Abstract
Background
Hyperglycemia is one of the most dangerous factors causing diabetic nephropathy. Salidroside is considered to have the effects of reducing oxidative stress damage and improving cell viability. This study was performed to investigate whether and how salidroside reduces high-glucose (HG)-induced apoptosis in mouse podocytes.
Material/Methods
We examined whether salidroside could decrease HG-induced podocyte oxidative stress and podocyte apoptosis in vitro. The potential signaling pathways were also investigated. Podocytes (immortalized mouse epithelial cells) were treated with normal glucose (5.5 mM) as control or HG (30 mM), and then exposed to salidroside treatment.
Results
HG enhanced the generation of intracellular reactive oxygen species (ROS) and apoptosis in podocytes. Salidroside reduced HG-induced apoptosis-related consequences via promoting HO-1 expression. Salidroside increased the expression level of phosphorylated Akt (p-Akt) and phosphorylated ILK (p-ILK), p-JNK, and p-ERK and localization of Nrf-2. JNK inhibitor and ILK inhibitor decreased HO-1 expression to different degrees. Moreover, specific siRNAs of ILK, Nrf-2, and HO-1, and inhibitors of HO-1 and ILK significantly increased ROS generation and Caspase9/3 expression in the presence of salidroside and HG.
Conclusions
The results suggest that salidroside reduces HG-induced ROS generation and apoptosis and improves podocytes viability by upregulating HO-1 expression. ILK/Akt, JNK, ERK1/2, p38 MAPK, and Nrf-2 are involved in salidroside-decreased podocyte apoptosis in HG condition.
MeSH Keywords: Apoptosis, Heme Oxygenase-1, Reactive Oxygen Species
Background
Diabetic nephropathy (DN) is one of the most serious complications of kidney diseases and is the end-stage event of type 1 and type 2 diabetes mellitus [1–3]. Recent studies revealed that podocyte injury is a typical characteristic of DN [4], but the pathogenesis of podocyte dysfunction remains poorly understood. Some research reported that high glucose enhances intracellular reactive oxygen species (ROS) generation and induces apoptosis [5–8], and a high concentration of glucose can promote apoptosis through inducing caspase family members such as Caspase-3 and -9 [9–10]. Caspase-3 and -9 activation was reported to be used as an index for pro-apoptotic processes in vitro [11,12].
Salidroside is the major active ingredient in the plant Rhodiola rosea. It reduces oxidative stress damage and promotes cell viability [13–15]. It also has therapeutic effects in a variety of diseases [16], such as diabetes, pneumonitis, cerebral artery occlusion [17], coronary heart disease [18], and malignant tumors. It is reported that salidroside can protect adrenal neuronal pheochromocytoma cells, hippocampal neurons [19,20], and neuroblastoma cells from oxidative stress injury and apoptosis through regulating the expression of heme oxygenase-1 (HO-1). HO-1 is regarded as the rate-limiting enzyme during the conversion of heme into free iron and plays an important role as an antioxidant [21,22]. There have been increasing findings showing that HO-1 expression is regulated at the transcriptional level, and the combination of activated Nrf-2 and antioxidant response element (ARE) can promote HO-1 expression [23,24]. It has also been reported that HO-1 induction might involve phosphatidylinositol-3-kinases/protein kinase B (PI3K/Akt), p38 mitogen-activated protein kinase (p38MAPK), and c-Jun N-terminal kinases (JNK) signaling pathways [24–27]. Akt was also found to be a marker for cell survival [11,12].
Recently, salidroside was shown to possess hypoglycemic activity and play protective roles in experimental diabetes, but its mechanism is not clear [10,17,28]. We hypothesized that salidroside promotes HO-1 expression to protect podocytes from high glucose. In the present study, we investigated whether and how salidroside reduced high-glucose (HG)-induced apoptosis in mouse podocytes. The involvement of PI3K/Akt, p38MAPK, JNK, extracellular regulated kinase (ERK), and nuclear factor-like 2 (Nrf-2) was also studied.
Material and Methods
Cell culture
Mouse podocytes from the Cell Culture Center, Peking Union Medical College, Chinese Academy of Medical Sciences (PUMC, CAMS, Beijing, China) were used and cultured as reported previously. Podocytes were put into RPMI 1640 medium (Gibco BRL, Gaithersburg, MD) containing 10% fetal bovine serum (FBS, Gibco BRL, USA), 100 mg/ml streptomycin, and 100 U/ml penicillin (Invitrogen, Carlsbad, CA) in a humidified atmosphere (5% CO2). The cells were cultured in plastic dishes coated with type I collagen in RPMI 1640, with supplements of fetal bovine serum (10%), penicillin (100 U/ml), streptomycin (100 mg/ml), and mouse recombinant γ-interferon (IFN-γ, 10 U/ml). To induce differentiation, podocytes were cultured at 37°C without IFN-γ for 1–2 weeks.
Podocyte phenotype was verified by analyzing mRNA level of the podocytes markers podocin and synaptopodin [12]. After achieving cell synchronization, the podocytes were cultured in normal glucose (NG, 5.5 nM) or high glucose (HG, 30 nM) condition for further studies. As osmotic control, cells were treated with NG and mannitol (24.5 nM) [9]. There was no decreased cell viability or elevated ROS production in the podocytes cultured in mannitol (24.5 nM) for 48 h.
