Abstract
Background
This study investigated the prognostic factors of bone metastases from lung cancer.
Material/Methods
From March 2014 to March 2015, 168 patients with bone metastases from lung cancer treated at our hospital were included and the clinical data were reviewed. The Kaplan-Meier survival curves were calculated and analyzed using the log-rank univariate test. Multivariate regression analysis was conducted using Cox’s regression model.
Results
The overall median survival of the 168 patients was 13 months. The 1-year survival was 54.3% and the 2-year survival was 12.9%. Univariate regression analysis indicated that the pathologic types, number of bone metastases, clinical stage, ECOG scores, and serum ALP levels were significantly correlated with survival (P<0.05). Multivariate regression analysis indicated that the number of bone metastases, clinical stage, and serum ALP levels were significantly correlated with prognosis (P<0.05). The risk associated with multiple bone metastases was 1.72 times of that of single bone metastasis (P=0.029); the risk associated with advanced clinical stage was 1.49 times of that of early clinical stage (P=0.001); and the risk associated with a high serum ALP level was 1.75 times of that of the low serum ALP level (P=0.006).
Conclusions
Pathologic types, number of bone metastases, clinical stage, ECOG scores, and serum ALP levels were the prognostic factors for bone metastases from lung cancer.
MeSH Keywords: Kaplan-Meier Estimate, Lung Neoplasms, Neoplasm Metastasis
Background
Bone tumor metastasis occurs when a malignant tumor of the primary non-hematopoietic system is transferred to the secondary bone malignant tumor of the bone by blood or lymphatic pathways. Bone metastasis of a malignant tumor is one of the common complications of late-stage cancer. Breast cancer, prostate cancer, and lung cancer are the 3 that are most prone to bone metastases of malignant tumors [1]. Tumor cells release cytokines and chemical mediators to stimulate periosteum and bone, combined with the mechanical stress caused by tumor tissue in the osteolytic lesions, causing serious bone pain, which is the main symptom for which patients with bone metastases demand treatment. Pain treatments include systemic and analgesic therapy. Combined chemotherapy with radiotherapy [2] is the major therapy, supplemented with bisphosphonates and nuclide [3].
Lung cancer is the leading cause of cancer-related deaths worldwide [4]. About 1.5 million people are diagnosed with lung cancer every year and 1.3 million of these die [5]. The incidence and mortality of lung cancer rank first among all types of cancer in China. The incidence of lung cancer is about 53.6/100 000 population every year, and the mortality is 45.6/100 000 every year [6].
Non-small cell lung cancer (NSCLC) accounts for about 80% of all lung cancer cases, and small cell lung cancer (SCLC) accounts for 20%. P53, P63, MiR-30a, MiR-31, c-erb-B2, and p-glycoprotein are prognostic factors for lung cancer [7–10]. Bone is the most common and the earliest site of metastases from lung cancer [11]. Other types of cancer that can lead to bone metastases are breast cancer, prostate cancer, liver cancer, ovarian cancer, and thyroid cancer. About 30–70% of bone metastases are associated with lung cancer [12], and 20–30% of lung cancer patients already have bone metastases upon initial diagnosis. Bone metastases can cause bone pain, pathological fracture, spinal instability, spinal cord compression, and hypercalcemia [13]. Bone metastases usually occur at an advanced stage and adversely affects patient quality of life.
Many factors influence bone metastases from lung cancer, including, age, sex, pathologic types, number of primary lesions, number of bone metastases, treatment regimens, and serum markers. However, few studies have focused on identifying the prognostic factors for bone metastases from lung cancer. Therefore, we conducted a retrospective analysis of the clinical characteristics of bone metastases from lung cancer and discus the prognostic factors.
Material and Methods
Subjects
From March 2014 to March 2015, 168 patients with bone metastases from lung cancer treated at West part of the third hospital of Hebei Medical University were recruited, including 102 males and 66 females. They were ages 32–87 years, with an average of 60.45 years. All cases were pathologically confirmed as lung cancer with bone metastases by CT or MRI scan. We recorded clinical data, including sex, age, pathologic types, number of primary lesions, number of bone metastases, and clinical stage. Double data entry was used to enter the data records. Ethics approval was obtained from the Ethics Committee of the Third Hospital, Hebei Medical University.
