Abstract
The creation of auditory threat Pavlovian memory requires an initial learning stage in which a neutral conditioned stimulus (CS), such as a tone, is paired with an aversive one (US), such as a shock. In this phase, the CS acquires the capacity of predicting the occurrence of the US and therefore elicits conditioned defense responses. Norepinephrine (NE), through β-adrenergic receptors in the amygdala, enhances threat memory by facilitating the acquisition of the CS–US association, but the nature of this effect has not been described. Here we show that NE release, induced by the footshock of the first conditioning trial, promotes the subsequent enhancement of learning. Consequently, blocking NE transmission disrupts multitrial but not one-trial conditioning. We further found that increasing the time between the conditioning trials eliminates the amplificatory effect of NE. Similarly, an unsignaled footshock delivered in a separate context immediately before conditioning can enhance learning. These results help define the conditions under which NE should and should not be expected to alter threat processing and fill an important gap in the understanding of the neural processes relevant to the pathophysiology of stress and anxiety disorders.
Pavlovian aversive conditioning (PTC) (Pavlov 1927) is a widely used behavioral paradigm to study a basic and universal form of associative learning. In this paradigm, which will be referred to here as Pavlovian threat conditioning (LeDoux 2014), a neutral conditioned stimulus (CS), a tone in the case of auditory conditioning, is paired with an innately aversive unconditioned stimulus (US), usually a mild footshock. The CS then acquires predictive value over the delivery of the US and can elicit conditioned defensive responses (CRs), such as freezing. Quantification of the time the animal freezes during CS presentations provides an objective and noninvasive measure of the degree of threat learning and memory induced by pairing of the CS and US (LeDoux 2000).
During learning, neural signals encoding the sensory representations of the CS and the US converge in the lateral nucleus of the amygdala (LA) (LeDoux 2007). Neurons in this region respond to both stimuli, and the CS–US pairing induces increased single-unit activity, potentiated auditory-evoked field potentials, activation of molecular cascades, and morphological changes that eventually underlie the consolidation of the learning as a long-term memory (LTM) (Weisskopf et al. 1999; Bauer et al. 2001; Doyère et al. 2003; Maren and Quirk 2004; Ostroff et al. 2010; Johansen et al. 2011). These changes are the result of a combination of Hebbian and neuromodulatory mechanisms (Bailey et al. 2000; McGaugh et al. 2002; Pawlak et al. 2010; Tully and Bolshakov 2010; Johansen et al. 2014).
Norepinephrine (NE), which is released widely in the brain during situations involving stress, emotional arousal, attention, and alertness (for reviews, see Rodrigues et al. 2009; Sara and Bouret 2012; Raio and Phelps 2015) is one of the key neuromodulators that regulate the degree of learning and memory (Roozendaal et al. 2009; Joëls et al. 2011; McEwen 2012). The amygdala receives direct noradrenergic innervation from the locus coeruleus (LC) (Uematsu et al. 2015), and β-adrenergic receptors (βARs) are highly expressed in LA (Farb et al. 2010). The standard view of the effect of NE in the amygdala, largely based on studies using inhibitory avoidance, is that NE acting through βARs potentiates the consolidation of the learning (McGaugh 2000, 2015; McGaugh et al. 2002; Packard and Wingard 2004; Roozendaal and McGaugh 2011) without having any effect on the acquisition of the association itself. However, we have recently reported that NE, also acting through βARs, must be present during the initial CS–US training (acquisition) to create robust memory of the CS–US association (Sears et al. 2013; Johansen et al. 2014). In line with these results, our laboratory and others have found that the blockade of βARs by propranolol disrupts long-term memory when infused into the LA before the training session (acquisition) but not immediately after (Miserendino et al. 1990; Lee et al. 2001; Dębiec and LeDoux 2004; Bush et al. 2010; Schiff et al. 2017). This suggests that the action of NE in LA results in an enhanced memory of the CS–US by facilitating the acquisition of the association.
How might NE achieve these effects on acquisition? The NE system projects widely throughout the brain (Swanson and Hartman 1975). It is involved in many adaptive physiological and pathophysiological processes, being released in response to a large variety of stimuli (Benarroch 2009). While tones and shocks both activate LC and elicit NE release, shock is a particularly effective stimulus (Aston-Jones et al. 1996; Sara 2009). In the present study, we explore the possibility that the footshock US is the trigger responsible for an increase of the NE release during acquisition. This hypothesis leads to the prediction that the basal release of NE has no influence on the first pairing trial of multitrial learning, but should affect subsequent trials, provided the presentation of these trials occur before NE levels return to baseline.
Results
NE facilitates learning only when conditioning involves multiple trials
We previously reported that NE, acting through βARs in the LA, is involved in the acquisition but not in the consolidation of cued threat memories, as long-term memory is only reduced when the βAR-antagonist propranolol is infused into the LA before training but not after training (Bush et al. 2010). In accordance, systemic injection of propranolol after training does not affect LTM (Lee et al. 2001; Dębiec and LeDoux 2004). However, to our knowledge, the effect of a pretraining systemic administration of a βAR-blocker in rodents has never been reported.
We tested whether pretraining systemic propranolol administration would similarly diminish acquisition of PTC. Rats were randomly administered an intraperitoneal (i.p.) injection of propranolol (10 mg/kg) or its vehicle (saline 1 mL/kg) 30 min before the beginning of the conditioning session (Fig. 1A). To account for the possibility that NE activity could differ depending on prior exposure to the CS–US pairing, during the training session half of the animals received a single CS–US presentation (1Trial group) while the other half were presented with a second CS–US 2 min after the first one (2Trial group). All animals stayed a total duration of 9 min in the conditioning box (Fig. 1A).