Cell viability assay
Podocyte viability was checked by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma, St. Louis, Missouri, USA). After being treated with HG for 24 h, podocytes were exposed to salidroside (50 μM) for 24 h. Then, MTT (0.5μg/μl; Sigma, St. Louis, Missouri, USA) was added to the culture medium. Four hours later, cells viability was evaluated according to the manufacturer’s instruction. The absorbance was assessed at 570 nm using an ELISA plate reader.
Measurement of ROS level
Dichlorofluorescein diacetate (DCFH-DA, Biotium, Hayward, CA, USA) was used to assess the ROS level in cells according to the manufacturer’s instructions. Podocytes were cultured in 6-well plates with HG for 24 h and then treated with salidroside (50 μM) for 24 h. Then, the podocytes were incubated with DCFH-DA (20 μM) for 30 min. The relative fluorescence degree (fluorescence intensity) was examined at 485 nm excitation and 530 nm emission.
Measurement of podocyte apoptosis
Apoptosis was assessed using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL; Promega, Madison, USA) assay according to the manufacturer’s instructions. Cells were prepared and viewed with fluorescence microscopy. The percentage of podocytes that were TUNEL-positive was determined as: [TUNEL-positive cells/(TUNEL-positive cells + TUNEL-negative cells)] ×100%. At least 200 cells in 5 fields of view were examined.
Knockdown of integrin-linked kinase (ILK), Nrf2, and HO-1 by siRNA
Podocytes were cultured in 6-well plates and were transfected with specific siRNA (10 nM, Santa Cruz, USA) and scrambled control siRNA (10 nM, Santa Cruz, USA) separately [29,30]. After transfection for 12 h, the podocytes were treated with HG and salidroside. Western blot analysis was carried out to confirm knockdown of the target gene.
Western blot analysis
Western blot analysis was carried out to check the expression of apoptosis-related proteins. Protein was extracted and processed as described previously. Briefly, podocytes were lysed in buffer (2.5 mM Tris-HCl, 2% SDS, 10% glycerol, 1 mM PMSF, 2 μg/ml aprotinin, 2 μg/ml pepstatin, and 2 μg/ml leupeptin). A total protein (30 μg) was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (12%), followed by electroblotting onto nitrocellulose membrane. The membranes were detected with mouse antibodies directed to Caspase-3 and Caspase-9 (R&D system, Inc., Minneapolis, USA; concentration: 1: 500), or mouse antibodies directed to p-ILK, ILK, p-Akt, Akt, MAPKs (Abcam Co, Nanjing, China; concentration: 1: 500) and HO-1 (Abcam; concentration: 1: 1000), then incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibody for 1.5 h at room temperature. Finally, the membranes were washed with Tris-buffered saline including Tween-20 (0.1%). The signals were assessed using a chemiluminescence detection system (NEN Life Science, Boston, MA, USA). The intensity of the bands was measured using LabWorks 4.5. (UVP, Upland, CA, USA).
Statistical analysis
All tests were done at least 3 times. All data are expressed as means ± standard deviation. The comparison between 2 groups was done by independent-samples t test; the comparison among 3 or more groups was analyzed by ANOVA using SPSS software (version 15.0, Chicago, IL, USA). P<0.05 was considered as statistical significance.
Results
Salidroside improved HG-cultured podocytes viability via reducing apoptosis-related events
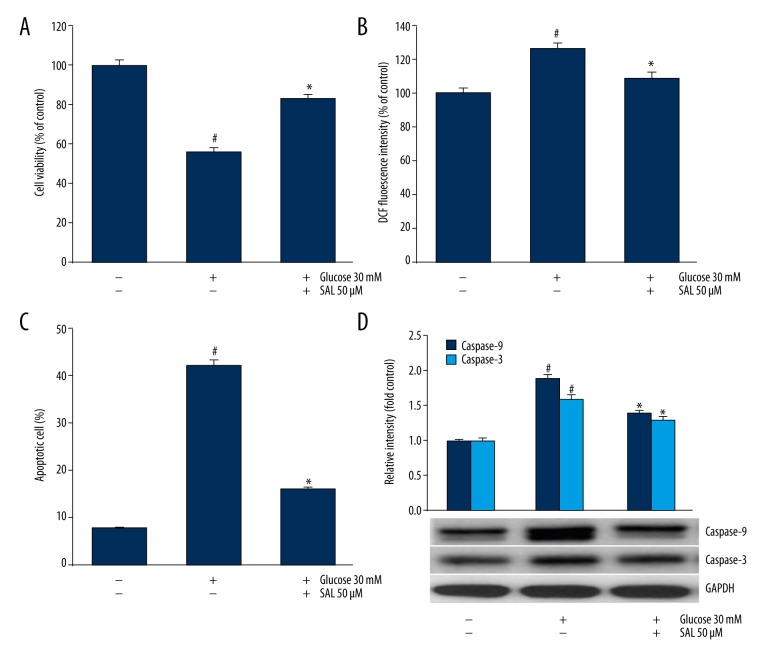
Podocytes cultured in HG showed lower cell viability (Figure 1A), higher ROS level (Figure 1B) and higher apoptosis cell rate (Figure 1C) compared with the control. HG induced ROS generation and apoptosis in podocytes. Podocytes cultured in HG plus salidroside showed higher cell viability, lower ROS level and lower apoptosis cell rate compared with that cultured in HG. This indicated that salidroside increased podocyte viability and reduced ROS level and apoptosis in HG environment. Besides, salidroside reduced the expression of Caspase-3 and Caspase-9 in HG condition (Figure 1D).
