.1 | 8.8± 6.6 | 0.12 | 4.5± 3.7 | 8.7± 7.1 | 0.006 ** | 13.7 ± 7.8 | 12.3 ± 8.2 | 0.43 |
| OHA use | 184 (93.9) | 192 (92.3) | 0.53 | 74 (94.9) | 68 (88.3) | 0.14 | 37 (90.2) | 47 (95.9) | 0.4 | 37 (94.9) | 40 (88.9) | 0.44 | 36 (94.7) | 37 (100.0) | 0.49 |
| Insulin use | 71 (36.2) | 73 (35.1) | 0.81 | 26 (33.3) | 24 (31.2) | 0.77 | 16 (39.0) | 14 (28.6) | 0.29 | 2 (5.1) | 9 (20.0) | 0.05 | 27 (71.1) | 26 (70.3) | 0.94 |
| OHA and insulin use | 64 (32.7) | 65 (31.3) | 0.76 | 26 (33.3) | 22 (28.6) | 0.52 | 12 (29.3) | 13 (26.5) | 0.77 | 1 (2.6) | 4 (8.9) | 0.36 | 25 (65.8) | 26 (70.3) | 0.67 |
| Microvascular complications | |||||||||||||||
| Retinopathy | 51 (94.4) | 54 (93.1) | 1 | 23 (29.5) | 25 (32.5) | 0.79 | 6 (14.6) | 8 (16.3) | 1 | 6 (15.4) | 5 (11.1) | 0.59 | 16 (42.1) | 16 (43.2) | 0.92 |
| Neuropathy | 67 (35.3) | 71 (34.6) | 0.54 | 47 (60.3) | 42 (54.5) | 0.13 | 3 (7.3) | 1 (2.0) | 0.55 | 7 (17.9) | 15 (33.3) | 0.1 | 10 (26.3) | 13 (35.1) | 0.38 |
| CKD | 1 (0.5) | 1 (0.5) | 1 | - | - | - | 1 (2.4) | 1 (2.0) | 1 | - | - | - | - | - | - |
| Cardio metabolic risk factors | |||||||||||||||
| FBG | 182.9± 67.2 | 181.1± 77.1 | 0.34 | 188.8± 66.1 | 185.3± 75.9 | 0.49 | 179.8± 71.1 | 178.8± 57.8 | 0.99 | 168.1± 56.1 | 166.6± 69.2 | 0.75 | 189.4± 75.2 | 193.1± 105.9 | 0.61 |
| HbA1c | 9.3± 2.0 | 9.0± 1.9 | 0.12 | 9.6± 2.1 | 9.3± 1.8 | 0.24 | 9.4± 1.7 | 9.3± 1.6 | 0.91 | 7.6± 1.2 | 7.6± 1.1 | 0.82 | 10.2 ± 1.8 | 9.8± 2.1 | 0.3 |
| SBP | 131.8± 15.4 | 132.6± 17.1 | 0.7 | 128.5± 16.4 | 131.4± 17.5 | 0.32 | 132.6± 15.7 | 133.4± 18.9 | 0.89 | 138.2± 10.9 | 134.8± 15.0 | 0.14 | 131.2± 15.1 | 131.4± 16.5 | 0.92 |
| DBP | 80.1 ± 10.5 | 80.4 ± 9.7 | 0.86 | 80.5 ± 9.0 | 81.2 ± 9.4 | 0.77 | 79.4 ± 12.2 | 81.9 ± 10.7 | 0.18 | 85.9 ± 7.7 | 83.1 ± 6.4 | 0.04** | 74.3 ± 11.0 | 73.1 ± 9.3 | 0.85 |
| LDL-c | 100.4± 38.2 | 101.3± 38.1 | 0.91 | 109.8± 37.8 | 108.7± 33.6 | 0.84 | 91.7 ± 31.2 | 95.0 ± 30.1 | 0.69 | 112.1± 40.1 | 117.5± 44.0 | 0.55 | 79.3 ± 33.6 | 75.0 ± 33.9 | 0.55 |
| BMI | 26.9 ± 5.2 | 26.6 ± 5.2 | 0.7 | 26.0 ± 5.5 | 25.9 ± 5.8 | 0.7 | 28.3 ± 6.2 | 27.4 ± 4.0 | 0.51 | 26.0 ± 3.9 | 25.5 ± 4.5 | 0.43 | 28.0 ± 4.2 | 28.4 ± 5.7 | 0.77 |
| History of CVD | 8 (4.1) | 11 (5.3) | 0.5 | 1 (1.3) | 4 (5.2) | 0.16 | 4 (9.8) | 6 (12.2) | 0.7 | - | - | - | 3 (7.9) | 1 (2.7) | 0.31 |
| Current smoker | 7 (3.6) | 5 (2.4) | 3 (3.8) | 1 (1.3) | 2 (4.9) | 4 (8.2) | - | - | 2 (5.3) | - | |||||
| Former smoker | 8 (4.1) | 6 (2.9) | 0.62 | 3 (3.8) | 2 (2.6) | 0.67 | 5 (12.2) | 2 (4.1) | 0.32 | - | 1 (2.2) | 1 | - | 1 (2.7) | 0.4 |
| Never smoked | 181 (92.3) | 197 (94.7) | 72 (92.3) | 74 (96.1) | 34 (82.9) | 43 (87.8) | 39 (100.0) | 44 (97.8) | 36 (94.7) | 36 (97.3) | 9 | ||||
Abbreviations: I, intervention; UC, usual care; MDRF, Madras Diabetes Research Foundation; AIIMS, All India Institute of Medical Sciences; EDC, Endocrine and Diabetes Centre; DIACON, Diabetes Care and Research Center;