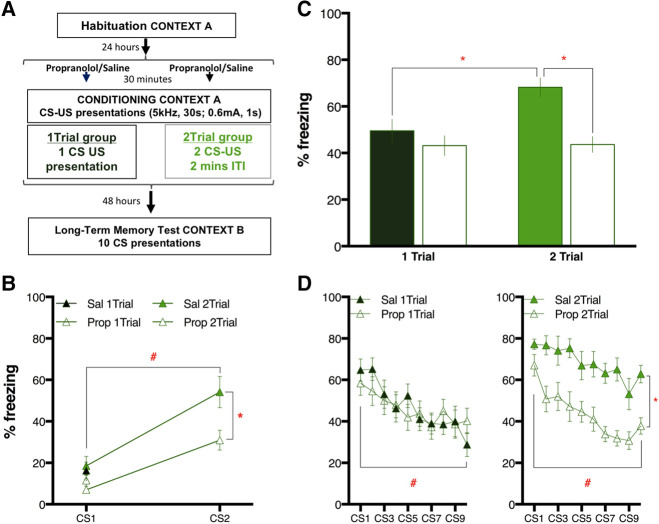
Figure 1.
Long-term threat memory (LTM) is impaired by pretraining propranolol (10 mg/kg) in animals conditioned with 2 CS–US pairing trials but not in animals that received 1 CS–US trial. (A) Schematic of the experimental design. Animals were habituated to the conditioning context for 30 min. Twenty-four hours later, rats received propranolol (10 mg/kg: Prop: empty symbols) or saline vehicle (1 mL/kg, Sal: filled symbols) 30 min before the conditioning session, which consisted of either 1 CS–US pairing trial (1Trial: dark green) or two trials (2Trial: light green) with a 2-min intertrial interval (ITI). Forty-eight hours later, LTM was tested in a modified context with 10 CS-alone presentations. (B) Graph showing the average percentage of freezing (mean ± SEM) to each CS presentations during threat conditioning session for each set of animals. In the group conditioned by presenting two CS–US pairings, the animals treated with propranolol froze less than their controls to the CS2. (C) Percentage of freezing (mean ± SEM) averaged across all 10 CSs presented to test LTM. Post hoc tests after finding significant main Drug Treatment and Trial effects as well as Treatment by Trial interaction revealed that the saline animals conditioned with 2Trials froze more that the 1Trial ones. Propranolol reduced the amount of freezing only in the 2Trial group. (D) The percentage of freezing (mean ± SEM) to each of the 10 CSs presented for LTM test divided by treatment. The 1Trial (left graph) and the 2Trial (right graph) groups showed main effects of CS presentation indicating normal within-session extinction learning. 2Trial animals also showed a main effect of the treatment. (*) P < 0.05, (#) P < 0.05 for CS main effect.
The freezing to the context was assessed by measuring the freezing during the 30 sec immediately before the first CS–US pairing in the conditioning session or the first CS presentation in the long-term memory test. For all the described experiments, no significant difference was observed between saline and propranolol treatment or between behavioral manipulations, therefore detailed contextual freezing data is not shown. Instead only the statistics and the average contextual freezing of all rats in each experiment are shown.
Baseline freezing to the conditioning context before CS–US1 did not differ among the four conditions (F(3,38) = 1.37, P = 0.27), and freezing in all conditions was low (combined average: 1.5 ± 0.5%, mean ± SEM). A one-way ANOVA with six levels compared freezing during CS–US1 and CS–US2 across the four conditions. The six levels included freezing to CS–US1 for all four conditions and freezing to CS–US2 for the two conditions that received the second trial. (Fig. 1B) This ANOVA showed a significant effect (F(5,54) = 14.21, P < 0.01). Post hoc Sidak tests showed no pairwise differences between conditions in freezing to CS–US1 (adjusted P values: Sal 1Trial versus Prop 1Trial P = 0.99; Sal 1Trial versus Sal 2Trial P > 0.99; Sal 1Trial versus Prop 2Trial P = 0.74; Prop 1Trial versus Sal 2Trial P = 0.93; Prop 1Trial versus Prop 2Trial P > 0.99; Sal 2Trial versus Prop 2Trial P = 0.55). Conditioning was effective in the 2Trial group, as shown by Sidak tests comparing freezing to the first tone with freezing to the second tone in both Sal 2Trial and Prop 2Trial (both P < 0.01). Interestingly, Prop 2Trial animals showed significantly less freezing than Sal 2Trial to the CS–US2 presentation (P < 0.01). These results indicate that propranolol reduced freezing to CS–US2, but did not affect freezing to CS–US1 or to the context prior to CS–US1. This suggests that propranolol may be selectively affecting the brain processing of the first CS–US presentation or affecting freezing only to a learned CS, rather than decreasing freezing behavior in general.
Long-term memory was tested 2 d later by 10 CS-alone presentations in a modified context (Fig. 1A). A two-way ANOVA comparing treatment (Propranolol or Saline) and groups (1Trial or 2Trial) revealed no difference in the contextual freezing level (Treatment effect: F(1,38) = 1.51 P = 0.23; Group effect: F(1,38) = 0.01, P = 0.93; Interaction: F(1,38) = 0.63, P = 0.43) and conditioning did not induce freezing to the context since averaged pre-CS freezing of all tested animals (5.0 ± 1.3%) was negligible. A two-way ANOVA test comparing average freezing across the 10 CS presentations (Fig. 1C) showed an interaction between treatment and group (F(1,38) = 4.21, P < 0.05), a main effect of the treatment (F(1,38) = 12.17, P < 0.01) and a main effect of the group (F(1,38) = 4.70, P < 0.05). As expected, Sal 2Trial animals showed significantly more freezing than Sal 1Trial (P < 0.05). However, Prop 2Trial animals displayed significantly less freezing than Sal 2Trial (P < 0.05), while the freezing of Prop 1Trial animals did not differ from their controls (P = 0.86).