Figure 1.
Cell viability, apoptosis, ROS production, and expression of apoptosis-related proteins were assessed in podocytes. (A) Podocytes were cultured in normal concentration of glucose (5 mM) or high glucose (30 mM) with the presence or absence of salidroside (50 μM), and the cell viability was assessed using MTT. (B) ROS generation was evaluated by DCFH-DA. (C, D) Apoptosis rate and caspase-3 and caspase-9 expression were assessed using TUNEL assay and Western blot, separately. # Indicates P<0.05. compared with control using the independent-samples t test. * Indicates P<0.05 compared with high glucose group using independent-samples t test. SAL (salidroside). At least 3 independent experiments with 3 replicates per experiment were conducted.
Salidroside promoted HO-1 expression
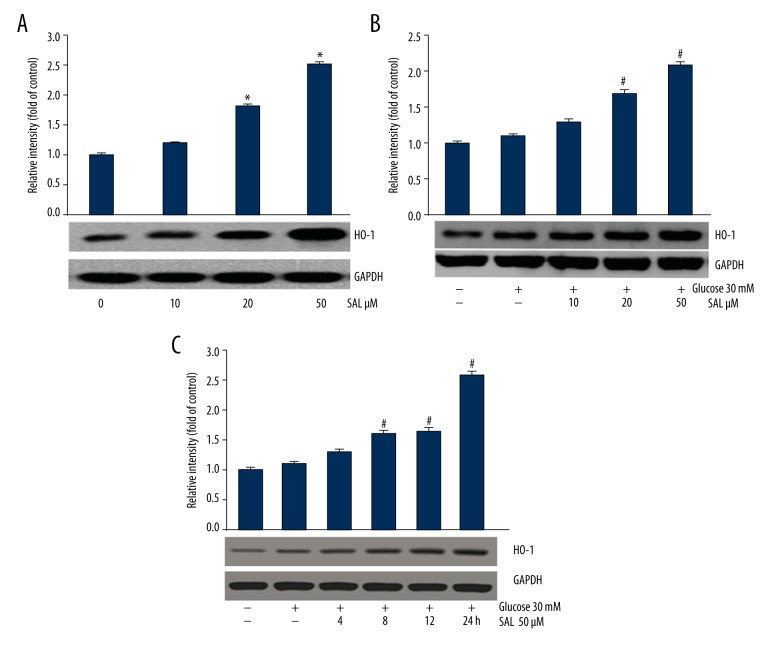
HO-1 expression level in podocytes increased after treatment with salidroside (10, 20, and 50 μM) for 24 h (Figure 2A). In addition, salidroside promoted HO-1 expression in a dose-dependent (Figure 2B) and time-dependent manner (Figure 2C). Thus, in the following experiments, the expression level of HO-1 was assessed at the condition of 50-μM salidroside treatment for 24 h.
Figure 2.
Evaluation of HO-1 expression in podocytes. (A, B) HO-1 expression was assessed after culturing with salidroside (0, 10, 20, and 50 μM) for 24 h. The data were analyzed by ANOVA. (C) HO-1 expression was assessed after culturing with salidroside (50 μM) for 0, 4, 8, 12, and 24 h. The results were analyzed by ANOVA. * P<0.05 compared with control. # P<0.05 compared with high glucose group. SAL (salidroside). At least 3 independent experiments with 3 replicates per experiment were conducted.
Salidroside decreased ROS generation and Caspase-3 and Caspase-9 expression by promoting HO-1 expression
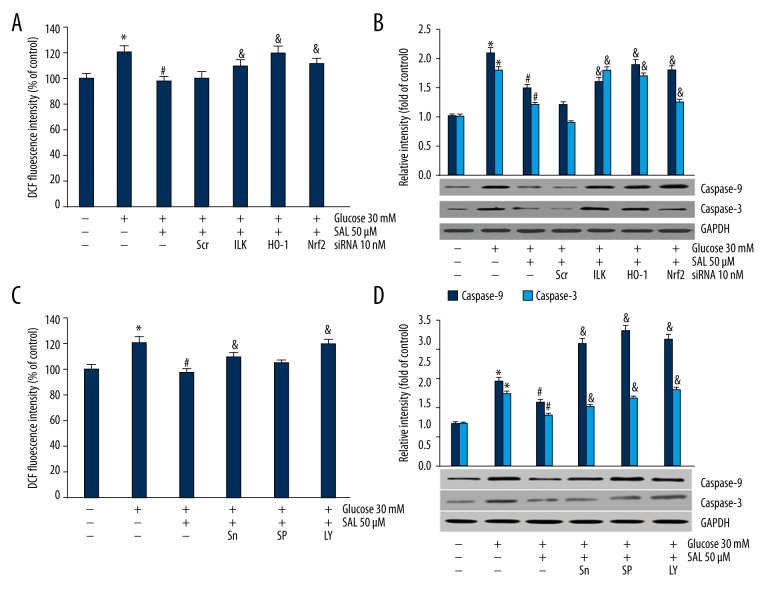
To investigate the relationship between ROS generation, Caspase-3 and Caspase-9 expression, and salidroside-induced HO-1 expression in an HG environment, we used HO-1 inhibitor SnPPIX, and HO-1 siRNA. Podocytes were treated with HO-1 siRNA (10 nM) for 12 h or HO-1 inhibitor SnPPIX (10 μM) for 1 h [29,30] and then cultured in the presence of salidroside for 24 h. The result showed higher ROS level and Caspase-3 and Caspase-9 expression HO-1 when podocytes were treated with siRNA (Figure 3A, 3B). Moreover, ROS level and Caspase-3 and Caspase-9 expression level increased in the presence of SnPPIX (Figure 3C, 3D). These results suggest that salidroside decreases ROS generation and Caspase-3 and Caspase-9 expression via promoting HO-1 expression.