The Madras Diabetes Research Foundation, Endocrine and Diabetes Centre, and Diabetes Care and Research Center are private diabetes clinics in Chennai, Vishakhapatnam, and Bangalore, respectively, while the final site is an outpatient clinic at the All India Institute of Medical Sciences, a large public hospital in Delhi. Minor differences existed between intervention and usual care groups within clinics. At AIIMS and EDC, participants were differentially distributed across the income categories between groups. At EDC differences also existed in participants’ mean duration of diabetes and mean DBP. The mean duration of diabetes was 4.5±3.7 years in the intervention group, compared to 8.7±7.1 years in the usual care group. Mean DBP was slightly higher in the intervention group compared to the usual care group (85.9±7.7 vs 83.1±6.4 mmHg). There were no significant differences between groups at MDRF and DIACON.
DISCUSSION
Depressive disorders are the second leading cause of disability worldwide and are twice as likely to exist among individuals with chronic conditions, such as diabetes.1,12 The co-occurrence of these conditions is therefore substantial in India where an estimated 50 million individuals suffer from common mental health conditions like depression and over 69 million are affected by diabetes.64 Yet there is an acute shortage of mental health professionals, with approximately 1 psychiatrist for every 300,000 individuals.26–28
Given the adverse interactions between diabetes and depression, and the fact that diabetes physicians often serve as routine care providers for people with diabetes in India, integrating depression screening and care into diabetes clinics may provide efficient opportunities to reduce morbidity and improve physical and social functioning among patients with these co-morbidities.62,63 Indeed, evidence suggests that for individuals with diabetes and depression, using patient-centered care models to target different levels of barriers to care at the same time can enhance health outcomes and satisfaction.65–72 The INDEPENDENT integrated care model is therefore a promising strategy to improve access to mental health care and also improve mental health and cardiometabolic outcomes.
To date, the research team has trained and provides ongoing support in culturally-tailored, patient-centered depression and diabetes care to seven locally-based care coordinators. The research team developed a decision-support electronic health record system that functions as clinical trial management software, an electronic health record system, and decision support system. The decision support feature use updated algorithms for cardiometabolic risk factor management and a newly developed algorithm to guide diabetes physicians on how to effectively manage antidepressant medication treatment. Case review meetings have provided opportunities for reciprocal learning between senior diabetes physicians and psychiatrists. The case review process has been integrated into medical practice in four diabetes clinics in distinct environments (northern/southern states and public/private clinics) in India. Recruitment has been completed and 404 individuals with co-morbid depression and diabetes with at least one poorly controlled cardiometabolic risk factor were successfully enrolled in the study and randomized. Randomization was balanced within clinic sites. Together, these data establish the feasibility of recruitment, and implementation (including training and ongoing implementation support of the clinical teams) of the INDEPENDENT study.