Treatment regimen
Of the 168 patients, 79 received surgery after the diagnosis, 103 received radiotherapy, and 137 received chemotherapy. After the diagnosis of bone metastases, 128 patients received radiotherapy, 123 received chemotherapy, and 139 received bisphosphonate treatment.
Follow-up
The patients were followed up by telephone for 30 months. Survival referred to the interval from the date of diagnosis of bone metastases to the date of death or the last follow-up.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics 21.0 software. The survival rate was calculated using the Kaplan-Meier method. Univariate regression analysis was performed using the log-rank test, and multivariate regression analysis was performed using Cox’s regression model. The significance level was set as α=0.05.
Results
Sites of bone metastases for different pathologic types
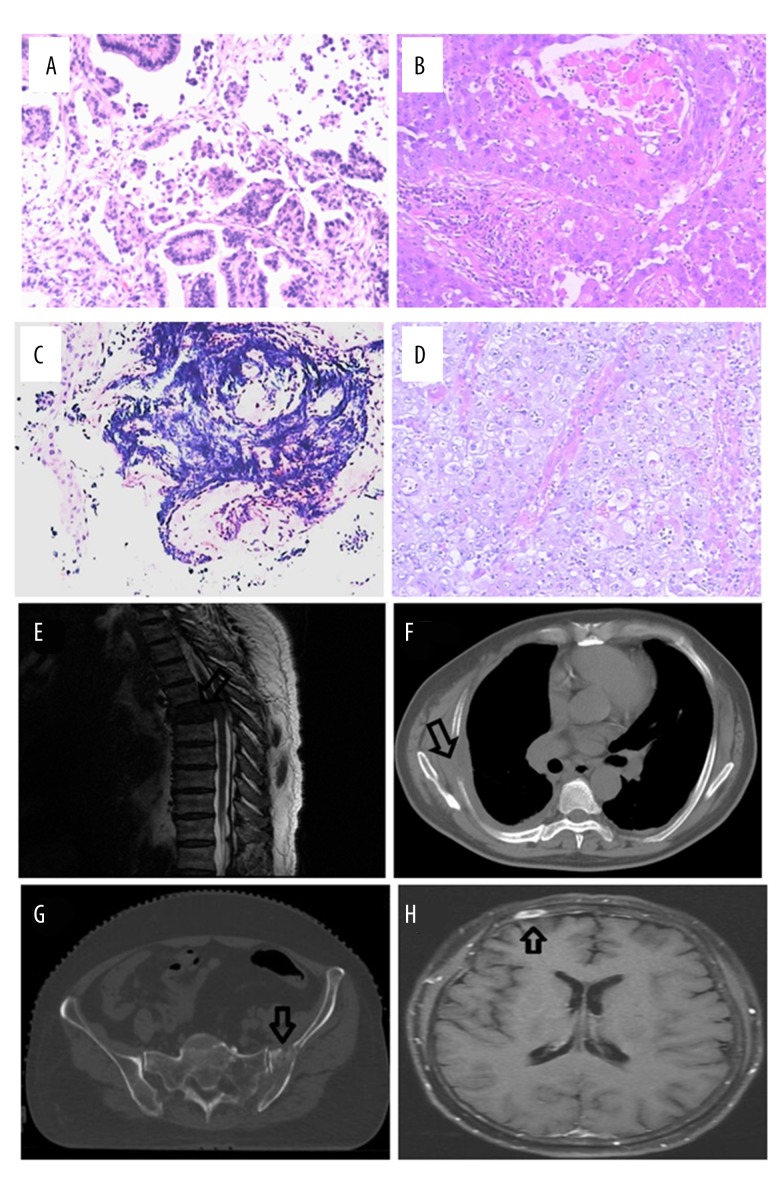
Four pathologic types were identified for the lung cancer cases, including adenocarcinoma in 74 cases, squamous cell carcinoma in 45 cases, SCLC in 33 cases, and large cell carcinoma in 16 cases. The spine (cervical, thoracic, and lumbar) is the most common site of bone metastases, accounting for 59.5%, followed by the chest (scapula and ribs) (23.2%), pelvis (15.5%), and skull (2.4%). There was no statistical difference among the pathological types of different lesions (χ2=8.173, P=0.976) (Table 1, Figure 1).
