Abstract
The role of genetic mutations in cancer is indisputable: They are a key source of tumor heterogeneity and drive its evolution to malignancy. But, the success of these new mutant cells relies on their ability to disrupt the homeostasis that characterizes healthy tissues. Mutated clones unable to break free from intrinsic and extrinsic homeostatic controls will fail to establish a tumor. Here, we will discuss, through the lens of mathematical and computational modeling, why an evolutionary view of cancer needs to be complemented by an ecological perspective to understand why cancer cells invade and subsequently transform their environment during progression. Importantly, this ecological perspective needs to account for tissue homeostasis in the organs that tumors invade, because they perturb the normal regulatory dynamics of these tissues, often coopting them for its own gain. Furthermore, given our current lack of success in treating advanced metastatic cancers through tumor-centric therapeutic strategies, we propose that treatments that aim to restore homeostasis could become a promising venue of clinical research. This ecoevolutionary view of cancer requires mechanistic mathematical models to both integrate clinical with biological data from different scales but also to detangle the dynamic feedback between the tumor and its environment. Importantly, for these models to be useful, they need to embrace a higher degree of complexity than many mathematical modelers are traditionally comfortable with.
A tumor invades and evolves into a tightly regulated homeostatic tissue environment. An ecoevolutionary perspective of cancer, based on mathematical models, can offer insight into tissue homeostasis, its disruption during tumor invasion, and its restoration during therapy.
Historically, cancer biology has been an almost entirely empirical field where mathematical theory has been largely neglected. But, theoretical frameworks not only allow us to make sense of (often) counterintuitive experimental data but also help define new hypotheses and identify holes in our understanding that need to be addressed (Kuhn 2012). Mathematical models allow us to formally define (and combine) biological mechanisms in such a way that the logical consistency can be checked, the assumptions thoroughly analyzed, and scenarios tested at a speed and cost unmatched by experimental models (Möbius and Laan 2015). This is particularly important given the long timescales and multiple interconnected spatial scales involved in the evolution of cancer. Without the aid of mathematical and computational models, we have no easy way to understand cancer evolution (Byrne et al. 2006; Servedio et al. 2014).
Mathematical and computational models of cancer have seen rapid growth in the last two decades. The emerging field of mathematical oncology, embraces mathematical modeling as a key tool to systematically understand the mechanisms underlying all aspects of cancer progression and treatment and, perhaps even more importantly, to make predictions about future outcomes. In recent years, there have been some excellent reviews on mathematical models of cancer (Anderson and Quaranta 2008; Byrne 2010; Jackson 2014; Korolev et al. 2014; Altrock et al. 2015); our focus here, however, is more explicitly tied to models that consider an ecological view of cancer.
Normal tissues contain a multitude of cell types, molecular signals, and microenvironmental features that work in symphony to ensure tissue function, as well as maintain homeostasis. This homeostasis is dynamic and naturally emerges from the interplay between life and the environment (Dyke and Weaver 2013). The majority of cancer models, mathematical or experimental, neglect both the microenvironment in which cancer originates and to which it metastasizes. This is in part owing to the dynamic and complex nature of the dialogue between the tumor and its environment. However, because the cancer microenvironment has a strong selective influence on the course of cancer evolution (Mueller and Fusenig 2004; Tlsty 2008; Lu et al. 2012), we cannot continue to ignore it. Although genetic mutations can partially explain the diversity that is required for somatic evolution, the physical microenvironment and the interactions with other cells are the source of selection that drives evolution. The importance of the environment in evolution may even justify a new view of evolution based on niche construction, which would replace the standard genetic-based view (Turner 2016).
Although normal tissues are robust against many perturbations (Basanta et al. 2008a; Gerlee et al. 2011), tumors that will grow to become clinically relevant can disrupt tissue homeostasis beyond the point of recovery. This can lead to a process of clonal evolution (Nowell 1976; Gerlinger et al. 2012; Greaves 2015), in which an initial aberrant cell grows, adapts to (and alters) the environment, and diversifies. In fact, this diversity, known as intratumor heterogeneity (ITH), is a pervasive feature of most cancers and is increasingly correlated with poor prognoses (Gay et al. 2016). There are multiple sources of heterogeneity in cancer (Welch 2016); ITH is not only limited to genetic changes but can include epigenetic (Heng et al. 2009; Easwaran et al. 2014; Angermueller et al. 2016), metabolic (Damaghi et