Figure 3.
We investigated the relationship between ROS generation, caspase-3 and caspase-9 expression, HO-1 induction, and Akt/ILK, Nrf2, and JNK signaling pathways. (A, B) After treatment with siRNAs of ILK, HO-1 and Nrf-2, ROS level and caspase-9/3 expressions were assessed. The data were analyzed by independent-samples t test. (C, D) After podocyte synchronization, cells were treated with 10 uM SnPPIX for 1 h, 20 uM SP600125 for 0.5 h, and 50 uM LY294002 for 1 h. ROS level and caspase-3 and caspase-9 expressions in podocytes were evaluated. The data were analyzed by independent-samples t test. * Compared with control, # compared with high glucose group, & compared with high glucose+salidroside group P<0.05. Sn (HO-1 inhibitor SnPPIX), SP (JNK inhibitor SP600125), LY (ILK inhibitor LY294002). At least 3 independent experiments with 3 replicates per experiment were conducted.
Salidroside regulates Akt/ILK, MAPKs signaling pathway and modulates Nrf-2 localization
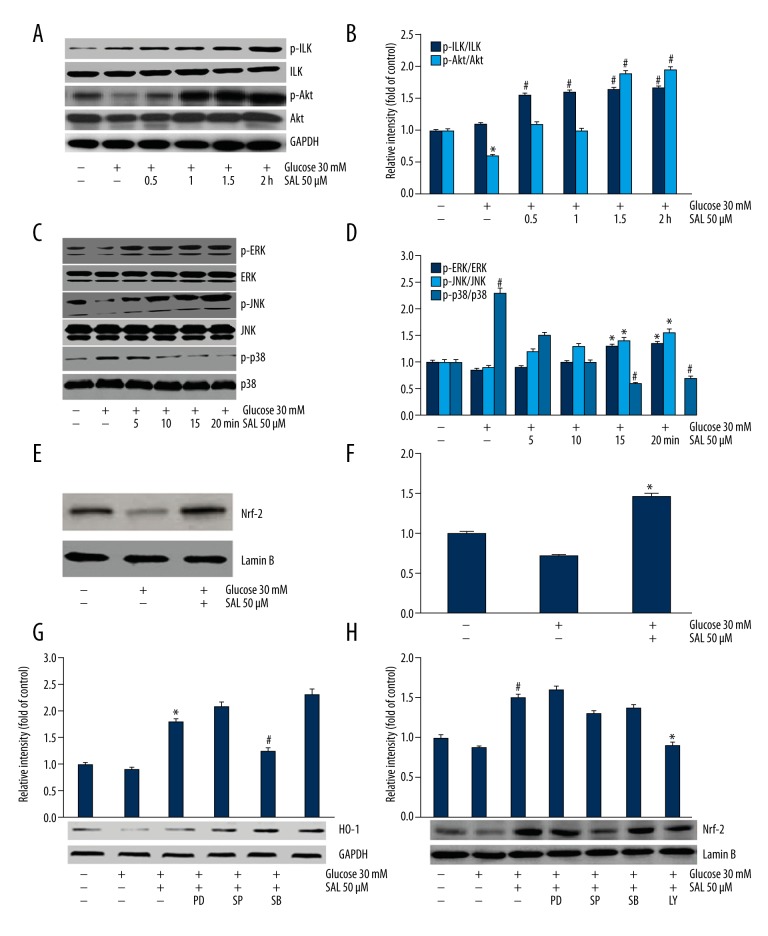
To investigate the signaling pathways involved in salidroside promoting HO-1 expression, we proposed PI3K/Akt and ILK pathways as candidates. After culturing in salidroside for 0.5–2 h, the expression level of phosphorylated Akt (p-Akt) and phosphorylated ILK (p-ILK) increased in a time-dependent manner (Figure 4A, 4B). The MAPK family members JNKs, ERKs, and p38 MAPK were also investigated to determine if they were involved in podocytes cultured in HG and salidroside. The result showed that the expression of phosphorylated p38 (p-p38) decreased after cells were cultured with salidroside for 5, 10, 15, and 20 min, and p-JNK and p-ERK expression levels increased (Figure 4C, 4D). p38 inhibitor (SB203580, 10uM) was further used to investigate the role of p38 MAPK, and the result was accordance with the above results (Figure 4G). JNK inhibitor (SP600125, 20 uM) decreased HO-1 expression (Figure 4G).