Table 1.
Sites of bone metastases for different pathologic types.
| Pathologic types | N | Skull | Cervical spine | Thoracic spine | Lumbar spine | Scapula | Ribs | Pelvis |
|---|---|---|---|---|---|---|---|---|
| Adenocarcinoma (%) | 74 | 2 (2.7) | 10 (13.5) | 13 (17.6) | 15 (20.3) | 8 (10.8) | 10 (13.5) | 16 (21.6) |
| Squamous cell carcinoma (%) | 45 | 1 (2.2) | 6 (13.3) | 11 (24.4) | 12 (26.7) | 6 (13.3) | 4 (8.9) | 6 (13.2) |
| SCLC (%) | 33 | 1 (3.0) | 6 (18.2) | 8 (24.2) | 7 (21.2) | 3 (9.1) | 5 (15.2) | 3 (9.1) |
| Large cell carcinoma (%) | 16 | 0 (0.0) | 3 (18.8) | 5 (31.3) | 4 (25.0) | 1 (6.2) | 2 (12.5) | 1 (6.2) |
| Total (%) | 168 | 4 (2.4) | 25 (14.9) | 37 (22.0) | 38 (22.6) | 18 (10.7) | 21 (12.5) | 26 (15.5) |
Figure 1.
The lung cancer and bone metastases patients were diagnosed by histopathology, CT, or MRI. (A) Adenocarcinoma; (B) squamous cell carcinoma; (C) small cell lung carcinoma; (D) large cell carcinoma; (E) thoracic spine bone metastases; (F) chest bone metastases; (G) pelvis bone metastases; (H) skull bone metastases.
Survival and univariate regression analysis of prognostic factors
The median survival estimated by the Kaplan-Meier method was 13 months. We assessed the correlation of potential prognostic factors to survival, finding that pathologic type, number of bone metastases, clinical stage, ECOG scores, and serum ALP levels were significant factors. Among different pathologic types, adenocarcinoma was associated with the shortest survival, which was 11.83 months (10.21–13.46), and squamous cell carcinoma was associated with the longest survival, which was 18.47 months (16.25–20.68). The survival was longer for single bone metastasis compared to multiple bone metastases. More advanced clinical stage was associated with shorter survival, and survival was longer for early clinical stage compared to advanced clinical stage. Lower ECOG scores were associated with longer survival compared to higher ECOG scores. Lower serum ALP levels were associated with longer survival compared to higher serum ALP levels. Age, sex, number of primary lesions, time after diagnosis of bone metastases, treatment regimens used for bone metastases, and serum calcium levels were not correlated with prognosis. We used the log-rank test to calculate the P value of the difference between prognostic factors (Table 2). Figure 2 shows the overall survival curve and univariate survival analyses for significant factors.
Table 2.
Baseline information of subjects and univariate analysis of prognostic factors.