Two-way ANOVAs comparing the average freezing per treatment during each of the 10 tones (Fig. 1D) for the 1Trial group (left graph) showed an overall effect of the CS trial (F(9,220) = 4.44, P < 0.01), no effect of treatment (F(1,220) = 0.16, P = 0.69) nor a treatment by trial interaction (F(9,220) = 0.68, P = 0.73). This confirms that NE activity at βARs is not required for learning in the single-trial group. In contrast, the animals conditioned with two CS–US trials (Fig. 1D, right graph) showed a significant effect of treatment (F(1,160) = 101.10, P < 0.01) and trial (F(9,160) = 5.54, P < 0.01) but no treatment by trial interaction (F(9,220) = 0.64, P = 0.77). This indicates that the animals treated with propranolol on conditioning day showed reduced freezing on test day. There was a main effect of Trial in both panels (Fig. 1) indicating no difference in extinction over the 10 CSs regardless of the number of trials on conditioning day. Similarly, the lack of interaction indicates that within-session extinction was unaffected by pretraining drug administration.
Overall the above results reveal that (1) the first conditioning trial affects the following trial within the conditioning session (Fig. 1B); (2) when tested for LTM, saline-treated animals conditioned with two trials show higher freezing than the ones conditioned with a single trial (Fig. 1C); (3) propranolol diminishes the freezing response only for the animals that experienced the second CS–US (Fig. 1C,D). This pattern of results suggests that the first conditioning trial induces an increase in the release of NE that strengthens the learning of the following CS–US presentation.
An unsignaled footshock before acquisition enhances learning through a mechanism involving β-adrenergic receptors
Several studies using in vivo microdialysis have reported that the release of NE in the Amygdala is increased by the presentation of an unsignaled footshock (Galvez et al. 1996; Quirarte et al. 1998; Hatfield et al. 1999). According to these findings, the concentration of NE remains elevated for nearly 30 min. Given this evidence, we hypothesize that the footshock delivered with the first CS–US presentation is the necessary trigger that, by increasing the release of NE, facilitates learning on following trials.
To test this idea, we designed an experiment in which rats were exposed to an unsignaled footshock (U-US) before conditioning (Fig. 2A). The U-US was delivered in a different context (shuttlebox in a different room) than the one used for conditioning to prevent contextual freezing. Rats were assigned randomly to one of four experimental groups (Fig. 2A). In a control group, “No U-US”, rats were placed in the shuttlebox and did not experience a U-US. “High U-US” and “Early U-US” received a 1-mA intensity footshock with a duration of 5 sec, while the shock presented to the “Low U-US” group had the same intensity (1 mA) but only 1 sec duration (Fig. 2A). Thirty minutes before the presentation of the U-US, the animals were injected with propranolol or saline. After the unsignaled shock, “Early U-US” rats spent 40 min in their home cages before conditioning, while the three other groups were conditioned immediately in the new context for a single CS–US conditioning trial.
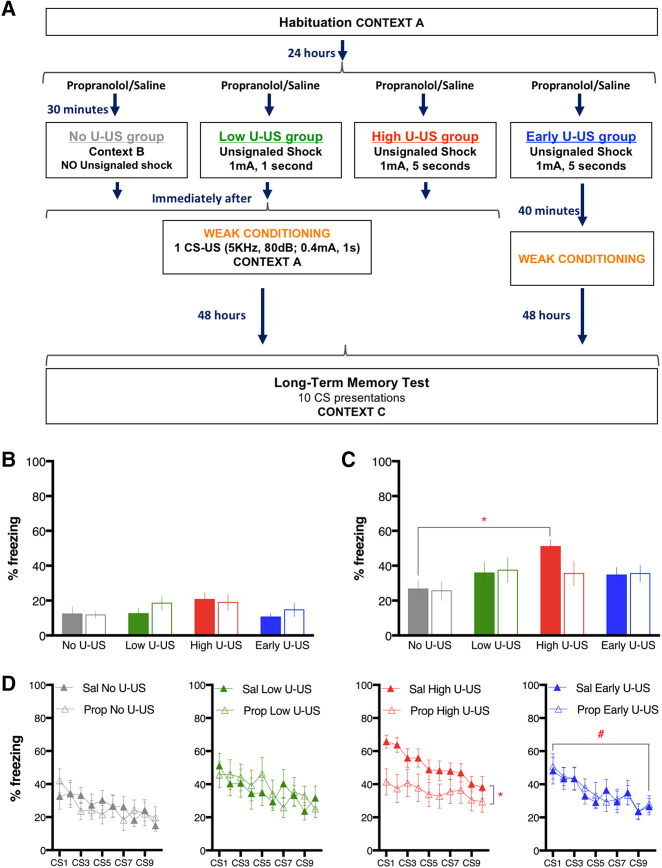
Figure 2.