The clinical characteristics of the INDEPENDENT study population are similar to those of participants in the CARRS Trial which took place in outpatient clinics in India and Pakistan and successfully improved diabetes and cardiometabolic risk factor control through a nonphysician care coordinator and decision support intervention.38 Noteworthy differences between INDEPENDENT and CARRS study populations include lower LDL-c and lower proportion with a history of CVD among INDEPENDENT participants at enrollment.38 The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study was a representative study of glycemic control in three states and one union territory in India.73 Average HbA1c is higher among INDEPENDENT participants at baseline, although INDEPENDENT used an older age cutoff in its eligibility criteria and did not enroll any type 1 diabetics.73
There are some limitations that should be acknowledged. The usual care physicians treat participants in both the intervention and usual care arms and are not blinded to participants’ status due to the nature of the intervention. This will likely lead to conservative between-group findings (which was accounted for in sample size estimation) as the usual care providers may enhance the treatment they deliver to participants in the usual care arm as well. In an effort to avoid bias in the measurements, study visits are conducted by an outcomes assessor blinded to the participant’s status. Of note, the care coordinators in the trial have wide-ranging backgrounds and levels of experience; this is an aspect of our trial resembling real-world practice, and will be examined in by-site analyses for heterogeneity of effect. Our study is confined to urban settings (where most of the country’s psychiatrists are located) and further study would be needed to assess whether this care model can be effectively implemented in primary care and rural settings.
Conclusions
This is the first study to test an intervention for co-morbid depression and diabetes in diabetes clinics in India. If the INDEPENDENT intervention proves effective in improving depression and cardiometabolic indicators, it may be relevant to efforts to increase access to mental health care in other countries with limited mental health resources and improve chronic disease management among complex patients.
Acknowledgments
The authors would like to acknowledge and thank a number of individuals without whom this study would not have been possible. The study is funded by the National Institute of Mental Health of the US National Institutes of Health (R01MH100390) and is registered at clinicaltrials.gov (trial number NCT02022111). The National Institute of Mental Health had no role in the study design, data collection, analysis and interpretation of data, in writing the manuscript, and in the decision to submit the manuscript for publication. We would like to thank Cygnis Media for working with us to develop the DS-EHR system. We would like to thank the patients for their participation and time and the study staff at each of the clinic sites listed below.
Authors MKA, MLH, and KMVN were also partially supported by the Georgia Center for Diabetes Translation Research (National Institute of Diabetes Digestive and Kidney Disorders: P30DK111024).
Site personnel:
-
Madras Diabetes Research Foundation (MDRF), Chennai
Site PI: Dr. V. Mohan
Co-I: Dr. Radha Shankar, Dr. Ranjit Mohan Anjana, Dr. Sethuraman Jagdish, Dr. Selvam Kasthuri (offline Diabetologist)
Lead Study Coordinator: Dr. Subramani Poongothai
Study Coordinator: Mr. Parthasarathy Nandakumar
Medical Officer: Dr. Muthu Ramu
Care Coordinator (CC): Ms. Balasundaram Bhavani Sundari
Outcomes Assessor (OA): Ms. Kulasegaran Karkuzhali
Data Manager: Mrs. Chandrasekaran Anitha
-
All India Institute of Medical Sciences (AIIMS), New Delhi
Site PI: Dr. Nikhil Tandon
Co-I: Dr. Rajesh Khadgawat and Dr. Rajesh Sagar
Study Coordinator: Jijo Joseph
Care Coordinator (CC): Ms. Chandni Chopra and Ms. Bhanvi Grover
Outcomes Assessor (OA): Ms. Priyanka Rawat and Tania Bhardwaj
-
Endocrine and Diabetes Centre (EDC), Vishakhapatnam
Site PI: GR Sridhar, Kosuri Madhu
Study Coordinator: Tejomurthula Dhanalakshmi
Care Coordinator (CC): Sikha Aruna Sree
Outcomes Assessor (OA): Chiramana Venkateswarlu
-
DIACON Hospital, Diabetes Care and Research Center, Bangalore
Site PI: Dr. Sosale Ramachandran Aravind
Co-I: Dr. Bhavana Sosale and Dr. Kallur Somaiah Sunitha
Study Coordinator: Dr. Velkoor Teju
Care Coordinator (CC): Raja Karthik
Outcomes Assessor (OA): Narshimamurthy Pooja and Rangawamy Geethanjali
-
Data Safety and Monitoring Board
Dr. Pallab Maulik
Dr. Usha Pingali
Dr. K.M. Prasannakumar