| Prognostic factor | Case (%) | Median survival time | Survival time (95%CI) | P | |
|---|---|---|---|---|---|
| Age | <60 | 83 (49.4) | 14 | 15.06 (13.46–16.67) | 0.479 |
| ≥60 | 85 (50.6) | 13 | 14.09 (12.36–15.81) | ||
| Gender | Male | 102 (60.7) | 14 | 15.15 (13.64–16.69) | 0.201 |
| Female | 66 (39.3) | 13 | 13.67 (11.82–15.52) | ||
| Pathologic types* | Adenocarcinoma | 74 (44.0) | 9 | 11.83 (10.21–13.46) | <0.001 |
| Squamous cell carcinoma | 45 (26.8) | 17 | 18.47 (16.25–20.68) | ||
| SCLC | 33 (19.6) | 13 | 14.93 (12.21–17.66) | ||
| Large cell carcinoma | 16 (9.6) | 15 | 15.25 (12.34–18.16) | ||
| Number of primary lesions | Single | 119 (70.8) | 13 | 14.49 (13.07–15.91) | 0.970 |
| Multiple | 49 (29.2) | 13 | 14.77 (12.66–16.88) | ||
| Number of bone metastases* | Single | 42 (25.0) | 18 | 19.98 (17.80–22.16) | <0.001 |
| Multiple | 126 (75.0) | 12 | 12.78 (11.53–14.03) | ||
| Clinical stage* | I | 17 (10.1) | 20 | 20.23 (17.01–23.44) | <0.001 |
| II | 44 (26.2) | 16 | 17.75 (15.65–19.86) | ||
| III | 70 (41.7) | 13 | 14.18 (12.49–15.88) | ||
| IV | 37 (22.0) | 7 | 8.80 (6.93–10.67) | ||
| ECOG scores* | 0–1 | 47 (28.0) | 16 | 17.57 (15.42–19.71) | 0.008 |
| 2–4 | 121 (72.0) | 12 | 13.38 (12.02–14.73) | ||
| Time after diagnosis of bone metastases (months) | ≤4 | 110 (65.5) | 13 | 14.20 (12.77–15.62) | 0.363 |
| >4 | 58 (34.5) | 13 | 15.38 (13.26–17.50) | ||
| Chemotherapy | Yes | 123 (73.2) | 13 | 14.31 (12.98–15.64) | 0.443 |
| No | 45 (26.8) | 14 | 15.24 (12.77–17.71) | ||
| Radiotherapy | Yes | 128 (76.2) | 14 | 14.61 (13.27–15.96) | 0.993 |
| No | 40 (23.8) | 13 | 14.40 (11.96–16.83) | ||
| Bisphosphonate therapy | Yes | 139 (82.7) | 13 | 1423 (12.93–15.53) | 0.272 |
| No | 29 (17.3) | 15 | 16.25 (13.48–19.02) | ||
| Serum ALP(U/L)* | <140 | 55 (32.7) | 17 | 18.34 (16.22–20.45) | <0.001 |
| ≥140 | 113 (67.3) | 12 | 12.71 (11.43–13.99) | ||
| Serum Ca+ (mmol/L) | <2.4 | 44 (26.2) | 13 | 14.82 (12.48–17.16) | 0.970 |
| ≥2.4 | 124 (73.8) | 14 | 14.47 (13.10–15.83) | ||
Significant differences. Survival time and median survival time were performed by Kaplan-Meier; and P value is obtained by Log-Rank test.
Figure 2.
Overall survival curve and univariate survival analyses for significant factors. (A) Overall; (B) pathologic types; (C) number of bone metastases; (D) clinical stage; (E) ECOG scores; (F) serum ALP.
Multivariate regression analysis of prognostic factors
Multivariate analysis was conducted using Cox’s regression model. Number of bone metastases, pathologic type, clinical stage, and serum ALP were significantly correlated with prognosis (P<0.05). The risk of squamous cell carcinoma was 0.389 times that of adenocarcinoma (P<0.001). There was no significant difference in the risk of adenocarcinoma compared with that of the other pathological types. The risk associated with multiple bone metastases was 2.159 times that of single bone metastasis (P=0.004<0.05). Referenced to stage I, the risk of stage IV clinical stage was 3.41 times that of stage I (P=0.002<0.05), while there was no significant difference in the risk of stage II and III compared with that of the stage I. The risk associated with high serum ALP levels was 1.55 times that of low serum ALP levels (P=0.035<0.05) (Table 3).
Table 3.