The enhancement of freezing during long-term memory test induced by the preexposure to an unsignaled footshock (U-US) before single-trial weak conditioning is prevented by pretraining propranolol. (A) Schematic of experimental design. On the day following habitation to the conditioning context, animals received propranolol (10 mg/kg: Prop: empty symbols) or saline vehicle (1 mL/kg, Sal: filled symbols) 30 min before placement in a shuttlebox. Three seconds after the beginning of the shuttlebox protocol, rats received either no shock (No U-US: gray), a 1-sec shock (Low U-US: green), or a 5-sec shock (High U-US: red; or Early U-US: blue). All groups were immediately transferred to the conditioning context after offset of the U-US, except for the Early U-US group that instead waited in their home cages for 40 min before conditioning. Conditioning consisted of a single trial of tone–shock pairing with a weak shock (0.4 mA). Forty-eight hours later, an LTM test was done by giving 10 CS-alone presentations in a modified context. (B) Graph showing percentage of freezing (mean ± SEM) during the CS presentation in the conditioning session for each group of animals. Note that this CS is a novel stimulus at this point, not having been paired with shock until the final second of this CS presentation. No significant effects or differences were observed on this measure. (C) Results from LTM test expressed as percentage freezing (mean ± SEM) to CSs averaged across all 10 CS-alone presentations. A main effect of U-US condition was observed, and post hoc testing revealed that Sal High U-US froze more than the Sal No U-US control. (D) Percentage freezing (mean ± SEM) to each CS in the LTM session. A main effect of drug treatment was seen only in the High U-US condition. A main effect of CS presentation was observed only for the Early U-US condition. (*) P < 0.05, (#) P < 0.05 for CS main effect
Pre-CS freezing for both the conditioning session and the LTM test showed no group or treatment effect nor an interaction between group and treatment (Conditioning: F(3,75) = 1.83, P = 0.35; F(1,75) < 0.01, P = 0.95; F(3,75) = 1.1, P = 0.34. LTM test: F(3,75) = 2.40, P = 0.07; F(1,75) = 3.30, P = 0.07; F(3,75) = 0.79, P = 0.50). The averaged pre-CS freezing was 4.0 ± 1% (Conditioning) and 6.9 ± 1.3 (LTM test). All animals were conditioned with a single CS–US trial. The intensity of the footshock was 0.4 mA with the aim of inducing weak learning in the animals, which is ideal for detecting increases in freezing due to manipulations when decreases relative to the control condition are not expected. Freezing to the CS presented during conditioning was unaffected by drug treatment and the U-US precondition, and there was no interaction between the two as shown by a two-way ANOVA (Fig. 2B) (U-US: F(3,75) = 1.92, P = 0.13; Drug: F(1,75) = 0.54, P = 0.47; U-US × Drug F(3,75) = 0.50, P = 0.68). Thus, neither systemic propranolol administration nor prior exposure to a U-US in a distinct context affected freezing in response to a novel auditory cue (the CS).
In contrast, the comparison of the averaged freezing across the 10 CSs presented to test LTM 48 h after conditioning (Fig. 2C) showed a main effect of group (F(3,75) = 3.20, P < 0.05). There was no main effect of treatment (F(1,75) = 0.80, P = 0.37) and no group by treatment interaction (F(3,75) = 1.07, P = 0.37). In the saline condition, only the animals that were exposed to the high intensity U-US and conditioned immediately after (High U-US) froze significantly more than the animals that were not exposed to the U-US before training (post hoc Sidak pairwise test P < 0.05). This finding indicates that prior exposure to an unsignaled shock in a distinct context can enhance one-trial learning, perhaps relying on a similar mechanism as that recruited by two-trial learning.
The analysis of the effect of propranolol in each U-US condition was performed comparing the mean percent freezing scores across each test trial (Fig. 2D). The animals that were exposed to high intensity U-US and treated with propranolol exhibited significantly less freezing than their vehicle controls (F(1,210) = 26.87, P < 0.01). Interestingly, only animals that did not experience the conditioning session immediately after the U-US (Early U-US condition) showed significant within-session extinction over the course of 10 CS presentations (F(9,180) = 3.51, P < 0.01). No interactions between treatment and CS trial were significant for any of the groups.
These results indicate that an unsignaled footshock, depending on its intensity, potentiates learning when conditioning occurs close in time. The effect of the unsignaled footshock is prevented by propranolol, providing further evidence for the hypothesis that the first shock during conditioning can be the trigger of the NE-induced facilitation of learning.
The enhancement of learning induced by NE depends on the time interval between training trials
If the initial shock during the threat conditioning session is what induces the release of NE that further enhances the acquisition of the CS–US association, then we predict that this facilitative effect must be circumscribed to the temporal window in which NE concentration is increased in the LA (Galvez et al. 1996; Quirarte et al. 1998; Hatfield et al. 1999). To test this, 30 min after systemic propranolol or saline, rats underwent two-trial conditioning with ITIs of 2 or 40 min (Fig. 3A). All animals stayed in the conditioning context for a total time of 60 min.
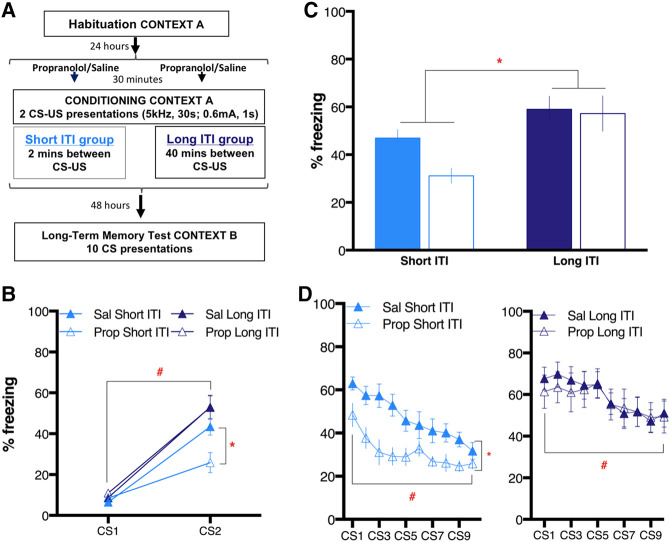
Figure 3.