Dr. Sreekumaran Nair
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care. 2002;25(3):464–470. doi: 10.2337/diacare.25.3.464. [DOI] [PubMed] [Google Scholar]
- 3.Golden SH, Lazo M, Carnethon M, et al. Examining a bidirectional association between depressive symptoms and diabetes. JAMA. 2008;299(23):2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golden SH, Williams JE, Ford DE, et al. Depressive symptoms and the risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care. 2004;27(2):429–435. doi: 10.2337/diacare.27.2.429. [DOI] [PubMed] [Google Scholar]
- 5.Knol M, Twisk J, Beekman A, Heine R, Snoek F, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 6.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31(12):2383–2390. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan A, Keum N, Okereke OI, et al. Bidirectional Association Between Depression and Metabolic Syndrome. Diabetes Care. 2012;35(5):1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llorente MD, Urrutia V. Diabetes, Psychiatric Disorders, and the Metabolic Effects of Antipsychotic Medications. Clinical Diabetes. 2006;24(1):18–24. [Google Scholar]
- 9.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 10.Sridhar G, Madhu K. Psychosocial and cultural issues in diabetes mellitus. Curr Sci. 2002;83:1556–1564. [Google Scholar]
- 11.Sridhar GR. Psychiatric co-morbidity & diabetes. The Indian Journal of Medical Research. 2007;125(3):311–320. [PubMed] [Google Scholar]
- 12.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 13.Bot M, Pouwer F, Zuidersma M, van Melle JP, de Jonge P. Association of Coexisting Diabetes and Depression With Mortality After Myocardial Infarction. Diabetes Care. 2012;35(3):503–509. doi: 10.2337/dc11-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davydow DS, Russo JE, Ludman E, et al. The association of comorbid depression with intensive care unit admission in patients with diabetes: a prospective cohort study. Psychosomatics. 2011;52(2):117–126. doi: 10.1016/j.psym.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27(5):1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 16.Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katon WJ, Lin EH, Williams LH, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med. 2010;25(5):423–429. doi: 10.1007/s11606-009-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katon WJ, Russo JE, Heckbert SR, et al. The relationship between changes in depression symptoms and changes in health risk behaviors in patients with diabetes. Int J Geriatr Psychiatry. 2010;25(5):466–475. doi: 10.1002/gps.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin EH, Heckbert SR, Rutter CM, et al. Depression and increased mortality in diabetes: unexpected causes of death. Ann Fam Med. 2009;7(5):414–421. doi: 10.1370/afm.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 21.Unutzer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically Ill fee-for-service medicare participants. J Am Geriatr Soc. 2009;57(3):506–510. doi: 10.1111/j.1532-5415.2008.02134.x. [DOI] [PubMed] [Google Scholar]
- 22.Engel CC, Kroenke K, Katon WJ. Mental health services in Army primary care: the need for a collaborative health care agenda. Military Medicine. 1994;159(3):203–209. [PubMed] [Google Scholar]
- 23.Shea KK, Shih A, Davis K. Internet: The Commonwealth Fund. 2007. Health Care Opinion Leaders’ Views on the Transparency of Health Care Quality and Price Information in the United States. [Google Scholar]
- 24.Brown JB, Nichols GA, Perry A. The Burden of Treatment Failure in Type 2 Diabetes. Diabetes Care. 2004;27(7):1535. doi: 10.2337/diacare.27.7.1535. [DOI] [PubMed] [Google Scholar]
- 25.Schmittdiel JA, Uratsu CS, Karter AJ, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23(5):588–594. doi: 10.1007/s11606-008-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel V, Xiao S, Chen H, et al. The magnitude of and health system responses to the mental health treatment gap in adults in India and China. Lancet. 2017;388(10063):3074–3084. doi: 10.1016/S0140-6736(16)00160-4. [DOI] [PubMed] [Google Scholar]
- 27.Thirunavukarasu M, Thirunavukarasu P. Training and National deficit of psychiatrists in India - A critical analysis. Indian Journal of Psychiatry. 2010;52(Suppl 1):S83–88. doi: 10.4103/0019-5545.69218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Mental Health Atlas Country Profile 2014: India. World Health Organization; 2015. [Google Scholar]
- 29.World Bank Group, World Health Organization. [Accessed July 19, 2016];Out of the Shadows: Making Mental Health a Global Priority. 2016 http://www.worldbank.org/en/events/2016/03/09/out-of-the-shadows-making-mental-health-a-global-priority#1.