Multivariate regression analysis of prognostic factors (using COX regression analysis).
| Prognostic factors | Regression coefficient | Standard error | P | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||
| Gender | .126 | .186 | .500 | 1.134 | .787 | 1.634 |
| Agge | .009 | .007 | .184 | 1.009 | .996 | 1.022 |
| Adenocarcinoma | .001 | |||||
| Squamous cell carcinoma | −.944 | .235 | .000 | .389 | .245 | .617 |
| small cell carcinoma | −.368 | .245 | .133 | .692 | .428 | 1.119 |
| Large cell carcinoma | −.239 | .307 | .437 | .788 | .432 | 1.438 |
| Number of primary foci | .005 | .201 | .979 | 1.005 | .677 | 1.492 |
| Number of bone metastases | .770 | .265 | .004 | 2.159 | 1.285 | 3.630 |
| Stage I | .002 | |||||
| Stage II | .401 | .353 | .256 | 1.494 | .748 | 2.985 |
| Stage III | .399 | .348 | .253 | 1.490 | .753 | 2.950 |
| Stage IV | 1.227 | .389 | .002 | 3.410 | 1.589 | 7.316 |
| ECOG score | .282 | .203 | .165 | 1.325 | .890 | 1.973 |
| Transfer time | −.002 | .054 | .964 | .998 | .898 | 1.108 |
| chemotherapy | −.063 | .207 | .761 | .939 | .626 | 1.409 |
| radiotherapy | .034 | .224 | .878 | 1.035 | .667 | 1.606 |
| Double phosphate treatment | −.316 | .246 | .199 | .729 | .450 | 1.181 |
| Serum ALP | .437 | .207 | .035 | 1.548 | 1.031 | 2.324 |
| Blood calcium | −.047 | .213 | .826 | .954 | .629 | 1.448 |
Discussion
The survival of lung cancer patients has been prolonged by advances in healthcare technology. However, the risk of bone metastases from lung cancer has increased [14,15], which predicts poor prognosis. Therefore, it is vital to identify the prognostic factors. This study retrospectively reviewed the clinical characteristics of 168 patients with bone metastases from lung cancer and discusses the prognostic factors.
Adenocarcinoma was the pathologic type associated with the highest incidence of bone metastases. The spine was the most common site of bone metastases, followed by the chest, pelvis, and skull. This finding was consistent with the reports by Wang et al. [16–18]. Bone metastases from lung adenocarcinoma are most commonly found in the ribs and thoracic vertebrae [19], probably because they are very close to the lungs. Moreover, the tumor cells migrate via the blood circulation and proliferate in the bones of the trunk, which are rich in red bone marrow, rather than in the bones of limbs, which are rich in yellow bone marrow. The vertebral bones contain abundant capillary vessels, which provide a favorable condition for the colonization and migration of tumor cells. Because the chest is close to the primary lung lesions and contains abundant capillary vessels, the tumor cells are very likely to spread to thoracic bones [20].
Kaplan-Meier analysis showed that the overall median survival of these168 patients was 13 months, 1-year survival was 54.3%, and the 2-year survival was 12.9%. Bone metastases in lung cancer patients usually predicts a short survival and poor prognosis.
Univariate analysis indicated that pathologic types, number of bone metastases, clinical stage, ECOG scores, and serum ALP levels were the prognostic factors of bone metastases in lung cancer. Multivariate analysis further identified the number of bone metastases, pathologic types, clinical stage, and serum ALP levels as the significant prognostic factors. Adenocarcinoma is usually associated with multiple bone metastases, and the survival for adenocarcinoma is shorter than that of large cell carcinoma, SCLC, and squamous cell carcinoma; therefore, the pathologic type of adenocarcinoma is considered as an independent prognostic factor, as indicated by the univariate analysis and by other studies [21,22]. In multivariate analysis, the risk of squamous cell carcinoma was significantly different from that of adenocarcinoma. There was no statistically significant difference between the risk of large and small carcinomas and that of adenocarcinoma, suggesting that pathological type is an important prognostic factor for bone metastasis of lung cancer. The prognosis of adenocarcinoma may be worse than that of other pathological types.