al. 2015; Robertson-Tessi et al. 2015; Hensley et al. 2016), and microenvironmental (Lalonde et al. 2014; Mumenthaler et al. 2015; Natrajan et al. 2016). Importantly, this heterogeneity is dynamic (Fisher et al. 2013), which means that where and when mutations occur is equally important (Swanton 2012; Sottoriva et al. 2015). These facts highlight the degree of complexity that makes studying somatic evolution very challenging, especially if we restrict ourselves to experimental techniques alone. Mathematical models can help understand cancer growth (Gatenby and Maini 2003; Michor et al. 2004; Anderson and Quaranta 2008; Byrne 2010; Altrock et al. 2015) and cancer evolutionary dynamics and shed light on the role of ITH in both progression and treatment response (Anderson et al. 2006; Iwasa and Michor 2011).
In this paper, we will describe, through the lens of mathematical modeling, how homeostasis disruption can explain cancer initiation, help determine its evolution, and suggest novel treatment approaches.
MODELS OF NORMAL HOMEOSTASIS AND ITS DISRUPTION
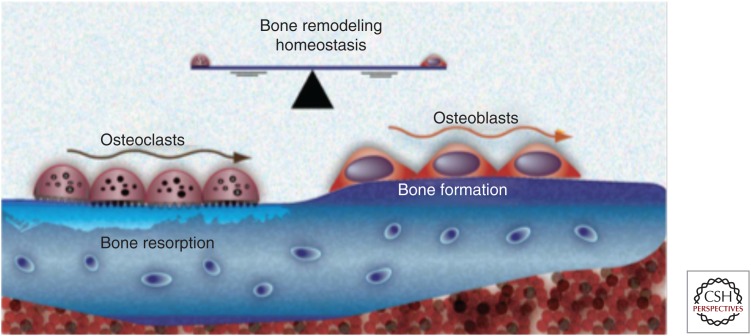
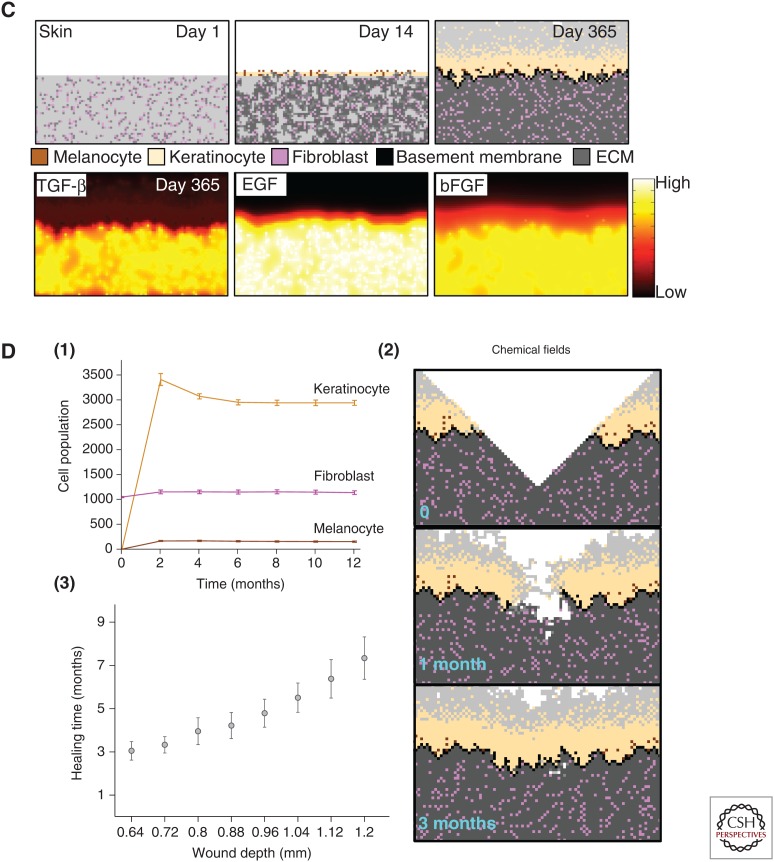
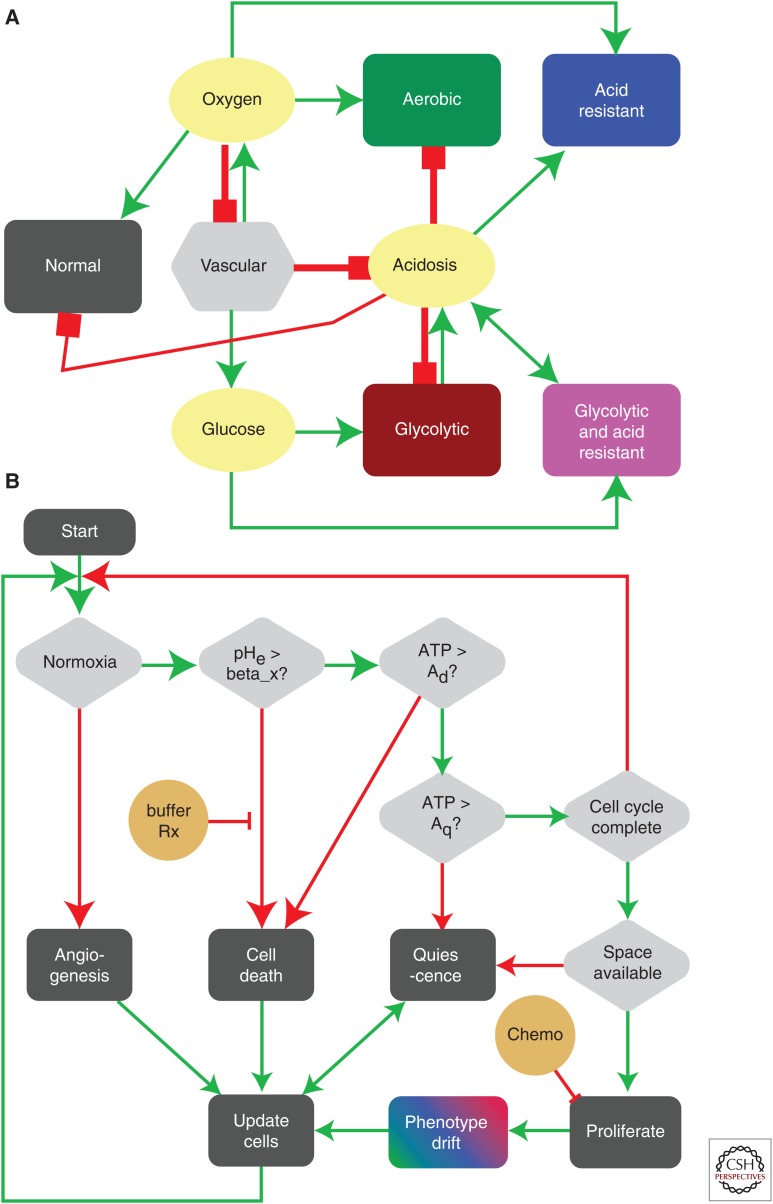
The human organism is a complex multiple scale biological system in which multiple organs act as a unified organ system to maintain both health and function. In turn, individual organs are made up of multiple tissues that form specific structures for defined tasks and these tissues contain billions of individual cells that perform diverse functions using many distinct cell types. Much of the communication between individual components of this multiscale system are driven by autocrine and paracrine signals that are either produced by individual cells or as a result of interactions between cells. This communication drives a tight dialogue between individual components of the system that regulates and maintains organ and whole organism homeostasis. For example, in the case of bone homeostasis (Figs. 1,4A), the interactions between bone-destroying osteoclasts and bone-producing osteoblasts are mediated by molecules such as transforming growth factor β (TGF-β) and Receptor activator of nuclear factor κB ligand (RANKL) and results in bone remodeling and maintenance. In the skin (Fig. 4C–D), keratinocytes (composing 95% of the epidermis) and melanocytes (located on the basement membrane) interact together with factors (epidermal growth factor [EGF], TGF-β, and basic fibroblast growth factor [bFGF]) secreted by dermal fibroblasts to regulate cell turnover and maintain homeostasis. Homeostasis is also important at the microenvironmental level and key elements like the extracellular matrix (ECM) are maintained by a process that, if disrupted, can lead to fibrosis, cancer initiation, and metastasis (Cox and Erler 2011).
Figure 1.
Homeostasis in organs like the bone is dynamic and orchestrated by a variety of cell types and signaling factors. This allows the organ to maintain form and function even in the presence of genetic or environmental insults. (From Strand et al. 2010; reprinted, with permission, from Bentham Science Publishers © 2016.)
Figure 4.
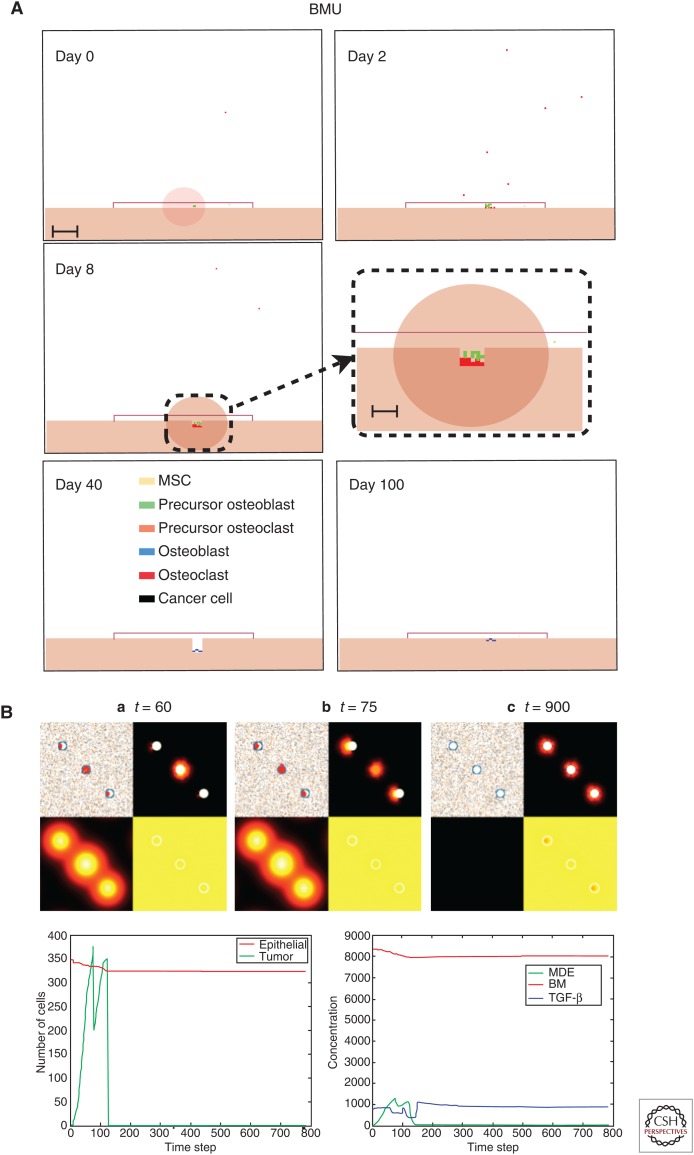
Agent-based modeling of homeostasis in bone and skin. (A) Agent-based models such as the hybrid cellular automaton (HCA) can describe complex homeostasis like the one in the bone, orchestrated by several cell types and involving a number of signaling molecules (Araujo et al. 2014). (B) An HCA model of prostate cancer with homeostasis of the epithelial cells. Although not always the case, in some simulations, homeostasis can be restored even after cancer initiation. (C) An HCA model of melanoma initiation showing a homeostatic layer of skin growing over the course of a year via interactions between three cell populations (melanocytes, keratinocytes, and fibroblasts) modulated by growth factors (transforming growth factor β [TGF-β], epidermal growth factor [EGF], and basic fibroblast growth factor [bFGF]). (D) (1) Cell population dynamics highlight homeostatic fractions; (2) an emergent property of the homeostatic skin structure is its ability to heal in response to (3) wounding events of differing depths.
Homeostasis, at its simplest, is the balance between cell proliferation and apoptosis that preserves the architecture and functionality of the organ. Homeostasis is very robust, because tissues remain fully functional, maintaining shape and size even under significant genetic perturbations and environmental insults.
Organ ecology and homeostasis varies from organ to organ as the mechanisms involved depend on the importance of the organ (Thomas et al. 2016) and the purpose they serve. Mathematical models allow us to explore the mechanisms of homeostasis of relevant tissues, understand how different tumor phenotypes can disrupt this homeostasis, explore how tumor heterogeneity changes through space and time, and understand the impact of new competitive and cooperative interactions on tumor growth and progression. As we discussed previously (Basanta and Anderson 2013), mathematical tools like evolutionary game theory (with its focus on simplicity and cell–cell interactions) and agent-based modeling (with strengths in capturing heterogeneity and the physical microenvironment) are ideal frameworks in which to capture these processes.