- 30.Chakraborty K, Avasthi A, Kumar S, Grover S. Attitudes and beliefs of patients of first episode depression towards antidepressants and their adherence to treatment. Social Psychiatry and Psychiatric Epidemiology. 2009;44(6):482–488. doi: 10.1007/s00127-008-0468-0. [DOI] [PubMed] [Google Scholar]
- 31.Nagpal J, Bhartia A. Quality of diabetes care in the middle- and high-income group populace: the Delhi Diabetes Community (DEDICOM) survey. Diabetes Care. 2006;29(11):2341–2348. doi: 10.2337/dc06-0783. [DOI] [PubMed] [Google Scholar]
- 32.Raheja BS, Kapur A, Bhoraskar A, et al. DiabCare Asia--India Study: diabetes care in India--current status. J Assoc Physicians India. 2001;49:717–722. [PubMed] [Google Scholar]
- 33.Menon VU, Guruprasad U, Sundaram KR, Jayakumar RV, Nair V, Kumar H. Glycaemic status and prevalence of comorbid conditions among people with diabetes in Kerala. The National Medical Journal of India. 2008;21(3):112–115. [PubMed] [Google Scholar]
- 34.Kakuma R, Minas H, van Ginneken N, Dal Poz MR, et al. Human resources for mental health care: current situation and strategies for action. Lancet. 2011;378(9803):1654–1663. doi: 10.1016/S0140-6736(11)61093-3. [DOI] [PubMed] [Google Scholar]
- 35.Grover S, Avasthi A, Bhansali A, Chakrabarti S, Kulhara P. Cost of ambulatory care of diabetes mellitus: a study from north India. Postgraduate Medical Journal. 2005;81(956):391–395. doi: 10.1136/pgmj.2004.024299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shobhana R, Rama Rao P, Lavanya A, Williams R, Vijay V, Ramachandran A. Expenditure on health care incurred by diabetic subjects in a developing country--a study from southern India. Diabetes Research and Clinical Practice. 2000;48(1):37–42. doi: 10.1016/s0168-8227(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 37.Kapur A. Economic analysis of diabetes care. The Indian Journal of Medical Research. 2007;125(3):473–482. [PubMed] [Google Scholar]
- 38.Ali MK, Singh K, Kondal D, et al. Effectiveness of a Multicomponent Quality Improvement Strategy to Improve Achievement of Diabetes Care Goals: A Randomized, Controlled Trial. Annals of Internal Medicine. 2016 doi: 10.7326/M15-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katon W, Lin EH, Von Korff M, et al. Integrating depression and chronic disease care among patients with diabetes and/or coronary heart disease: the design of the TEAMcare study. Contemp Clin Trials. 2010;31(4):312–322. doi: 10.1016/j.cct.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–2620. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah S, Singh K, Ali MK, et al. Improving diabetes care: multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia--the CARRS multi-center translation trial. Diabetes research and clinical practice. 2012;98(2):285–294. doi: 10.1016/j.diabres.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao D, Lipira L, Kumar S, Mohanraj R, Poongothai S, Tandon N, Sridhar G, Katon W, Narayan KMV, Chwastiak L, Mohan V, Ali MK. Input of Stakeholders on Reducing Depressive Symptoms and Improving Diabetes Outcomes in India: Formative Work for the INtegrated DEPrEssioN and Diabetes TreatmENT (INDEPENDENT) Study. International Journal of Non-Communicable Diseases. 2016;1(2):65–75. doi: 10.4103/2468-8827.191979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 44.Lewis N. Populations, Population Health, and the Evolution of Population Management: Making Sense of the Terminology in US Health Care Today. [Accessed Jan 24, 2017];IHI Leadership Blog. 2014 http://www.ihi.org/communities/blogs/_layouts/ihi/community/blog/itemview.aspx?List=81ca4a47-4ccd-4e9e-89d9-14d88ec59e8d&ID=50.