Survival in patients with single bone metastasis was longer than in those with multiple bone metastases. Multiple bone metastases in lung cancer usually indicate high activity and malignancy of tumor cells and, therefore, poor prognosis [23,24]. A more advanced clinical stage is associated with shorter survival. The clinical implication is that timely and effective treatment after the diagnosis of bone metastases is crucial for prolonging survival.
ECOG performance scores are a quantification of the general well-being and activities of daily life for cancer patients. Higher ECOG scores indicate poor general health of cancer patients, who usually show less tolerance to radiotherapy and chemotherapy and gain little benefit from the treatment. These patients have increased prognostic risk [25,26]. In the univariate analysis, the survival of the low-score patients was much longer than that of the high-score patients. Therefore, ECOG scores are another prognostic factor for bone metastases from lung cancer.
Bone regeneration in normal bone tissue is accomplished by a dynamic balance of bone resorption and formation, which is destroyed after bone metastasis. Bone resorption and formation of metabolic factors change, such as the change in serum alkaline phosphatase ALP. Serum ALP is a type of phosphomonoesterase secreted by the osteoblasts and occurs abundantly in bone and liver. The serum ALP level usually increases following bone fracture and bone metastases [27], especially for bone metastases. Univariate and multivariate analyses indicated that serum ALP was a prognostic factor for bone metastases from lung cancer; higher serum ALP level is associated with shorter survival and worse prognosis.
Tumor cells can damage the inorganic constituents in bone and induce the release of a large amount of calcium into the extracellular fluid via osteolysis, thus increasing serum calcium levels. Changes in serum calcium and free calcium levels are closely associated with bone metastases from lung cancer [28]. However, we did not find a correlation between serum calcium levels and prognosis, and more studies are needed to confirm this.
Conclusions
Pathological type, number of bone metastases, clinical stage, ECOG score, and serum ALP can be used as independent prognostic risk factors for bone metastasis of lung cancer, but a large multicenter clinical follow-up is needed for further validation of our results.
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–49s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Mertsoylu H, Köse F, Sümbül AT, et al. Concurrent chemoradiotherapy with vinorelbine plus split-dose cisplatin may be an option in inoperable stage III non-small cell lung cancer: A single-center experience. Med Sci Monit. 2015;21:661–66. doi: 10.12659/MSM.892730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeVasseur N, Clemons M, Hutton B, et al. Bone-targeted therapy use in patients with bone metastases from lung cancer: A systematic review of randomized controlled trials. Cancer Treat Rev. 2016;50:183–93. doi: 10.1016/j.ctrv.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Kim ST, Uhm JE, Lee J, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer (Amsterdam, Netherlands) 2012;75:82–88. doi: 10.1016/j.lungcan.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7.Bozcuk H, Gumus A, Ozbilim G, et al. Cluster analysis of p-glycoprotein, c-erb-B2 and P53 in relation to tumor histology strongly indicates prognosis in patients with operable non-small cell lung cancer. Med Sci Monit. 2005;11:HY11–20. [PubMed] [Google Scholar]
- 8.Ferda Bir AAA, Satiroglu-Tufan Naciye Lale, Kaya Seyda, et al. Potential utility of p63 expression in differential diagnosis of non-small-cell lung carcinoma and its effect on prognosis of the disease. Med Sci Monit. 2014;20:219–26. doi: 10.12659/MSM.890394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang R, Liang L, Luo D, et al. Downregulation of MiR-30a is associated with poor prognosis in lung cancer. Med Sci Monit. 2015;21:2514–20. doi: 10.12659/MSM.894372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan HJ, Ma JY, Wang L, et al. Expression and significance of circulating microRNA-31 in lung cancer patients. Med Sci Monit. 2015;21:722–26. doi: 10.12659/MSM.893213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebert W, Muley T, Herb KP, et al. Comparison of bone scintigraphy with bone markers in the diagnosis of bone metastasis in lung carcinoma patients. Anticancer Res. 2004;24:3193–201. [PubMed] [Google Scholar]