We previously used a combination of agent-based modeling and a genetic algorithm to “evolve” homeostatic organisms capable of first growing and then maintaining a target shape and size (Basanta et al. 2008a). Our results show that evolution of homeostasis also selects for robustness such that the organisms that achieved a higher degree of homeostasis were also better at restoring it after external insult or “genetic” aberrations. In subsequent work (Gerlee et al. 2011), we used a hybrid cellular automaton (HCA) model to evolve homeostasis and showed that evolution may select for two different strategies to achieve homeostasis: wasteful (with high proliferation and death rates) and conservative (low proliferation and death rates). Both strategies are successful in achieving homeostasis but show different types of susceptibility to mutations, which might happen at any stage of the cell’s life cycle or during division. Specifically, wasteful phenotypes were more suited to low mutation rates during replication and higher spontaneous mutation rates (i.e., cosmic ray mutations that can occur at any stage of the cell’s life cycle), whereas the conservative phenotype is better suited to higher mutation rates during replication and low spontaneous mutation rates. Both pieces of research highlight how homeostasis is dynamic and robust to many types of perturbation. More recently, Csikász-Nagy et al. (2013) have used a combination of game theoretical and network modeling to model homeostasis and how disruption of the interactions between normal cells could help a newly established cancer grow.
THE CANCER ECOSYSTEM
Tumors are not simply collections of mutated cells that grow in isolation of the environment in which they live. They interact with and modify both the physical microenvironment and a variety of nontumor cells that make up the organ in which the cancer originated. Given the importance of these interactions, it is useful to consider cancer as an ecosystem, a view that highlights the importance of noncancer cells and physical microenvironmental features (Merlo et al. 2006; Kareva 2011; Greaves 2015). This view also allows us to better understand cancer initiation, its evolution, and the impact of treatment (Pienta et al. 2008; Chen and Pienta 2011; Basanta and Anderson 2013). Perhaps the most important, but usually overlooked, feature of organ ecosystems is that before cancer developed, the organ was a dynamic and functional homeostatic system. An ecological view of cancer envisions tumor cells as a new species invading a healthy ecosystem disrupting its homeostasis (Lloyd et al. 2016). Not all invading species are successful, but those that are, can take advantage of their local environment as well as find weaknesses in the mechanisms that control homeostasis. Thus, homeostatic mechanisms not only constitute a barrier that needs to be overcome by an evolving tumor, but also means that understanding them is the first step in understanding the evolutionary trajectory of a tumor (Frank and Rosner 2012). This is especially important considering that early mutations might have a disproportionate impact on how tumors evolve (Sottoriva et al. 2015).
Like many cancers, prostate cancer becomes lethal when it metastasizes and, in 90% of the patients, there is evidence of metastases to the bone. The elements that make up the bone ecosystem that prostate cancer might subsequently disrupt are well known (Mundy 2002). Figure 2 shows some of the elements that characterize the normal bone ecosystem (left) and how a successful (and heterogeneous) metastasis can disrupt that homeostasis leading to a vicious cycle promoting metastatic growth (center). Mathematical models (Araujo et al. 2014) have allowed us to explore which phenotypes usually lead to successful metastases.
Figure 2.
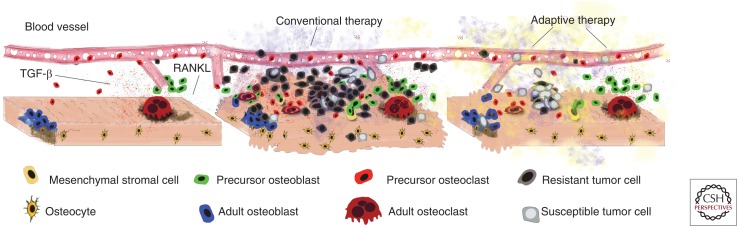
Tissue homeostasis, disruption, and restoration. An example of dynamic tissue homeostasis in the bone including bone resorpting osteoclasts and osteoblasts (left). With the introduction of tumor cells (center), this homeostasis is disrupted leading to an altered microenvironment and increasingly malignant tumor. Conventional application of treatments leads to an increasingly resistant tumor. (Right) Ecoevolutionary enlightened treatments aim to restore some degree of homeostasis while allowing the tumor to remain treatable.
EVOLUTION AND SELECTION
It is well known that selection acts on the phenotype, not the genotype. Hanahan and Weinberg (2011) summarized decades of research in cancer genetics to identify six, later extended to ten, phenotypic hallmarks that characterize the emergence of aggressive metastatic cancers. Axelrod et al. (2006) suggested that, given the right ITH, a group of cancer cells could collectively gain all the hallmarks to be an aggressive metastatic cancer. Although it is assumed that competition is the main type of interaction between cancer cells in a tumor, cooperative behavior could help the tumor achieve all the hallmarks much faster than in the case in which every cell has to gain all the phenotypic traits described by Hanahan and Weinberg. Together with spatial heterogeneity (Komarova 2014; Kaznatcheev et al. 2015), the ability of the tumor to evolve is limited by phenotypic heterogeneity (Korolev et al. 2014; McGranahan and Swanton 2015). It is clear then that, although we are now beginning to understand some of the mechanisms that underpin heterogeneity (Sutherland and Visvader 2015) and how this heterogeneity correlates with prognosis (Gay et al. 2016) and treatment response (Junttila and de Sauvage 2013), it is nontrivial to properly understand how evolution impacts cancer progression (Miller et al. 1989), which further emphasizes the need for mathematical modeling. Mathematical tools like evolutionary game theory (Smith 1982; Basanta and Deutsch 2008; Hummert et al. 2014) can be used to investigate how the interactions between tumor cells, with different phenotypic strategies, can impact selection (Basanta 2015). Tomlinson investigated angiogenesis as a public good (Tomlinson and Bodmer 1997), work that was followed by Bach et al. (2006), examining how an effort that requires cooperation (such as angiogenesis) can emerge in a tumor population. The results in both models show that this type of cooperation would allow for one of the cancer hallmarks to emerge even when many tumor cells are nonangiogenic. Further work by Archetti et al. (2015), using evolutionary game theory and in vitro modeling, explains how cooperation and ITH can be maintained with other factors like IGF (a growth factor) that can effectively be considered as public goods.
Evolutionary game theory can provide further insights on the interplay between heterogeneity and evolution. For instance, we explored the emergence of invasive phenotypes in cancer using a simple game between two tumor cell phenotypes, purely proliferative and motile cells (Fig. 3) (Basanta et al. 2008b). In this game, the only factor driving the emergence of invasive phenotypes was the cost of motility (which depends on the type of cancer such that the cost is lower in cancers like leukemia and higher in solid tumors). The introduction of a new phenotypic strategy, that of tumor cells with a glycolytic metabolism (resulting in acidification of the microenvironment), leads to selection for invasive phenotypes when we consider the scenarios that paradoxically should favor glycolytic cells (e.g., when the cost of environmental acidification is higher for proliferative cells). Even if glycolytic cells were not abundant in the game, the results show that they played a key role in the evolutionary dynamics of the cancer in ways that would be hard to elucidate with experimental data alone.
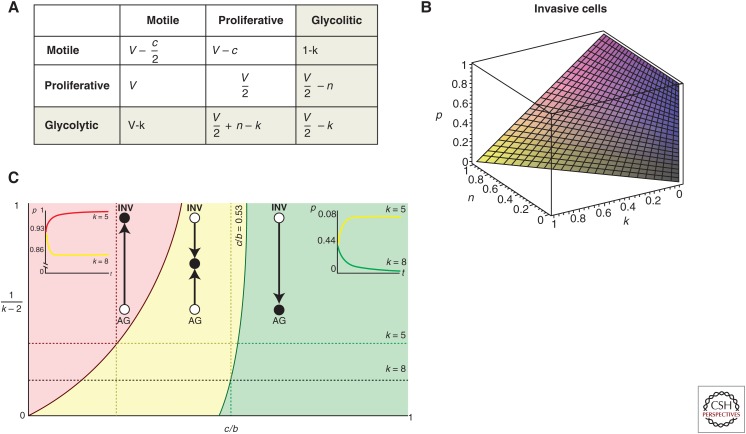
Figure 3.
Game theory is a tool to understand the impact of interactions in selection. (A) Payoff table describing the interactions between proliferative, invasive, and glycolytic cells. (B) Proportion of invasive cells goes up as we increase the heterogeneity of the original game to consider glycolytic cells. (C) Space also plays a role in selection; using an Ohtsuki–Nowak transform, we investigated the impact of hard edges (like bone) on evolutionary dynamics. Here, we plot level of viscosity 1/(k-2) versus relative cost of motility c/b and highlight three regions with qualitatively different dynamics. In the red, the population evolves toward all invasive (INV); in the yellow, toward a polyclonal tumor of INV and proliferative cells (autonomous growth [AG]); and in the green, the tumor.