- 45.Kindig D, Stoddart G. What is population health? American Journal of Public Health. 2003;93(3):380–383. doi: 10.2105/ajph.93.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Education and Counseling. 2013;93(2):157–168. doi: 10.1016/j.pec.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behavioural and Cognitive Psychotherapy. 2009;37(2):129–140. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 48.Rollnick S, Butler CC, Kinnersley P, Gregory J, Mash B. Motivational interviewing. BMJ. 2010;340:c1900. doi: 10.1136/bmj.c1900. [DOI] [PubMed] [Google Scholar]
- 49.Arean P, Hegel M, Vannoy S, Fan MY, Unuzter J. Effectiveness of problem-solving therapy for older, primary care patients with depression: results from the IMPACT project. The Gerontologist. 2008;48(3):311–323. doi: 10.1093/geront/48.3.311. [DOI] [PubMed] [Google Scholar]
- 50.Harpole LH, Williams JW, Jr, Olsen MK, et al. Improving depression outcomes in older adults with comorbid medical illness. Gen Hosp Psychiatry. 2005;27(1):4–12. doi: 10.1016/j.genhosppsych.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348–353. doi: 10.1370/afm.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wittkampf KA, Naeije L, Schene AH, Huyser J, van Weert HC. Diagnostic accuracy of the mood module of the Patient Health Questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29(5):388–395. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 54.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Modern Problems of Pharmacopsychiatry. 1974;7(0):79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 55.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288(22):2836–2845. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 56.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 57.Little RJA. A Test of Missing Completely at Random for Multivariate Data with Missing Values. Journal of the American Statistical Association. 1988;83(404):1198–1202. [Google Scholar]
- 58.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Statistical Methods in Medical Research. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 59.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? International Journal of Methods in Psychiatric Research. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.The Hindu. Chennai: Dec 1, 2015. Chennai misses new rain record by a whisker. [Google Scholar]
- 61.Chaitanya SVK. Deccan Chronicle. Nov 14, 2015. Chennai receives highest rainfall in Tamil Nadu. Current Affairs. [Google Scholar]
- 62.Express News Service. The New Indian Express. Chennai: Nov 17, 2015. The Day Chennai Drowned: City Experiences ‘Sunk Monday’. [Google Scholar]
- 63.Sivakumar B, Ayyappan V. The Times of India. India: Dec 1, 2015. Rain batters Chennai, parts of Tamil Nadu: Normal life hit, flights and trains delayed. [Google Scholar]
- 64.Ministry of Health and Family Welfare Department of Health and Family Welfare. Starred Question No. 253: Infrastructure to Treat Mental Illnesses. Government of India; 2015. [Google Scholar]
- 65.Fera T, Bluml BM, Ellis WM, Schaller CW, Garrett DG. The Diabetes Ten City Challenge: interim clinical and humanistic outcomes of a multisite community pharmacy diabetes care program. Journal of the American Pharmacists Association. 2008;48(2):181–190. doi: 10.1331/JAPhA.2008.07166. [DOI] [PubMed] [Google Scholar]
- 66.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 67.Hunkeler EM, Katon W, Tang L, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259–263. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Institute of Medicine Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press; 2001. [Google Scholar]
- 69.Katon WJ, Von Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]
- 70.Narayan KM, Benjamin E, Gregg EW, Norris SL, Engelgau MM. Diabetes translation research: where are we and where do we want to be? Annals of Internal Medicine. 2004;140(11):958–963. doi: 10.7326/0003-4819-140-11-200406010-00037. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor PJ, Rush WA, Davidson G, et al. Variation in quality of diabetes care at the levels of patient, physician, and clinic. Preventing Chronic Disease. 2008;5(1):A15. [PMC free article] [PubMed] [Google Scholar]
- 72.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. The Milbank Quarterly. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 73.Unnikrishnan R, Anjana RM, Deepa M, et al. Glycemic control among individuals with self-reported diabetes in India--the ICMR-INDIAB Study. Diabetes Technol Ther. 2014;16(9):596–603. doi: 10.1089/dia.2014.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]