Figure 4.
The involvement of p38, Akt/ILK, JNK and ERK signaling pathways, and Nrf2 nucleus localization was investigated. (A, B) The expression of p-Akt and p-ILK was evaluated using Western blot analysis after culturing in salidroside (50 μM) for 0, 0.5, 1, 1.5, and 2 h. The data were analyzed by ANOVA. * P<0.05 compared with control. # P<0.05 compared with high glucose group. (C, D) The expression of phosphorylated MAPK family members was assessed after culturing in salidroside (50 μM) for 0, 5, 10, 15, and 20 min. The data were analyzed by ANOVA. # P<0.05 compared with control. * P<0.05 compared with high glucose group. (E, F) Nrf-2 nuclear localization was evaluated after treatment with salidroside (50 μM). The data were analyzed by independent-samples t test. * P<0.05 compared with high glucose group. (G) HO-1 expression was assessed after podocytes were treated with ERK1/2 inhibitor PD98059 (25 uM, 0.5 h), JNK inhibitor SP600125 (20 uM, 0.5 h), and p38 inhibitor SB203580 (10 uM, 1 h). The data were analyzed by independent-samples t test. * Compared with high glucose group, # compared with high glucose+salidroside group P<0.05. (H) Nrf2 localization was assessed. * Compared with high glucose+salidroside group. # Compared with high glucose group P<0.05. PD (ERK1/2 inhibitor PD98059), SP (JNK inhibitor SP600125), SB (p38 inhibitor SB203580), LY (ILK inhibitor LY294002). At least 3 independent experiments with 3 replicates per experiment were conducted.
We further investigated the localization manner of Nrf-2, which is regarded as an upstream nuclear factor to activate HO-1 expression. It showed that salidroside increased localization of Nrf-2 (Figure 4E, 4F). Moreover, Nrf2 localization was reduced by LY294002 (PI3K/AKT inhibitor, 50uM) and SP600125 (20 uM) to different degrees (Figure 4H).
Salidroside induced HO-1 expression through Akt/ILK signaling pathway
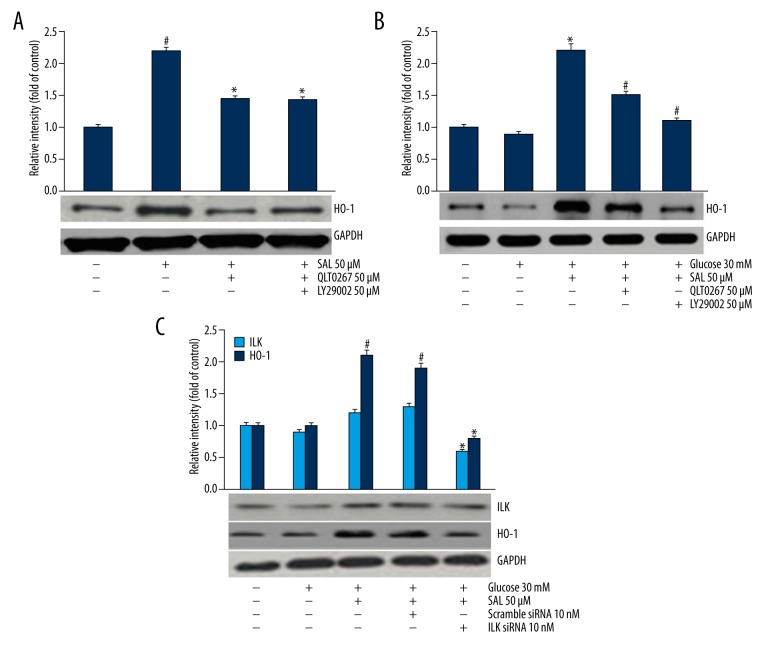
Specific siRNA or inhibitor were used to verify the signaling pathway in salidroside-induced HO-1 expression. Pre-treatment with LY294002 (50uM) or QLT0267 (the ILK inhibitor, 50 μM) decreased salidroside-induced HO-1 expression in the absence or presence of HG (Figure 5A, 5B). Additionally, podocytes transfected with 10nM ILK siRNA showed decreased expression level of HO-1 in the presence of salidroside and HG, while the scramble siRNA did not show any reducing effect (Figure 5C).
Figure 5.
The relationship between HO-1 expression and Akt/ILK and JNK signaling pathways was investigated. The data were analyzed by independent-samples t test. (A) HO-1 expression was evaluated after treatment of 50 μM LY294002 for 1 h and 50 uM QLT0267 for 0.5 h (ILK inhibitor) in normal concentration of glucose (5 mM). # Compared with control, * compared with salidroside group P<0.05. (B) HO-1 expression was assessed after treatment with ILK inhibitor (50μM LY294002, 1 h) in high glucose (30 mM). * Compared with control, # compared with high glucose+salidroside group P<0.05. (C) HO-1 expression was assessed after treatment with ILK siRNA. * Compared with high glucose+salidroside group, # compared with high glucose group P<0.05.