The norepinephrine enhancement of acquisition of long-term threat memory depends on the length of ITI during training. (A) Schematic of the experimental design. The conditioning session included two CS–US pairings, which were either 2 min apart (short ITI: light blue) or 40 min apart (long ITI: dark blue). All animals were injected with propranolol (10 mg/kg: Prop: empty symbols) or saline vehicle (1 mL/kg: Sal: filled symbols) 30 min before conditioning. Habituation and LTM test sessions were identical to the other experiments. (B) Percentage of freezing (mean ± SEM) to each CS in the conditioning session for each condition. Post hoc pairwise comparisons showed Prop Short ITI animals froze less to CS2 than their saline controls. (C) Graph showing freezing (mean ± SEM) averaged across all 10 CS presentations in the LTM test. A main effect of group was observed. (D) Analysis of freezing in the LTM test to each CS within each group showed that pretraining propranolol reduced freezing only for the Short ITI group but not for Long ITI. Both conditions showed a main effect of CS, demonstrating within-session extinction learning. (*) P < 0.05, (#) P < 0.05 for CS main effect.
Pre-CS freezing for both the conditioning session and LTM test showed no main effect of ITI group or drug treatment, nor was there an interaction (conditioning, one-way ANOVA: F(3,38) = 2.21, P = 0.10; LTM test, two-way ANOVA: group: F(1,38) = 0.24, P = 0.63; treatment: F(1,38) = 1.73, P = 0.20; interaction: F(1,38) = 0.02, P = 0.89). The averaged pre-CS freezing of all conditions together was 1.2 ± 0.3% (Conditioning) and 6.6 ± 2.4 (LTM test). During training, a one-way ANOVA on the freezing response of each group to CS–US1 (Fig. 3B) showed no significant difference among conditions in their response to the tone before it was paired with a shock (F(3,38) = 1.66, P = 0.19). Next, a two-way ANOVA including the four conditions as a between-subjects factor and CS–US trial as the other factor revealed a significant effect of CS–US trial (F(1,76) = 173.90, P < 0.01), indicating that after a single CS–US presentation, subjects were conditioned. In addition, a significant interaction suggests that this conditioning varied in strength between different conditions (F(3,76) = 5.38, P < 0.01). There was also a main effect of condition (F(1,76) = 6.51, P < 0.01; Fig. 3B). Post hoc Sidak testing revealed that Prop Short ITI animals froze significantly less during the second CS compared with their vehicle controls (P < 0.05), confirming our previous finding with two-trial conditioning (Fig. 1B).
During LTM, we observed a main effect of group (F(1,38) = 14.05 P < 0.01) on freezing (Fig. 3C). There was no overall effect of the treatment (F(1,38) = 3.00, P = 0.09), nor was there a significant group by treatment interaction (F(1,38) = 1.91, P = 0.17). Sidak's post hoc tests along the group dimension revealed a difference between ITI group only for the propranolol treatment (Prop Short ITI versus Prop Long ITI; P < 0.01), while saline-treated animals did not differ significantly (Sal Short ITI versus Sal Long ITI; P = 0.19)
The effect of treatment across the 10 CS presentations of the LTM session was assessed with a separate treatment-by-CS trial ANOVA for each group (Fig. 3D). Both Short ITI and Long ITI groups experienced a significant effect of CS trial (F(9,200) = 7.11, P < 0.01; F(9,180) = 2.04, P < 0.05, respectively). Within the Short ITI group, propranolol-treated animals exhibited significantly lower freezing than their saline controls (F(1,200) = 64.95, P < 0.01), while the freezing in the long ITI group was not affected by treatment (F(1,180) = 0.11, P > 0.99). Neither group showed a treatment by CS trial interaction (short ITI: F(9,200) = 0.95, P = 0.49; long ITI: F(9,180) = 0.31, P = 0.58). This result indicates that propranolol only affects memory formation when the second CS–US pairing occurs within a certain time following the first CS–US pairing.
Discussion
The results obtained here confirm that the NE transmission greatly contributes to the acquisition of Pavlovian threat associations. Building on this, the studies presented here show that the NE-induced facilitation of learning is governed by precise temporal dynamics initiated by an increase in the NE release triggered by the footshock presented as part of the first conditioning association.
Few studies have used systemic administration of propranolol and other βARs antagonist in rats or mice to study the role of NE transmission in memory formation during auditory threat conditioning. In those reports, the drug was only administered immediately after training (Miserendino et al. 1990; Lee et al. 2001; Dębiec and LeDoux 2004). Their conclusion, based on the lack of effect observed during the short-term memory and/or long-term memory tests, was that βAR activity is not involved in the consolidation of learning. When applied directly to the LA through local administration, blockade of βARs with propranolol reduces acquisition (Bush et al. 2010), but whether systemic propranolol would have the same effect was unknown. Here we show that systemic propranolol similarly reduces acquisition, indicating that NE, acting through βARs, serves to enhance memory formation by modulating acquisition processes. This suggests that the NE effects observed here occur in the LA and supports the conclusion that the LA is a critical site for PTC learning. Using threat-potentiated startle, Davis and his collaborators have reported contradictory results since systemic pretraining propranolol disrupted the expression of cued potentiated startle in rats (Walker and Davis 2002); however, this was not replicated in humans (Grillon et al. 2004).
This study provides evidence that NE must already be present at the time of a CS–US pairing in order to play its modulatory role in acquisition. The amount of freezing during the memory test was not modified by propranolol in the animals exposed to only one CS–US. In contrast, in the animals that were exposed to a second conditioning trial, propranolol diminished the CS-evoked freezing during the second CS–US presentation and the freezing when long-term memory was tested 48 h later. This implies that NE is not involved when learning occurs after a single CS–US pairing.