Evolution is driven by stochastic processes and is notoriously difficult to predict (Lipinski et al. 2016); this is especially true for cancer, even with the help of a mathematical model. This does not mean cancer evolution is entirely stochastic. There are constraints that could help define potential evolutionary trajectories (Venkatesan and Swanton 2016). Some of these constraints are related to the specific location of the tumor cells with regard to the rest of the cancer (Lloyd et al. 2016), and homeostasis itself may dictate which intrinsic and extrinsic barriers need to be broken first. Therefore, by understanding the microenvironmental context in which tumors emerge and the homeostatic mechanisms that need to be disrupted (Gerlee et al. 2011; Araujo et al. 2014), we can better understand the evolutionary trajectories of successful tumor cells. As Hanahan and Weinberg's work shows, although the specific genetic mutations might not be clear, what is clear are the traits that need to be acquired by a successful cancer initiating cell or metastasis. In the case of prostate cancer to bone metastases for instance, being able to disrupt the dialogue between bone-resorbing osteoclasts and bone-producing osteoblasts. This is usually accomplished through the up-regulation of the signaling molecule TGF-β. Therefore, understanding the mechanisms that regulate tissue homeostasis will allow us to better predict the first steps in the evolutionary process, which in certain cancers could be the only steps that matter (Sottoriva et al. 2015).
In some tumors, it has been observed that mutations that drive evolution are present from the early stages (Sottoriva et al. 2015), restricting the number of evolutionary paths in subsequent progression, that is, first versus fittest (Robertson-Tessi and Anderson 2015). Even with the limitations of commonly used sequencing tools, such as Sanger sequencing (Schmitt et al. 2016), they are currently the best way to quantify ITH (McGranahan and Swanton 2015). Bioinformatic tools allow for the extensive interrogation of molecular data to uncover driver mutations (Gonzalez-Perez et al. 2013; Zhang et al. 2014; Forbes et al. 2015), molecular signatures that characterize a variety of cancer types, and evolutionary paths (Nielsen 2005; Sotiriou and Piccart 2007; Liberzon et al. 2011; Alexandrov et al. 2013; Alexandrov and Stratton 2014) and even potential immunogenicity (Snyder and Chan 2015). But, this analysis is fundamentally correlative, tumor-centric, and provides only a cursory understanding of the underlying biological mechanisms at play without exhaustive substantiation. However, we do not mean to imply they are without utility; in fact, there is an opportunity to integrate what we learn from the molecular scale with data from the tissue (histology) and organ (imaging) scales through mechanistic mathematical models that embrace the microenvironment. This type of integrated analysis and modeling is still in its infancy, but it has great potential to bridge the divide between the data rich molecular and data poor clinical aspects of cancer (Sottoriva et al. 2015; Williams et al. 2016).
ECOEVOLUTIONARY MODELS OF CANCER
Given the importance of homeostasis in regulating tissue form and function, it is surprising that models (both experimental and mathematical) typically assume that tumors are present at the start, have already disrupted tissue homeostasis, and ignore surrounding tissue interactions. The necessity to disrupt homeostasis represents a key adaptation for tumor cells and, in complex nonlinear systems, this transition can be sudden and difficult to observe experimentally (Trefois et al. 2015). Importantly, understanding homeostatic mechanisms can help predict robustness (Scheffer et al. 2012), identify tipping points (Scheffer et al. 2009), and anticipate the most likely evolutionary dynamics of the runaway cancer. In this section, we will discuss some models that consider normal tissue homeostasis as a prerequisite for tumor initiation. This invariably means the models are more complex and often multiscale, because they need to take into account both tumor-centric and environment-centric mechanisms as well as the dialogue between them.
Hybrid models provide a natural way to integrate both discrete and continuous variables that are used to represent individual cells and microenvironmental variables, respectively (Rejniak and Anderson 2011). Each discrete cell can also be equipped with submodels that drive cell behavior in response to microenvironmental cues. Moreover, the individual cells can interact with one another to form and act as an integrated tissue. In our previous work, we used such an HCA approach to characterize the glandular architecture of prostate tissue and its homeostasis through a layered epithelial homeostasis via TGF-β signaling regulated by surrounding stroma (Basanta et al. 2009). Using this model, we examined (see Fig. 4B) prostate cancer initiation and were able to capture prostatic intraepithelial neoplasia (PIN), as well as invasion, and make some interesting hypotheses regarding the differential effects of stroma and TGF-β. For example, in PIN, TGF-β recruits stromal cells, which structurally inhibit glandular breakdown through matrix production, whereas TGF-β is more likely to promote growth once tumor cells emerge from a contained PIN-like state and facilitate stromal activation. In subsequent work, we used a similar computational approach to explore bone homeostasis emerging from the interactions between osteoclasts, osteoblasts, and other bone-resident cells (Araujo et al. 2014). Using this model, we investigated how prostate cancer cells can disrupt bone remodeling and how this disruption leads to a vicious cycle that enables the tumor to grow.
To understand melanoma initiation, we developed an in silico model of normal skin that incorporates keratinocytes, melanocytes, fibroblasts, and other key microenvironmental components of the skin (Kim et al. 2015). The model recapitulates normal skin structure that is robust enough to withstand physical (Fig. 4C,D) as well as biochemical perturbations. An important prediction this model generated was that senescent fibroblasts can create a favorable environment for melanoma initiation by facilitating mutant melanocyte growth through growth factor production and matrix degradation. This suggests that senescent fibroblasts should be considered as a therapeutic target in the early stages of melanoma progression.
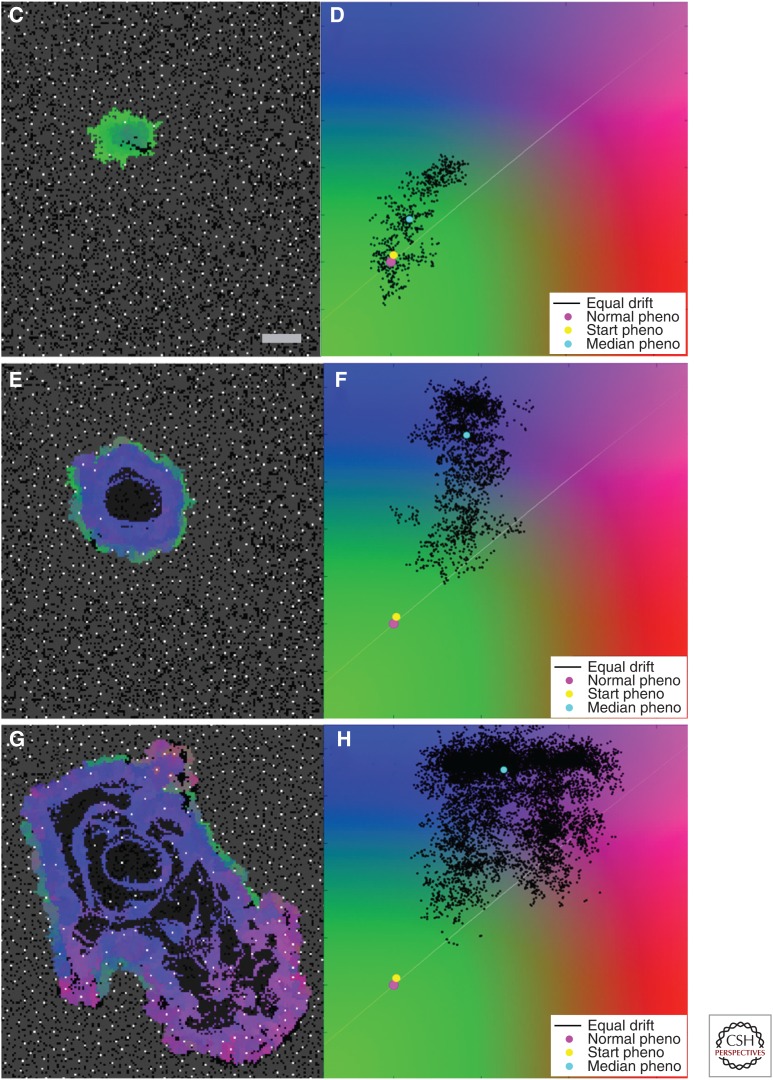
In a recent paper, we developed an HCA model to investigate the evolution of acid-mediated invasion (Robertson-Tessi et al. 2015). The model incorporates normal cells, blood vessels, aerobic, glycolytic, acid-resistant tumor cells, as well as environmental variables such as oxygen, glucose, and acidosis. Tumor cells in the model have two continuously variable, heritable traits: glucose consumption and resistance to extracellular pH (Fig. 5A). Cells interact through the HCA decision algorithm (Fig. 5B). Importantly, the normal cells and blood vessels, along with cell oxygen and glucose production/consumption form a homeostatic tissue. An emergent property of this model is that normal tissue can recover from both chemical and physical damage. The model examines the evolution of tumor phenotypes over time into this normal tissue space, finding that poor vasculature leads to acid resistance in the center of the tumor, followed by selection for aggressively glycolytic cells (Fig. 5C–F). Eventually, these metabolically atypical clones escape the niche that selected for them and interface with the normal tissue. The excess acidosis created by these cells destroys normal tissue and leads to rapid invasion (Fig. 5G,H). Varying the vessel density in time and space leads to different patterns of necrosis and invasive phenotypic distributions, a possible explanation for different histological patterns seen in different grades of cancers. This model also highlighted the risks of cytotoxic and antiangiogenic treatments in the context of tumor heterogeneity resulting from a selection for more aggressive behaviors.
Figure 5.