- 12.Katakami N. [Lung cancer with bone metastasis]. Gan To Kagaku Ryoho. 2006;33:1049–53. [in Japanese] [PubMed] [Google Scholar]
- 13.Tang Q, Zhao H, Jia R, et al. [Correlation of the levels of the bone turnover markers BAP and beta-CTX with bone metastasis progress in lung cancer patients]. Zhongguo fei ai za zhi. 2013;16:144–47. doi: 10.3779/j.issn.1009-3419.2013.03.05. [in Chinese] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook RJ, Major P. Multistate analysis of skeletal events in patients with bone metastases. Clin Cancer Res. 2006;12:6264s–69s. doi: 10.1158/1078-0432.CCR-06-0654. [DOI] [PubMed] [Google Scholar]
- 15.Ogihara S, Seichi A, Hozumi T, et al. Prognostic factors for patients with spinal metastases from lung cancer. Spine. 2006;31:1585–90. doi: 10.1097/01.brs.0000222146.91398.c9. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Ning L, Li H, et al. [Clinical observation of percutaneous osteoplasty in the treatment of 92 lung cancer patients with extraspinal bone metastases]. Tumor. 2014;34(5):443–49. [in Chinese] [Google Scholar]
- 17.Sugiura H, Yamada K, Sugiura T, et al. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res. 2008;466:729–36. doi: 10.1007/s11999-007-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson MA, Calhoun FW. The distribution of skeletal metastases in breast and pulmonary cancer: Concise communication. J Nucl Med. 1981;22:594–97. [PubMed] [Google Scholar]
- 19.Wang CY, Zhang XY. [(99m)Tc-MDP wholebody bone imaging in evaluation of the characteristics of bone metastasis of primary lung cancer]. Zhonghua Zhong Liu Za Zhi. 2010;32:382–86. [in Chinese] [PubMed] [Google Scholar]
- 20.Bae HM, Lee SH, Kim TM, et al. Prognostic factors for non-small cell lung cancer with bone metastasis at the time of diagnosis. Lung Cancer (Amsterdam, Netherlands) 2012;77:572–77. doi: 10.1016/j.lungcan.2012.05.094. [DOI] [PubMed] [Google Scholar]
- 21.Kadota K, Sima CS, Arcila ME, et al. KRAS Mutation Is a Significant Prognostic Factor in Early-stage Lung Adenocarcinoma. Am J Surg Pathol. 2016;40:1579–90. doi: 10.1097/PAS.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama N, Taguchi K, Yokoyama T, et al. Morphometric and cytomorphologic characterization of EGFR-mutated cancer cells-comparison between cultured lung cancer cell lines and lung adenocarcinoma clinical samples. Diagn Cytopathol. 2016;44:717–24. doi: 10.1002/dc.23514. [DOI] [PubMed] [Google Scholar]
- 23.Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: A review. Crit Rev Oncol Hematol. 2005;56(3):365–78. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 25.de Kock I, Mirhosseini M, Lau F, et al. Conversion of Karnofsky Performance Status (KPS) and Eastern Cooperative Oncology Group Performance Status (ECOG) to Palliative Performance Scale (PPS), and the interchangeability of PPS and KPS in prognostic tools. J Palliat Care. 2013;29:163–69. [PubMed] [Google Scholar]
- 26.Papagelopoulos PJ, Savvidou OD, Galanis EC, et al. Advances and challenges in diagnosis and management of skeletal metastases. Orthopedics. 2006;29:609–20. doi: 10.3928/01477447-20060701-01. quiz 621–22. [DOI] [PubMed] [Google Scholar]
- 27.Lumachi F, Marino F, Fanti G, et al. Serum N-telopeptide of type I collagen and bone alkaline phosphatase and their relationship in patients with non-small cell lung carcinoma and bone metastases. Preliminary results. Anticancer Res. 2011;31:3879–81. [PubMed] [Google Scholar]
- 28.Grzywacz A, Dziuk M, Niemczyk S. [Refractory hypercalcemia in patient with lung cancer]. Pol Merkur Lekarski. 2014;36:261–64. [in Polish] [PubMed] [Google Scholar]