Pavlovian learning is instructed by expectation, or rather, by violation of expectation. This prediction error can be the direct result of an unexpected US (Rescorla and Wagner 1972) or indirectly caused by the CS after the first CS–US presentation since this second CS commands more attention (Mackintosh 1975). Hence, the learning of the first CS–US association might be governed by different learning rules instructed by different brain areas and neurotransmitters. As an example, μ-opioid receptors in the ventrolateral quadrant of the midbrain periaqueductal gray mediate learning by altering the brain's processing of the US while having no effect on the CS attentional teaching signal (Cole and McNally 2007). Since NE blockade attenuates two-trial but not one-trial learning (Fig. 1), it is possible that NE facilitates learning by enhancing the attentional strength of the CS as a teaching signal. However, the facilitation of learning induced by the U-US is suggestive of an arousal mechanism, since in this case the facilitation of learning happens after only one CS–US presentation. The interval between a U-US or CS–US and a future CS–US seems to determine NE dependence, also consistent with an arousal mechanism.
We have not tested the effect of propranolol after single-trial learning; however, a previous article reported, using the same protocol and drug dose we use here, that after conditioning with 1 CS–US pairing neither systemic nor intra-LA propranolol impaired memory when tested 48 h later (Dębiec and LeDoux 2004). Together, these findings strongly suggest that NE is not necessary for single-trial learning.
The present results show that increased NE levels potentiate learning and this NE release can come from the US in the first CS–US pairing. However, learning is also potentiated in a β-AR-dependent manner when one-trial conditioning is preceded by an unsignaled US, indicating that the first CS–US pairing can be replaced by an unsignaled shock, or perhaps any strong enough stressor. We observed that long-term memory was strengthened only by preexposure to a long-duration unsignaled footshock immediately before conditioning and not by the short-duration one (Fig. 2), and this effect was prevented by propranolol. Confirming our prediction that was based on previous reports of a time-limited increase in NE release after an electrical shock (Galvez et al. 1996; Quirarte et al. 1998; Hatfield et al. 1999), the effect of the U-US did not occur if conditioning was delayed by 40 min. This temporal constraint on the NE-induced enhancement of learning was also observed when the interval between two consecutive conditioning trials was increased from 2 to 40 min (Fig. 3). The intensity of the U-US was not identical to the intensity of the US during conditioning. We chose the parameters of the unsignaled shock based on previous publications measuring the release of NE after the delivery of an unsignaled footshock (Galvez et al. 1996; Quirarte et al. 1998; Hatfield et al. 1999). Since we were not directly measuring NE release, and we wanted to be sure that in our paradigm were inducing an increase in NE we increased the intensity of the footshock from 0.6 to 1 mA. Of note, 1 mA, 1-sec U-US did not induce an effect on long-term memory while 1 mA, 5 sec did. An unsignaled shock increases the NE levels, but since the rodents in experiment 2 are exposed to the CS for the first time after the unsignaled shock, its salience is not comparable to the salience of the CS when it is presented in a second trial. This could explain the necessity of higher intensity of the U-US to facilitate learning.
It must be noted that the two-trial learning with a long ITI was robust despite the apparent lack of NE-dependence. Perhaps the same mechanisms that underlie single-trial learning have, over the 40 min ITI, induced an intermediate phase of memory formation that acts as scaffolding for further conditioning. It is also possible that other neuromodulators are involved that accomplish similar effects on a different time scale. For example, dopamine and serotonin have been shown to increase in the amygdala for well over an hour following tone–footshock pairings (Yokoyama et al. 2005).
Extensive evidence indicates that NE released into the amygdala from neurons in the LC has a direct and indirect (mediating the effects of other hormones and neurotransmitters) role in regulating stress effects on memory by acting through activation of β-adrenergic receptors (Morilak et al. 2005; Rodrigues et al. 2009; Roozendaal et al. 2009; Schwabe et al. 2012). Creating adaptive explicit and implicit memories during stress is vital for confronting future threats (Yehuda et al. 2010). We previously showed that NE, acting through βARs, enhances memory formation with temporally specific actions on extracellular regulated kinase (ERK) and AMPA receptor subunits within the LA (Schiff et al. 2017). Since unsignaled electrical shocks are used to generate stress responses, it is possible that the effect of the U-US is mediated by such mechanisms, priming the system for subsequent memory formation. Nonetheless, if the U-US facilitation of learning observed in the present experiments is a stress response remains to be evaluated by using other type of acute stressors.
Another question that comes out of our findings is whether prior U-US exposure could similarly enhance expression of a previously trained conditioned threat memory. According to our findings, the brain levels of NE would be elevated to levels that are capable of enhancing subsequent learning, but would it also enhance expression?
Pavlovian conditioning contributes to disorders like PTSD (Rau and Fanselow 2009; Cain et al. 2012; Mahan and Ressler 2012). Using this task, we have found that NE enhances memory by acting during learning. Our findings are consistent with clinical studies demonstrating that while βAR blockade does not prevent the consolidation of traumatic memory, it improves symptoms of stage fright (Dubovsky 1990). The description of the dynamics by which NE facilitates the acquisition of Pavlovian threat memories suggests that the timing in which NE agents are used to treat PTSD and related psychiatric conditions must be reevaluated for therapeutic success.
Materials and Methods
Subjects
Subjects were 167 male Sprague-Dawley rats (Hilltop Laboratory Animals; Scottdale, PA, USA) weighing 225–400 g at the beginning of the experiments. The animals were single housed in an environment that was temperature- and humidity-controlled and maintained on a 12/12 propranolol light–dark cycle. Rats had ad libitum access to food and water. All conditions and procedures followed the National Institutes of Health Guide for the Care and Use of Experimental Animals and all procedures were approved by the New York University Animal Care and Use Committee.