Multiscale metabolic cancer invasion model. (A) A hybrid cellular automaton (HCA) model interaction network highlights positive (green) and negative (red) cellular and microenvironmental interactions. (B) Cell life-cycle flowchart that every cell experiences, dictating cell proliferation, death, and mutation depending on the levels of ATP, oxygen, and pH. (C,E,G) Heterogeneous tumor growth and evolution at 2, 8, and 20 weeks. (D,F,H) Corresponding phenotypic (x-axis, glycolytic activity; y-axis, acid resistance) distribution at the same simulation time points.
BRIDGING THE DIVIDE
Clinical trials are the standard way in which basic science discoveries are translated into clinical practice. A major issue with these trials in cancer is their high failure rate, even though the trials were driven by data from successful equivalent preclinical studies. This is, in part, because of the intrinsic homogeneity of preclinical model systems and the contrasting heterogeneity of actual patient responses. It is becoming increasingly clear that not all preclinical outcomes scale to human populations and that mathematical models need to be developed with this in mind to allow researchers to explore how intrapatient heterogeneity in human cohorts would impact novel therapies developed and tested using in vitro and/or in vivo models (Kim et al. 2015).
We and others have used clinically and experimentally parameterized mathematical models to predict and to understand the impact of treatments in cancer. Mechanistic first-principles mathematical models help us not only to predict “when” treatments fail to work but also help us understand “how” they fail. However, this understanding has largely been focused on a tumor-centric perspective and, while this is certainly important, we only have a very limited understanding of how treatments impact normal cells and the microenvironment of the tumor (Medler et al. 2015). This further highlights the importance of mathematical models and how they could be used to inform clinical trials (Rockne et al. 2010; Mumenthaler et al. 2011; Scott 2012; Adair et al. 2014; Baldock et al. 2014; Gallaher et al. 2014; Leder et al. 2014; Kim et al. 2015; Walker and Enderling 2015; Poleszczuk et al. 2016).
These mathematical models can help us better understand how interventions impacting key genetic or epigenetic drivers, may or may not shape the evolutionary dynamics of the disease in the right direction. But, for these models to be useful, and to understand homeostatic mechanisms, they need to be able to account for the interactions between cells and between those cells and their environment. That is why network models, evolutionary game theory (Basanta 2015; Brown 2016), and especially hybrid agent-based models that explicitly account for environment and heterogeneity are so important (Anderson 2005; Anderson et al. 2006; Gerlee and Anderson 2008).
Hybrid agent-based models can also be used to investigate the impact of targeting specific aspects of the tumor system. Often, what looks like a key driver of an invasive process that can be therapeutically targeted but might result in a tumor that is more invasive or difficult to treat. For instance, in bone metastatic prostate cancer, stromal cells, like osteoclasts, can be targeted by treatments like bisphosphonates but experimental (Logothetis and Lin 2005) and mathematical research (Basanta et al. 2011a; Araujo et al. 2014) show that this approach is not sufficient to prevent successful metastatic growth. In other cases, treatments that have shown poor results in the clinic can be rejuvenated through different dosing or combination with others. Well-characterized molecules, such as TGF-β for instance, might play dual roles (Bierie and Moses 2006), but only through careful mathematical modeling are we be able to understand whether they present clear clinical targets (Basanta et al. 2009; Cook et al. 2016). In the multiscale metabolic model we discussed in Figure 5 above, targeting the vasculature through an antiangiogenic therapy leads to a more aggressive and invasive cancer (Robertson-Tessi et al. 2015). This is because the therapy actually induces hypoxia and accelerates the acquisition of the acid-resistant/glycolytic phenotype, which may be a key reason for the failure of many antiangiogenic therapies (Rapisarda and Melillo 2012; Quintieri et al. 2014).
ADAPTIVE THERAPY AND HOMEOSTASIS RESTORATION
In the context of cancer as an evolutionary process, it is sometimes easy to forget that treatments constitute selection. The prevalence of intratumor heterogeneity means that some clones will be negatively impacted by treatment but also that some will be left unaffected by it. Although treatment can reduce tumor burden and also, even if transiently, reduce heterogeneity (Gerlinger and Swanton 2010), the emergence of resistance is common. Relapse is usually driven by clones that are not the linear descendent of the most common clone at diagnosis (Wang et al. 2016). We need to understand how treatment-derived selection facilitates resistance (Hata et al. 2016) and changes the tumor in ways that make further treatment more difficult, if not impossible (e.g., multidrug resistance). Critical to this understanding are the costs that treatments represent to the different phenotypic strategies present in an evolving tumor. Because this will allow us to synergize treatments such that evolving resistance to one drug makes cells more susceptible to another (Basanta et al. 2011b, 2012; Orlando et al. 2012). Traditionally, cancer cells have been seen as competing with each other but, in reality, a variety of interactions could be taking place within a cancer (Axelrod et al. 2006; Strand et al. 2010; Thomas et al. 2012; Basanta and Anderson 2013; Brown 2016). Cooperative behaviors allow tumor cells to acquire cancer hallmarks faster but also suggest that disrupting this cooperation could seriously impact the tumor’s overall aggressiveness (Axelrod et al. 2006; Archetti 2013; McGranahan and Swanton 2015). Targeted treatments have been less successful than initially anticipated, in part due to their continuous delivery that inevitably leads to selection for resistance (Gillies et al. 2012). However, if used in combination with other treatments through the lens of evolution they could help to steer the evolutionary dynamics of the disease to facilitate long-term control.
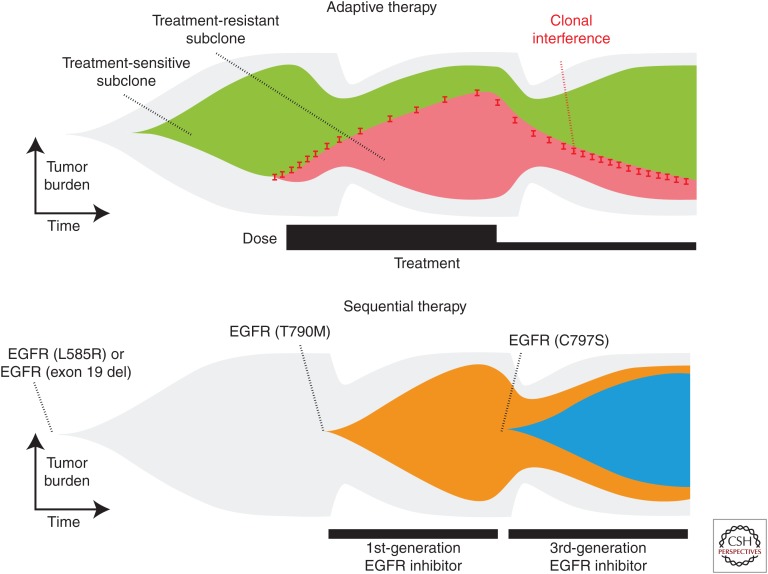
Until recently, most cancer treatments aimed to kill as many tumor cells as possible while minimizing damage to healthy ones. This approach does not take into consideration heterogeneity (Alfonso et al. 2014) or how they impact selection in somatic evolution (Miller et al. 1989; Gerlinger and Swanton 2010; Greaves and Maley 2012). Evolutionary enlightened therapies, such as adaptive therapy (Fig. 6), constitute one important step toward treatments that not only understand this impact but also exploit it. The theoretical framework underlying adaptive therapies (Gatenby et al. 2009) has been tested experimentally (Enriquez-Navas et al. 2016) and is now being used in a clinical trial at the Moffitt Cancer Center (NCT02415621). Gatenby and colleagues recognized that the standard maximum tolerated dose approach used in many treatments kills all the sensitive cells to allow for unopposed proliferation of any remaining resistant cells; this phenomenon is called competitive release (Adkins and Shabbir 2014). Their adaptive strategy instead is to design treatments that aim for control by leaving behind a residual population of drug-sensitive cells, rather than only resistant ones, which will regrow when treatment is paused and allow for subsequent future treatment applications. This ultimately means each patient’s treatment will be adapted based on their response rather than any single fixed regime. This effort is likely to be only the beginning of other mathematically led efforts with not only biological but also clinical impact. Subsequent work should look not only at the competitive interactions between resistant and susceptible tumor cells but also at the myriad interactions between a heterogeneous tumor with stromal and immune cells (Barron and Rowley 2012) as well as the microenvironment (Meades et al. 2009; Fedorenko and Smalley 2015; Hirata et al. 2015).
Figure 6.
Conventional sequential application of existing treatments almost inevitably select for resistant clones leading to untreatable tumors and uncontrolled growth. Evolutionary-enlightened treatments like adaptive therapies (top) aim to maintain a degree of homeostasis and control over the tumor growth. (From Venkatesan and Swanton 2016; reprinted, with permission, from the American Society of Clinical Oncology © 2016.)