Salidroside protects podocytes by promoting HO-1 expression via Akt/ILK, Nrf-2, and JNK pathways
A further series of experiments were conducted to confirm the salidroside-induced signaling pathways. First, we investigated whether the effect of salidroside on reducing HG-induced ROS generation could be weakened by specific siRNA or inhibitor. The results showed that specific siRNAs of ILK, Nrf-2, and HO-1 (Figure 3A, 3B), or SnPPIX and LY294002 could significantly increase ROS generation in the presence of salidroside and HG. Salidroside-inhibited Caspase-9/3 expression in HG was reversed by specific siRNAs of ILK, Nrf-2, and HO-1 or inhibitors of JNK, HO-1, and PI3K (Figure 3C, 3D).
Discussion
It is well known that hyperglycemia plays a crucial role in genesis and development of diabetic nephropathy in patients with diabetes mellitus [31]. Elevated ROS generation under hyperglycemia is the main cause of diabetic complications. It has been reported that excessive ROS generation induced by high glucose could start podocyte apoptosis, which plays a vital role in the pathogenesis of DN in animal models [10]. In accord with previous reports, the present study showed that HG enhanced ROS generation and cell apoptosis rate, and promoted the expression of apoptosis-related protein in podocytes.
Salidroside, a phenylpropanoid glycoside extracted from Rhodiola rosea, is a potent antioxidant. It was reported that salidroside suppresses oxidative stress in diabetic mice [32]. Moreover, Zhang et al. reported that salidroside provided antioxidant protection against hydrogen peroxide-dependent toxicity in neuronal cells via up-regulation of antioxidant genes such as HO-1, peroxiredoxin 1 (Nrf2-regulated genes), and anti-apoptotic genes [33]. Langer et al. studied the effect of metformin on cell apoptosis and on pro-/anti-apoptotic signaling in human podocyte and found metformin has an antioxidant effect of decreasing cell apoptosis [9]. In accordance with the above reports, salidroside also decreased HG-induced ROS generation and decreased cell apoptosis rate and increased podocytes viability.
HO-1 is an important member of antioxidation and anti-apoptosis family. It has been reported that, by upregulating HO-1 expression, hepatocyte apoptosis and oxidative stress can be greatly reduced [34] and endothelial ROS can also be reduced [35]. Moreover, cell death caused by oxidative stress was attenuated by up-regulation of HO-1 [36,37]. In the present study, salidroside induced HO-1 expression in a dose-dependent and time-dependent manner. Moreover, once HO-1 is interfered with or inhibited, the protective effect of salidroside was lost under HG condition, suggesting the important role of inducing HO-1 in antioxidation and anti-apoptosis.
PI3K/Akt is an important anti-apoptosis signaling pathway [9,11,12]. After being activated, PI3K/Akt upregulates the expression of HO-1, and has a synergistic effect with HO-1 in cell protection [38]. Some researchers have found that ILK promotes the expression of Caspase-3 and inhibits cell apoptosis through the PI3K/Akt pathway [39]. Eisenreich et al. showed that modulation of phospho-Akt was associated with changes in caspase-3 activation and apoptosis of podocytes [12]. This study also showed that salidroside induced p-Akt and p-ILK in a time-dependent manner. Moreover, co-incubation with PI3K inhibitor (LY294002) or ILK siRNA attenuates the effect of salidroside on reducing ROS and apoptosis-related protein, indicating that PI3K and ILK play important roles.
MAPKs consist of 3 primary signaling cascades: ERK, JNK and p38MAPKs; it regulates gene expression, cell proliferation, cell survival and death, and cell motility [40–42]. HO-1 can be induced by numerous stimuli that are known to enhance the activity of MAPKs. JNK mediates the induction of HO-1 gene expression by the glutathione depleter phorone in rat liver [43]. In our study, JNK inhibitor (SP600125) significantly decreased HO-1 expression, suggesting that salidroside probably protects podocytes through JNK.
Both Nrf2 and its downstream anti-oxidative protein HO-1 were involved in the antioxidant defense system in DN, and activation of the Nrf2 signaling pathway can promote HO-1 expression [24,26,44. After activation, Nrf-2 in cytoplasm moves to the nucleus, where it promotes the transcription and activation of HO-1 after binding with ARE [23,25,27]. We found that salidroside treatment increased Nrf2 location, and specific siRNA of Nrf2 significantly increased ROS level and the expression level of caspase-3 and caspase-9.
In the present study, the normal group received 5 mM of glucose and the HG group received 30 mM was used, consistent with previous studies [2,7–9]. The doses of salidroside used in previous in vitro studies were in the range of 10–200 mM. In the present study, we used 50 μM salidroside, as suggested by our pre-study experiment and as used in previous studies [13–15].
Conclusions
HG enhanced ROS generation, caspase-3 and caspase-9 expression, and apoptosis in podocytes. Salidroside treatment reduced HG-induced ROS generation and apoptosis via enhancing HO-1 expression. ILK/Akt, JNK, ERK1/2, p38 MAPK, and Nrf-2 are involved in salidroside-decreased podocyte apoptosis in HG condition. The present study suggests that salidroside has a potential role in protecting podocytes under HG condition. Our results show the important signaling pathways targeting on the induction of HO-1 are involved in the protective effect of salidroside on HG-cultured podocytes. However, the mechanism of salidroside in suppressing apoptosis is complex and needs further study. In vivo animal model studies are needed to verify the present findings.