Drug injections
(±) Propranolol hydrochloride (Sigma-Aldrich) was freshly prepared on each conditioning day. All rats were injected i.p. with 10 mg/mL of propranolol or its vehicle (saline). The volume was 1 mL/kg. For experiments 1 and 3, the treatments were administered 30 min before conditioning procedure. For experiment 2, the treatments were administered 30 min before the delivery of the U-US. This dose is commonly used in PTC experiments (Dębiec and LeDoux 2004; Rodriguez-Romaguera et al. 2009). All injections were done by the same experimenter.
Apparatus and stimuli
All experiments were conducted using a Habitest Linc system controlled by Graphic State 2 software. Conditioning boxes (Model H10-11R-TC; Coulbourn Instruments; 30 cm width, 25 cm depth, 30 cm height) were placed in sound-isolating cubicles (Model H10-24A; Coulbourn Instruments). For experiment 2, shuttleboxes were also used (Model H10-11R-SC; Coulbourn Instruments; 50 cm width, 25 cm depth, 30 cm height.) Every box had a rod flooring connected to shock generators (Model H13-15; Coulbourn Instruments), a house light, a speaker connected to a tone generator (Model A12-33; Coulbourn Instruments), and infrared cue lights.
The CS was a continuous tone (30 sec duration; 5 kHz; 80 dB). The US consisted of a mild electrical footshock (1 sec, 0.4 or 0.6 mA depending on the experiment). For experiment 2, we also used an unsignaled footshock (U-US; 1 or 5 sec, 1 mA) delivered in the shuttle boxes. During conditioning the US coterminated with the CS.
Behavioral procedures
On day 1, rats were habituated to the conditioning context by placing them in the conditioning boxes for 30 min. Twenty-four hours later the rats were weighed and randomly assigned to be injected i.p. with either propranolol (Prop) or saline vehicle (Sal) 30 min before the conditioning session (or 30 min before U-US delivery in experiment 2). Forty-eight hours later, long-term memory (LTM) was tested by presenting 10 unreinforced CS's in a modified context with a variable ITI ranging from 90 to 140 sec (mean ITI was 112 sec). The first CS was preceded by a 3-min acclimation period. This was in the same box as the conditioning session, but with peppermint odor, a smooth black plastic floor covering the rod flooring, and a dim red light instead of the usual house light. All sessions of all procedures were run for six rats at a time.
Experiment 1
In the conditioning session on day 2 (Fig. 1A), rats received either 1 or 2 CS–US pairing trials. A footshock (0.6 mA, 1 sec) was given during the final second of a 30 sec tone. The acclimation period was 5 min, and rats were in the conditioning context for 9 min total. For the rats that received 2 CS–US pairings, the ITI was 2 min. (Group sizes were Sal 1Trial: N = 12; Prop 1Trial: N = 12; Sal 2Trial: N = 9; Prop 2Trial: N = 9).
Experiment 2
Rats were exposed to an unsignaled 1.0-mA footshock in a shuttlebox before the conditioning session on day 2 (Fig. 2A). Four behavioral conditions were used: “No U-US” group was placed in the shuttlebox for the same duration as the other three groups but did not receive any footshock. After 3 sec, the other three groups were subjected to either 1 sec (Low U-US) or 5 sec (High U-US and Early U-US) of a 1.0-mA footshock. After this unsignaled shock procedure, rats were transferred to the conditioning context (No U-US, Low U-US, and High U-US groups) for the conditioning session, or returned to the home cage (Early U-US group) for a 40 min rest period before transfer to the conditioning session. (Group sizes were Sal No U-US: N = 10; Prop No U-US: N = 10; Sal Low U-US: N = 10; Prop Low U-US: N = 10; Sal High U-US: N = 11; Prop High U-US: N = 12; Sal Early U-US: N = 10; Prop Early U-US: N = 10)
For the conditioning procedure in experiment 2, a single CS–US trial was given, consisting of a weak footshock (0.4 mA) delivered during the last second of a 30-sec tone presentation. This trial was after 5 min of acclimation to context. Twenty seconds after the CS–US offset, animals were removed from the conditioning context and returned to the vivarium.
Experiment 3
Two CS–US pairings were delivered with an ITI that was either short (2 min; “Short ITI” group) or long (40 min; “Long ITI” group) (Fig. 3A). The pairings consisted of a 30-sec tone coterminating with a 1-sec 0.6-mA footshock. All rats were in the conditioning context for a total of 60 min, including a 3-min acclimation period, CS–US pairings with the ITI between, and a continued context-exposure period after the second pairing that brought the total length to 60 min. (Group sizes were Sal Short ITI: N = 11; Prop Short ITI: N = 11; Sal Long ITI: N = 10; Prop Long ITI: N = 10)
Assessment of freezing
Videos recorded during conditioning sessions and LTM test sessions were analyzed for freezing by at least one rater who was blind to drug treatment condition. Freezing was defined as cessation of all movement other than respiration. Freezing during each CS presentation was timed with a digital stopwatch and presented as a percentage of the CS duration. Baseline freezing in conditioning and test contexts was established by scoring the 30-sec period before the first CS.