Furthermore, an understanding of how treatments differentially impact tumor cells (Andor et al. 2015; Gao et al. 2015) and the microenvironment would allow mathematical models to play the long game: To use treatments not as ends in themselves but as a means to steer somatic evolution in a direction where the tumor either finds itself in an evolutionary dead end (Maley et al. 2004), its growth can be controlled for long periods of time (Gatenby et al. 2009) or its size reduced permanently (Foo and Michor 2009; Gallaher et al. 2014). Halting evolution once it has started is incredibly difficult (Greaves 2015; Robertson-Tessi and Anderson 2015; Sottoriva et al. 2015) but steering it might be an option for the next generation of oncologists with sufficient treatment options and time to deploy them (Nichol et al. 2015). Critically, all this information needs to be integrated into mathematical/computational frameworks in a way that is patient-specific—any steering or antievolutionary treatment strategy must be tailored to a specific cancer in a given patient, which inevitably means the end of fixed treatment regimens.
Together, these facts suggest that, in the future, we will implement clinical approaches that shape the evolutionary dynamics of tumors (rather than ignoring them) making them easier to treat, with fewer doses of drug and therefore reducing the impact on quality of life. It is possible that this might lead to a sequence of treatment combinations that completely eradicate the tumor. However, the lack of adequate targeted treatments or the presence of resistant clones that cannot be impacted either directly or indirectly (including other cell types or factors supporting their growth or resistance) means that we should focus on control rather than eradication, especially for disseminated disease. Adaptive therapies, as championed by Gatenby et al. (2009), represent a substantial step in this direction. Although not presented this way, adaptive therapies constitute an effort to accept that tumor cells cannot be eradicated and thus some sort of compromise needs to be enforced in which homeostasis is the goal. As shown in Figure 2 (left and center), tumors become a disease because they disrupt homeostasis. Novel treatment strategies could be designed and guided by mathematical and computational models (Gallaher et al. 2014) to steer evolution toward a return to homeostasis, even if different from the normal tissue before carcinogenesis (Fig. 2, right). This treatment-enforced homeostasis could give new hope to patients with the more heterogeneous and disseminated cancers, a hope that existing treatment strategies do not offer.
A DELICATE BALANCE BETWEEN COMPLEXITY AND UNDERSTANDING
The biological community has long acknowledged the complexity of cancer and has developed a dizzying array of tools to dissect, quantify, and test almost every aspect of it through all stages of progression and treatment. This reductionist paradigm has given us a detailed understanding of many of the component parts that define cancer as exemplified by the seminal hallmark papers (Hanahan and Weinberg 2000, 2011). However, we are now left with the fundamental problem of how to piece these individual components back together to define the cancer system. The enormous data sets already collated and currently being generated, largely at the molecular scale, need to not only be mined for correlations, but also to be integrated with data across biological scales to understand causation. Bridging the divide between genotype and phenotype, between cells and tissues, organs and organ systems, and individuals and populations remains an incredibly challenging and understudied question. However, this question is critical to the understanding and future success of cancer research and why integrated mathematical/experimental/clinical approaches are so important.
Taken together, the reductionist component parts and the multiscale nature of cancer, it is clear we have an overwhelming menu of potential players in building ecoevolutionary models of cancer—this begs the question what should we include and what should we leave out? One might be tempted to try to include everything and build the ultimate “kitchen sink” model of cancer. But building a model that is almost as complex as the real system would only provide a poor cartoon version of reality that may mimic it but provide little gain in our understanding of how it works. Aiming for truly minimal models is not an option if we aim, as this piece advocates, to understanding key aspects of cancer progression such as homeostasis disruption. A sufficient degree of realism and complexity is required to avoid biasing the evolutionary route that a tumor might take to that hard-coded into the model. Therefore, we need to be cautious and build models that walk the tightrope between complexity and understanding, to include sufficient details as to be useful and critically understandable.
Ultimately, this highlights the fact that building mathematical models of cancer is somewhat of an art that requires skill, thought, intuition, a delicate balance between complexity and simplicity, and, crucially, a dialogue with biologists. Placing these important variables in the right modeling framework can produce novel insights into the fundamentals of the cancer process and naturally lead to experimentally testable hypotheses. Therefore, it is clear that the key lies really in the biological question that needs to be answered. This should drive the model derivation and define the scales at which the model operates and bridges. In the context of this review, ecoevolutionary models will always need to be more complex because they integrate both tumor and microenvironmental aspects of cancer and are driven by the central premise of developing in normal homeostatic systems. However, this complexity, by constraining model dynamics, helps the model better forecast evolution and avoid dynamics that, although interesting, will have little relevance to those observed in biology or in the clinic. We believe that modular approaches to integrate multiple mathematical models into a more complex ecoevolutionary system will become one of the more important future directions in the mathematical oncology community.
ACKNOWLEDGMENTS
We thank Dr. Arturo Araujo for his help in producing the figures in this manuscript. We also thank Dr. Robert Gatenby for helpful discussions regarding adaptive therapies. Finally, D.B. acknowledges a Moffitt Team Science award and Grant National Institute of Cancer (NCI) U01CA202958-01, D.B. and A.R.A.A. acknowledge NCI U01CA151924, and A.R.A.A. acknowledges NCI U54CA193489.
Footnotes
Editors: Charles Swanton, Alberto Bardelli, Kornelia Polyak, Sohrab Shah, and Trevor A. Graham
Additional Perspectives on Cancer Evolution available at www.perspectivesinmedicine.org
REFERENCES
- Adair JE, Johnston SK, Mrugala MM, Beard BC, Guyman LA, Baldock AL, Bridge CA, Hawkins-Daarud A, Gori JL, Born DE, et al. 2014. Gene therapy enhances chemotherapy tolerance and efficacy in glioblastoma patients. J Clin Invest 124: 4082–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins S, Shabbir A. 2014. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag Sci 70: 1023–1029. [DOI] [PubMed] [Google Scholar]
- Alexandrov LB, Stratton MR. 2014. Mutational signatures: The patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev 24: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, et al. 2013. Signatures of mutational processes in human cancer. Nature 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso JC, Jagiella N, Núñez L, Herrero MA, Drasdo D. 2014. Estimating dose painting effects in radiotherapy: A mathematical model A. PLoS ONE 9: e89380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altrock PM, Liu LL, Michor F. 2015. The mathematics of cancer: Integrating quantitative models. Nat Rev Cancer 15: 730–745. [DOI] [PubMed] [Google Scholar]
- Anderson A. 2005. A hybrid mathematical model of solid tumour invasion: The importance of cell adhesion. Math Med Biol 22: 163–186. [DOI] [PubMed] [Google Scholar]
- Anderson A, Quaranta V. 2008. Integrative mathematical oncology. Nat Rev Cancer 8: 227–234. [DOI] [PubMed] [Google Scholar]
- Anderson AR, Weaver AM, Cummings PT, Quaranta V. 2006. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127: 905–915. [DOI] [PubMed] [Google Scholar]
- Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, Ji HP, Maley CC. 2015. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat Med 22: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angermueller C, Clark SJ, Lee HJ, Macaulay IC, Teng MJ, Hu TX, Krueger F, Smallwood SA, Ponting CP, Voet T, et al. 2016. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat Method 13: 229–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A, Cook LM, Lynch CC, Basanta D. 2014. An integrated computational model of the bone microenvironment in bone-metastatic prostate cancer. Cancer Res 74: 2391–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti M. 2013. Evolutionary game theory of growth factorproduction: Implications for tumourheterogeneity and resistance to therapies. Br J Cancer 109: 1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti M, Ferraro DA, Christofori G. 2015. Heterogeneity for IGF-II production maintained by public goods dynamics in neuroendocrine pancreatic cancer. Proc Natl Acad Sci 112: 1833–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod R, Axelrod DE, Pienta KJ. 2006. Evolution of cooperation among tumor cells. Proc Natl Acad Sci 103: 13474–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach LA, Helvik T, Christiansen FB. 2006. The evolution of n-player cooperation-threshold games and ESS bifurcations. J Theoret Biol 238: 426–434. [DOI] [PubMed] [Google Scholar]