Abbreviations
- DN
diabetic nephropathy
- ROS
reactive oxygen species
- HG
high glucose
- NG
normal glucose
- Ho-1
heme oxygenase-1
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Gui D, Guo Y, Wang F, et al. Astragaloside IV, a novel antioxidant, prevents glucose-induced podocyte apoptosis in vitro and in vivo. PLoS One. 2012;7(6):e39824. doi: 10.1371/journal.pone.0039824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Shon E, Kim CS, Kim JS. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res. 2012;2012(9):916–66. doi: 10.1155/2012/210821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szabó C, Biser A, Benko R, et al. Poly (ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55(11):3004–12. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 4.Khazim K, Gorin Y, Cavaglieri RC, et al. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiology. 2013;305(5):F691–700. doi: 10.1152/ajprenal.00028.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishibashi Y, Matsui T, Ohta K, et al. PEDF inhibits AGE-induced podocyte apoptosis via PPAR-gamma activation. Microvasc Res. 2013;85(1):54–58. doi: 10.1016/j.mvr.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Riedl E, Pfister F, Braunagel M, et al. Carnosine prevents apoptosis of glomerular cells and podocyte loss in STZ diabetic rats. Cell Physiol Biochem. 2011;28(2):279–88. doi: 10.1159/000331740. [DOI] [PubMed] [Google Scholar]
- 7.Toyonaga J, Tsuruya K, Ikeda H, et al. Spironolactone inhibits hyperglycemia-induced podocyte injury by attenuating ROS production. Nephrol Dial Transplant. 2011;26(8):2475–84. doi: 10.1093/ndt/gfq750. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Li Z, Xu P, Yang Z. Protective effects of leukemia inhibitory factor against oxidative stress during high glucose-induced apoptosis in podocytes. Cell Stress Chaperones. 2012;17(4):485–93. doi: 10.1007/s12192-012-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer S, Kreutz R, Eisenreich AL. Metformin modulates apoptosis and cell signaling of human podocytes under high glucose conditions. J Nephrol. 2016;29(6):765–73. doi: 10.1007/s40620-015-0258-1. [DOI] [PubMed] [Google Scholar]
- 10.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55(1):225–33. [PubMed] [Google Scholar]
- 11.Boltzen U, Eisenreich A, Antoniak S, et al. Alternatively spliced tissue factor and full-length tissue factor protect cardiomyocytes against TNF-α-induced apoptosis. J Mole Cell Cardiol. 2012;52(5):1056–65. doi: 10.1016/j.yjmcc.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenreich A, Langer S, Herlan L, Kreutz R. Regulation of podoplanin expression by microRNA-29b associates with its antiapoptotic effect in angiotensin II-induced injury of human podocytes. J Hypertens. 2016;34(2):323–31. doi: 10.1097/HJH.0000000000000799. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Ye X, Li X, et al. Salidroside protects against MPP(+)-induced apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res. 2011;1382(9):9–18. doi: 10.1016/j.brainres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Tang Y, Vater C, Jacobi A, et al. Salidroside exerts angiogenic and cytoprotective effects on human bone marrow-derived endothelial progenitor cells via Akt/mTOR/p70S6K and MAPK signalling pathways. Br J Pharmacol. 2014;171(9):2440–56. doi: 10.1111/bph.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu MC, Shi HM, Wang H, Gao XF. Salidroside protects against hydrogen peroxide-induced injury in HUVECs via the regulation of REDD1 and mTOR activation. Mol Med Rep. 2013;8(1):147–53. doi: 10.3892/mmr.2013.1468. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Deng A, Zhou H, Gu J. Neuroprotective effect of 2-(4-methoxyphenyl) ethyl-2-acetamido-2-deoxy-β-D-pyranoside against sodium nitroprusside-induced neurotoxicity in HT22 cells. Mol Cell Biochem. 2013;383(1):149–59. doi: 10.1007/s11010-013-1763-y. [DOI] [PubMed] [Google Scholar]
- 17.Tan CB, Gao M, Xu WR, et al. Protective effects of salidroside on endothelial cell apoptosis induced by cobalt chloride. Biol Pharm Bull. 2009;32(8):1359–63. doi: 10.1248/bpb.32.1359. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H, Xin H, Wu LX, Zhu YZ. Salidroside attenuates apoptosis in ischemic cardiomyocytes: A mechanism through a mitochondria-dependent pathway. J Pharmacol Sci. 2010;114(4):399–408. doi: 10.1254/jphs.10078fp. [DOI] [PubMed] [Google Scholar]