- Baldock A, Ahn S, Rockne R, Johnston S, Neal M, Corwin D, Clark-Swanson K, Sterin G, Trister AD, Malone H, et al. 2014. Patient-specific metrics of invasiveness reveal significant prognostic benefit of resection in a predictable subset of gliomas. PLoS ONE 9: e99057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DA, Rowley DR. 2012. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer 19: R187–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta D. 2015. Cell–cell interactions and evolution using evolutionary game theory. bioRxiv 028746; https://doi.org/10.1101/028746. [Google Scholar]
- Basanta D, Anderson AR. 2013. Exploiting ecological principles to better understand cancer progression and treatment. Interface Focus 3: 20130020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta D, Deutsch A. 2008. A game theoretical perspective on the somatic evolution of cancer. In selected topics on cancer modelling: Genesis, evolution, immune competition, therapy (ed. Bellomo N, et al. ), pp. 1–16. Birkhauser, Boston. [Google Scholar]
- Basanta D, Simon M, Hatzikirou H, Deutsch A. 2008a. Evolutionary game theory elucidates the role of glycolysis in glioma progression and invasion. Cell Prolif 41: 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta D, Miodownik M, Baum B. 2008b. The evolution of robust development and homeostasis in artificial organisms. PLoS Comput Biol 4: e1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta D, Strand DW, Lukner RB, Franco OE, Cliffel DE, Ayala GE, Hayward SW, Anderson AR. 2009. The role of transforming growth factor-mediated tumor-stroma interactions in prostate cancer progression: An integrative approach. Cancer Res 69: 7111–7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta D, Scott JG, Fishman MN, Ayala G, Hayward SW, Anderson AR. 2011a. Investigating prostate cancer tumour–stroma interactions: Clinical and biological insights from an evolutionary game. Br J Cancer 106: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta D, Ribba B, Watkin E, You B, Deutsch A. 2011b. Computational analysis of the influence of the microenvironment on carcinogenesis. Math Biosci 229: 22–29. [DOI] [PubMed] [Google Scholar]
- Basanta D, Gatenby RA, Anderson AR. 2012. Exploiting evolution to treat drug resistance: Combination therapy and the double bind. Mol Pharmaceut 9: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierie B, Moses HL. 2006. Tumour microenvironment: TGFβ: The molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520. [DOI] [PubMed] [Google Scholar]
- Brown JS. 2016. Why Darwin would have loved evolutionary game theory. Proc R Soc B 283: 20160847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne HM. 2010. Dissecting cancer through mathematics: From the cell to the animal model. Nat Rev Cancer 10: 1–10. [DOI] [PubMed] [Google Scholar]
- Byrne HM, Alarcon T, Owen MR, Webb SD, Maini PK. 2006. Modelling aspects of cancer dynamics: A review. Philos Trans A Math Phys Eng Sci 364: 1563–1578. [DOI] [PubMed] [Google Scholar]
- Chen KW, Pienta KJ. 2011. Modeling invasion of metastasizing cancer cells to bone marrow utilizing ecological principles. Theor Biol Med Model 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook LM, Araujo A, Pow-Sang JM, Budzevich MM, Basanta D, Lynch CC. 2016. Predictive computational modeling to define effective treatment strategies for bone metastatic prostate cancer. Sci Rep 6: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. 2011. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis Model Mech 4: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikász-Nagy A, Escudero LM, Guillaud M, Sedwards S, Baum B, Cavaliere M. 2013. Seminars in cancer biology. Semin Cancer Biol 23: 293–298. [DOI] [PubMed] [Google Scholar]
- Damaghi M, Tafreshi NK, Lloyd MC, Sprung R, Estrella V, Wojtkowiak JW, Morse DL, Koomen JM, Bui MM, Gatenby RA, et al. 2015. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat Commun 6: 8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke JG, Weaver IS. 2013. The emergence of environmental homeostasis in complex ecosystems. PLoS Comput Biol 9: e1003050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran H, Tsai HC, Baylin SB. 2014. Cancer epigenetics: Tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol Cell 54: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-Navas PM, Kam Y, Das T, Hassan S, Silva A, Foroutan P, Ruiz E, Martinez G, Minton S, Gillies RJ, et al. 2016. Exploiting evolutionary principles to prolong tumor control in preclinical models of breast cancer. Sci Transl Med 8: 327ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko IV, Smalley KSM. 2015. The complexity of microenvironment-mediated drug resistance. Genes Cancer 6: 367–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R, Pusztai L, Swanton C. 2013. Cancer heterogeneity: Implications for targeted therapeutics. Br J Cancer 108: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo J, Michor F. 2009. Evolution of resistance to targeted anti-cancer therapies during continuous and pulsed administration strategies. PLoS Comput Biol 5: e1000557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SA, Beare D, Gunasekaran P, Leung K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, et al. 2015. COSMIC: Exploring the world's knowledge of somatic mutations in human cancer. Nucl Acids Res 43: D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA, Rosner MR. 2012. Nonheritable cellular variability accelerates the evolutionary processes of cancer. PLoS Biol 10: e1001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher J, Cook LM, Gupta S, Araujo A, Dhillon J, Park JY, Scott JG, Pow-Sang J, Basanta D, Lynch CC. 2014. Improving treatment strategies for patients with metastatic castrate resistant prostate cancer through personalized computational modeling. Clin Exp Metastasis 31: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, et al. 2015. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med 21: 1318–1325. [DOI] [PubMed] [Google Scholar]
- Gatenby R, Maini P. 2003. Cancer summed up. Nature 421: 321. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Silva AS, Gillies RJ, Frieden BR. 2009. Adaptive therapy. Cancer Res 69: 4894–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay L, Baker AM, Graham TA. 2016. Tumour cell heterogeneity. F1000Res 5: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlee P, Anderson A. 2008. A hybrid cellular automaton model of clonal evolution in cancer: The emergence of the glycolytic phenotype. J Theor Biol 250: 705–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlee P, Basanta D, Anderson AR. 2011. Progress in biophysics and molecular biology. Prog Biophys Mol Biol 106: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M, Swanton C. 2010. How Darwinian models inform therapeutic failure initiated by clonal heterogeneity in cancer medicine. Br J Cancer 103: 139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, et al. 2012. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RJ, Verduzco D, Gatenby RA. 2012. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer 12: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez A, Perez-Llamas C, Deu-Pons J, Tamborero D, Schroeder MP, Jene-Sanz A, Santos A, Lopez-Bigas N. 2013. IntOGen-mutations identifies cancer drivers across tumor types. Nat Meth 10: 1081–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. 2015. Evolutionary determinants of cancer. Cancer Discov 5: 806–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M, Maley CC. 2012. Clonal evolution in cancer. Nature 481: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: The next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HE, Maruvka YE, Ji F, Bhang HE, Krishnamurthy Radhakrishna V, et al. 2016. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med 22: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng HH, Bremer SW, Stevens JB, Ye KJ, Liu G, Ye CJ. 2009. Genetic and epigenetic heterogeneity in cancer: A genome-centric perspective. J Cell Physiol 220: 538–547. [DOI] [PubMed] [Google Scholar]
- Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, et al. 2016. Metabolic heterogeneity in human lung tumors. Cell 164: 681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. 2015. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin b1/FAK signaling. Cancer Cell 27: 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummert S, Bohl K, Basanta D, Deutsch A, Werner S, Theissen G, Schroeter A, Schuster S. 2014. Evolutionary game theory: Cells as players. Mol BioSyst 10: 3044–3065. [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Michor F. 2011. Evolutionary dynamics of intratumor heterogeneity. PLoS ONE 6: e17866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T, Komarova N, Swanson K. 2014. Mathematical oncology: Using mathematics to enable cancer discoveries. Am Math Month 121: 840–856. [Google Scholar]
- Junttila MR, de Sauvage FJ. 2013. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501: 346–354. [DOI] [PubMed] [Google Scholar]
- Kareva I. 2011. What can ecology teach us about cancer? Transl Oncol 4: 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaznatcheev A, Scott JG, Basanta D. 2015. Edge effects in game-theoretic dynamics of spatially structured tumours. J R Soc Interface 12: 20150154–20150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Rebecca VW, Smalley KS, Anderson AR. 2015. Phase I trials in melanoma: A framework to translate preclinical findings to the clinic. Eur J Cancer 67: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova NL. 2014. Spatial interactions and cooperation can change the speed of evolution of complex phenotypes. Proc Natl Acad Sci 111: 10789–10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolev KS, Xavier JB, Gore J. 2014. Turning ecology and evolution against cancer. Nat Rev Cancer 14: 371–380. [DOI] [PubMed] [Google Scholar]
- Kuhn TS. 2012. The structure of scientific revolutions, University of Chicago Press, Chicago. [Google Scholar]
- Lalonde E, Ishkanian AS, Sykes J, Fraser M, Ross-Adams H, Erho N, Dunning MJ, Halim S, Lamb AD, Moon NC, et al. 2014. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: a retrospective cohort study. Lancet Oncol 15: 1521–1532. [DOI] [PubMed] [Google Scholar]
- Leder K, Pitter K, Laplant Q, Hambardzumyan D, Ross BD, Chan TA, Holland EC, Michor F. 2014. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell 156: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. 2011. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27: 1739–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski KA, Barber LJ, Davies MN, Ashenden M, Sottoriva A, Gerlinger M. 2016. Cancer evolution and the limits of predictability in precision cancer medicine. Trends Cancer 2: 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd MC, Cunningham JJ, Bui MM, Gillies RJ, Brown JS, Gatenby RA. 2016. Darwinian dynamics of intratumoral heterogeneity: Not solely random mutations but also variable environmental selection forces. Cancer Res 76: 3136–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis CJ, Lin SH. 2005. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer 5: 21–28. [DOI] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. 2012. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol 196: 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley CC, Reid BJ, Forrest S. 2004. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: Simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol Biomarkers Prev 13: 1375–1384. [PubMed] [Google Scholar]
- McGranahan N, Swanton C. 2015. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 27: 15–26. [DOI] [PubMed] [Google Scholar]
- Meades MB, Gatenby RA, Dalton WS. 2009. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat Rev Cancer 9: 665–674. [DOI] [PubMed] [Google Scholar]
- Medler TR, Cotechini T, Coussens LM. 2015. Immune response to cancer therapy: Mounting an effective antitumor response and mechanisms of resistance. Trends Cancer 1: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo LM, Pepper JW, Reid BJ, Maley CC. 2006. Cancer as an evolutionary and ecological process. Nat Rev Cancer 6: 924–935. [DOI] [PubMed] [Google Scholar]
- Michor F, Iwasa Y, Nowak MA. 2004. Dynamics of cancer progression. Nat Rev Cancer 4: 197–205. [DOI] [PubMed] [Google Scholar]
- Miller BE, Miller FR, Heppner GH. 1989. Therapeutic perturbation of the tumor ecosystem in reconstructed heterogeneous mouse mammary tumors. Cancer Res 49: 3747–3753. [PubMed] [Google Scholar]
- Möbius W, Laan L. 2015. Physical and mathematical modeling in experimental papers. Cell 163: 1577–1583. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. 2004. Friends or foes—Bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4: 839–849. [DOI] [PubMed] [Google Scholar]
- Mumenthaler SM, Foo J, Leder K, Choi NC, Agus DB, Pao W, Mallick P, Michor F. 2011. Evolutionary modeling of combination treatment strategies to overcome resistance to tyrosine kinase inhibitors in non-small cell lung cancer. Mol Pharm 8: 2069–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumenthaler SM, Foo J, Choi NC, Heise N, Leder K, Agus DB, Pao W, Michor F, Mallick P. 2015. The impact of microenvironmental heterogeneity on the evolution of drug resistance in cancer cells. Cancer Inform 14: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. 2002. Metastasis: Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593. [DOI] [PubMed] [Google Scholar]
- Natrajan R, Sailem H, Mardakheh FK, Arias Garcia M, Tape CJ, Dowsett M, Bakal C, Yuan Y. 2016. Microenvironmental heterogeneity parallels breast cancer progression: A histology–genomic integration analysis. PLoS Med 13: e1001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol D, Jeavons P, Fletcher AG, Bonomo RA, Maini PK, Paul JL, Gatenby RA, Anderson ARA, Scott JG. 2015. Steering evolution with sequential therapy to prevent the emergence of bacterial antibiotic resistance. PLoS Comput Biol 11: e1004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R. 2005. Molecular signatures of natural selection. Annu Rev Genet 39: 197–218. [DOI] [PubMed] [Google Scholar]
- Nowell PC. 1976. The clonal evolution of tumor cell populations. Science 194: 23–28. [DOI] [PubMed] [Google Scholar]
- Orlando PA, Gatenby RA, Brown JS. 2012. Cancer treatment as a game: Integrating evolutionary game theory into the optimal control of chemotherapy. Phys Biol 9: 065007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Sotiriou C. 2007. Adjuvant chemotherapy—yes or no? Prognostic markers in early breast cancer. Ann Oncol 18: xii2–xii7. [DOI] [PubMed] [Google Scholar]
- Pienta KJ, McGregor N, Axelrod R, Axelrod DE. 2008. Ecological therapy for cancer: Defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol 1: 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poleszczuk JT, Luddy KA, Prokopiou S, Robertson-Tessi M, Moros EG, Fishman M, Djeu JY, Finkelstein SE, Enderling H. 2016. Abscopal benefits of localized radiotherapy depend on activated T-cell trafficking and distribution between metastatic lesions. Cancer Res 76: 1009–1018. [DOI] [PubMed] [Google Scholar]
- Quintieri L, Selmy M, Indraccolo S. 2014. Metabolic effects of antiangiogenic drugs in tumors: Therapeutic implications. Biochem Pharmacol 89: 162–170. [DOI] [PubMed] [Google Scholar]
- Rapisarda A, Melillo G. 2012. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol 9: 378–390. [DOI] [PubMed] [Google Scholar]
- Rejniak KA, Anderson ARA. 2011. Hybrid models of tumor growth. WIREs Syst Biol Med 3: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson-Tessi M, Anderson AR. 2015. Big bang and context-driven collapse. Nat Genet 47: 196–197. [DOI] [PubMed] [Google Scholar]
- Robertson-Tessi M, Gillies RJ, Gatenby RA, Anderson AR. 2015. Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res 75: 1567–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockne R, Rockhill JK, Mrugala M, Spence AM, Kalet I, Hendrickson K, Lai A, Cloughesy T, Alvord EC Jr, Swanson KR. 2010. Predicting the efficacy of radiotherapy in individual glioblastoma patients in vivo: A mathematical modeling approach. Phys Med Biol 55: 3271–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes EH, Rietkerk M, Sugihara G. 2009. Early-warning signals for critical transitions. Nature 461: 53–59. [DOI] [PubMed] [Google Scholar]
- Scheffer M, Carpenter SR, Lenton TM, Bascompte J, Brock W, Dakos V, van de Koppel J, van de Leemput IA, Levin SA, van Nes EH, et al. 2012. Anticipating critical transitions. Science 338: 344–348. [DOI] [PubMed] [Google Scholar]
- Schmitt MW, Fox EJ, Prindle MJ, Reid-Bayliss KS, True LD, Radich JP, Loeb LA. 2015. Sequencing small genomic targets with high efficiency and extreme accuracy. Nat Meth 12: 423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. 2012. Phase I trialist. Lancet Oncol 13: 236. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Brandvain Y, Dhole S, Fitzpatrick CL, Goldberg EE, Stern CA, Van Cleve J, Yeh DJ. 2014. Not just a theory—The utility of mathematical models in evolutionary biology. PLoS Biol 12: e1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM. 1982. Evolution and the theory of games, Cambridge University Press, Cambridge. [Google Scholar]
- Snyder A, Chan TA. 2015. Immunogenic peptide discovery in cancer genomes. Curr Opin Genet Dev 30: 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D, et al. 2015. A big bang model of human colorectal tumor growth. Nat Genet 47: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand DW, Franco OE, Basanta D, Anderson AR, Hayward SW. 2010. Perspectives on tissue interactions in development and disease. Curr Mol Med 10: 95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland KD, Visvader JE. 2015. Cellular mechanisms underlying intertumoral heterogeneity. Trends Cancer 1: 15–23. [DOI] [PubMed] [Google Scholar]
- Swanton C. 2012. Intratumor heterogeneity: Evolution through space and time. Cancer Res 72: 4875–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Fisher D, Fort P, Marie JP, Daoust S, Roche B, Grunau C, Cosseau C, Mitta G, Baghdiguian S, et al. 2012. Applying ecological and evolutionary theory to cancer: A long and winding road. Evol Appl 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Nesse RM, Gatenby R, Gidoin C, Renaud F, Roche B, Ujvari B. 2016. Evolutionary ecology of organs: A missing link in cancer development? Trends Cancer 2: 409–415. [DOI] [PubMed] [Google Scholar]
- Tlsty T. 2008. Cancer: Whispering sweet somethings. Nature 453: 604–605. [DOI] [PubMed] [Google Scholar]
- Tomlinson IP, Bodmer WF. 1997. Modelling the consequences of interactions between tumour cells. Br J Cancer 75: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trefois C, Antony PM, Goncalves J, Skupin A, Balling R. 2015. Critical transitions in chronic disease: Transferring concepts from ecology to systems medicine. Curr Opin Biotechnol 34: 48–55. [DOI] [PubMed] [Google Scholar]
- Turner JS. 2016. Homeostasis and the physiological dimension of niche construction theory in ecology and evolution. Evol Ecol 30: 303–319. [Google Scholar]
- Venkatesan S, Swanton C. 2016. Tumor evolutionary principles: How intratumor heterogeneity influences cancer treatment and outcome. Am Soc Clin Oncol Educ Book 35: e141. [DOI] [PubMed] [Google Scholar]
- Walker R, Enderling H. 2015. From concept to clinic: Mathematically informed immunotherapy. Curr Probl Cancer 40: 68–83. [DOI] [PubMed] [Google Scholar]
- Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DI, Zairis S, Abate F, Liu Z, Elliott O, Shin YJ, et al. 2016. Clonal evolution of glioblastoma under therapy. Nat Genet 48: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch DR. 2016. Tumor heterogeneity—A contemporary concept founded on historical insights and predictions. Cancer Res 76: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Werner B, Barnes CP, Graham TA, Sottoriva A. 2016. Identification of neutral tumor evolution across cancer types. Nat Genet 48: 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu L-Y, Zhang X-S, Zhang S. 2014. Discovery of co-occurring driver pathways in cancer. BMC Bioinformat 15: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]