- 19.Hoi CP, Ho YP, Baum L, Chow AH. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother Res. 2010;24(10):1538–42. doi: 10.1002/ptr.3178. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Yu HX, Zhao XC, et al. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int. 2010;57(5):547–55. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Endale M, Park SC, Kim S, et al. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218(12):1452–67. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Tayem Y, Green CJ, Motterlini R, Foresti R. Isothiocyanate-cysteine conjugates protect renal tissue against cisplatin-induced apoptosis via induction of heme oxygenase-1. Pharmacol Res. 2014;81(2):1–9. doi: 10.1016/j.phrs.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Yang JJ, Tao H, Huang C, Li J. Nuclear erythroid 2-related factor 2: a novel potential therapeutic target for liver fibrosis. Food Chem Toxicol. 2013;59(8):421–27. doi: 10.1016/j.fct.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 24.Zeng T, Zhang CL, Song FY, et al. The activation of HO-1/Nrf-2 contributes to the protective effects of diallyl disulfide (DADS) against ethanol-induced oxidative stress. Biochim Biophys Acta. 2013;1830(10):4848–59. doi: 10.1016/j.bbagen.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Lii NJG. Dobutamine-mediated heme oxygenase-1 induction via P13K and p38 MAPK inhibits high mobility group box 1 protein release and attenuates rat myocardial ischemia/reperfusion injury in vivo. J Surg Res. 2013;183(2):509–16. doi: 10.1016/j.jss.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 26.Karube H, Inamura H, Matsuoka M. Zinc chloride exposure increases heme oxygenase-1 expression in MDPC-23 odontoblast-like cells. Arch Oral Biol. 2013;58(4):355–61. doi: 10.1016/j.archoralbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Tsai JR, Wang HM, Liu PL, et al. High expression of heme oxygenase-1 is associated with tumor invasiveness and poor clinical outcome in non-small cell lung cancer patients. Cell Oncol. 2012;35(6):461–71. doi: 10.1007/s13402-012-0105-5. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Liu M, Gu X, Ding F. Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol. 2008;28(8):1067–78. doi: 10.1007/s10571-008-9284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong C, Zheng H, Huang S, et al. Heme oxygenase-1 enhances autophagy in podocytes as a protective mechanism against high glucose-induced apoptosis. Exp Cell Res. 2015;337(2):146–59. doi: 10.1016/j.yexcr.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Lee SC, Han SH, Li JJ, et al. Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int. 2009;76(8):838–48. doi: 10.1038/ki.2009.286. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Dong H, Song E, et al. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem Biol Interacti. 2014;209(1):56–67. doi: 10.1016/j.cbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Tang H, Xiao F, et al. Protective effect of salidroside from Rhodiolae Radix on diabetes-induced oxidative stress in mice. Molecules. 2011;16(12):9912–24. doi: 10.3390/molecules16129912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Yu H, Sun Y, et al. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564(3):18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 34.Sass G, Seyfried S, Parreira SM, et al. Cooperative effect of biliverdin and carbon monoxide on survival of mice in immune-mediated liver injury. Hepatology. 2004;40(5):1128–35. doi: 10.1002/hep.20450. [DOI] [PubMed] [Google Scholar]
- 35.Tsai HY, Huang PH, Lin FY, et al. Ginkgo biloba extract reduces high-glucose-induced endothelial reactive oxygen species generation and cell adhesion molecule expression by enhancing HO-1 expression via Akt/eNOS and p38 MAP kinase pathways. Eur J Pharm Sci. 2013;48(4–5):803–11. doi: 10.1016/j.ejps.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Hur J, Kim S, Lee P, et al. The protective effects of oxyresveratrol imine derivative against hydrogen peroxide-induced cell death in PC12 cells. Free Radic Res. 2013;47(3):212–18. doi: 10.3109/10715762.2012.762769. [DOI] [PubMed] [Google Scholar]
- 37.Son Y, Byun SJ, Pae HO. Involvement of heme oxygenase-1 expression in neuroprotection by piceatannol, a natural analog and a metabolite of resveratrol, against glutamate-mediated oxidative injury in HT22 neuronal cells. Amino Acids. 2013;45(2):393–401. doi: 10.1007/s00726-013-1518-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin D. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279(10):8919–29. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 39.Verpoorten N, Verhoeven K, Weckx S, et al. Synaptopodin and 4 novel genes identified in primary sensory neurons. Mol Cell Neurosci. 2005;30(3):316–25. doi: 10.1016/j.mcn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 41.Diffley JM, Wu M, Sohn M, et al. Apoptosis induction by oxidized glycated LDL in human retinal capillary pericytes is independent of activation of MAPK signaling pathways. Mol Vis. 2009;15(12–13):135–45. [PMC free article] [PubMed] [Google Scholar]
- 42.Semaan S, Wu J, Gan Y, et al. Hyperactivation of BDNF-TrkB signaling cascades in human hypothalamic hamartoma (HH): A potential mechanism contributing to epileptogenesis. CNS Neurosci Ther. 2015;21(2):164–72. doi: 10.1111/cns.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oguro T, Hayashi M, Nakajo S, et al. The expression of heme oxygenase-1 gene responded to oxidative stress produced by phorone, a glutathione depletor, in the rat liver; the relevance to activation of c-jun n-terminal kinase. J Pharmacol Exp Ther. 1998;287(2):773–78. [PubMed] [Google Scholar]
- 44.Deng X, Wei R, Fang Z, Ding W. PM 2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol Toxicol. 2013;29(3):143–57. doi: 10.1007/s10565-013-9242-5. [DOI] [PubMed] [Google Scholar]