Statistical analysis
The freezing in the conditioning session (Figs. 1B, 2B, 3B) was analyzed differently between experiments because the nature of the manipulations differed. First, we used one-way analysis of variance (ANOVA) tests to check for differences (freezing during Pre-CS and CS–US1) before behavioral manipulations. This was done in experiments 1 and 3. We then confirm effectiveness of conditioning by analyzing increases in the percentage of freezing from CS–US1 to CS–US2. In experiment 1, only the 2Trial group was exposed to CS–US2, so in this case the one-way ANOVA included the freezing to the CS–US2 to simplify analysis. In experiment 3, a two-way ANOVA is used, one factor being CS–US1 or CS–US2 and the other factor being the combination of treatment and behavioral group. Given that the acquisition of learning is reflected in part by within-session change, we include CS–US1 in all ANOVAs involving CS–US2. We examined group and treatment effects on CS–US2 with post hoc Sidak tests. Experiment 2 involved only one CS–US pairing, and this was analyzed with a two-way group-by-treatment ANOVA because the manipulation resulting in the four groups occurred before conditioning. Pre-CS freezing is also analyzed by a two-way ANOVA in this case because it is also after behavioral manipulations.
Analysis of the long-term memory test was the same in all experiments. First, we performed a two-way ANOVA with group and treatment as factors and the average of all 10 freezing-to-CS measures for each rat as the dependent variable (Figs. 1C, 2C, 3C) followed by a Sidak post hoc test analyzing the biologically relevant factors depending on the experiment. To further analyze the effect of treatment we analyzed each group separately and including CS-presentation trial as a second variable in a treatment-by-CS two-way ANOVA.
Acknowledgments
This work was supported by a grant from the National Institute for Mental Health to J.E.L. (R01 MH046516).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.044412.116.
Freely available online through the Learning & Memory Open Access option.
References
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. 1996. Role of the locus coeruleus in emotional activation. Prog Brain Res 107: 379–402. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Giustetto M, Huang YY, Hawkins RD, Kandel ER. 2000. Is heterosynaptic modulation essential for stabilizing Hebbian plasticity and memory? Nat Rev Neurosci 1: 11–20. [DOI] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE, Nader K. 2001. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat Neurosci 4: 687–688. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. 2009. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73: 1699–1704. [DOI] [PubMed] [Google Scholar]
- Bush DEA, Caparosa EM, Gekker A, LeDoux JE. 2010. β-Adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci 4: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Maynard GD, Kehne JH. 2012. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs 21: 1323–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, McNally GP. 2007. Opioid receptors mediate direct predictive fear learning: evidence from one-trial blocking. Learn Mem 14: 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dębiec J, LeDoux JE. 2004. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129: 267–272. [DOI] [PubMed] [Google Scholar]
- Doyère V, Schafe GE, Sigurdsson T, LeDoux JE. 2003. Long-term potentiation in freely moving rats reveals asymmetries in thalamic and cortical inputs to the lateral amygdala. Eur J Neurosci 17: 2703–2715. [DOI] [PubMed] [Google Scholar]
- Dubovsky SL. 1990. Generalized anxiety disorder: new concepts and psychopharmacologic therapies. J Clin Psychiatry 51(Suppl): 3–10. [PubMed] [Google Scholar]
- Farb CR, Chang W, LeDoux JE. 2010. Ultrastructural characterization of noradrenergic axons and β-adrenergic receptors in the lateral nucleus of the amygdala. Front Behav Neurosci 4: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. 1996. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem 66: 253–257. [DOI] [PubMed] [Google Scholar]
- Grillon C, Cordova J, Morgan CA, Charney DS, Davis M. 2004. Effects of the β-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 175: 342–352. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. 1999. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res 835: 340–345. [DOI] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. 2011. Stress and emotional memory: a matter of timing. Trends Cogn Sci 15: 280–288. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. 2011. Molecular mechanisms of fear learning and memory. Cell 147: 509–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Diaz-Mataix L, Hamanake H, Ozawa T, Ycu E, Koivumaa J, Kumar A, Hou M, Deisseroth K, Boyden ES, et al. 2014. Hebbian and neuromodulatory mechanisms interact to trigger associative memory formation. Proc Natl Acad Sci 111: E5584–E5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. 2000. Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux J. 2007. The amygdala. Curr Biol 17: R868–R874. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. 2014. Coming to terms with fear. Proc Natl Acad Sci 111: 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Berger SY, Stiedl O, Spiess J, Kim JJ. 2001. Post-training injections of catecholaminergic drugs do not modulate fear conditioning in rats and mice. Neurosci Lett 303: 123–126. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. 1975. A theory of attention: variations in the associability of stimuli with reinforcement. Psychol Rev 82: 276–298. [Google Scholar]
- Mahan AL, Ressler KJ. 2012. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. 2004. Neuronal signaling of fear memory. Nat Rev Neurosci 5: 844–852. [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2012. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci 109(Suppl 2): 17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. 2000. Memory–a century of consolidation. Science 287: 248–251. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. 2015. Consolidating memories. Annu Rev Psychol 66: 1–24. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. 2002. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiol Learn Mem 78: 539–552. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. 1990. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 345: 716–718. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. 2005. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29: 1214–1224. [DOI] [PubMed] [Google Scholar]
- Ostroff LE, Cain CK, Bedont J, Monfils MH, LeDoux JE. 2010. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Nat Acad Sci 107: 9418–9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Wingard JC. 2004. Amygdala and “emotional” modulation of the relative use of multiple memory systems. Neurobiol Learn Mem 82: 243–252. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. 1927. Conditional reflexes. Dover, New York. [Google Scholar]
- Pawlak V, Wickens JR, Kirkwood A, Kerr JN. 2010. Timing is not everything: neuromodulation opens the STDP gate. Front Synaptic Neurosci 2: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. 1998. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res 808: 134–140. [DOI] [PubMed] [Google Scholar]
- Raio CM, Phelps EA. 2015. The influence of acute stress on the regulation of conditioned fear. Neurobiol Stress 1: 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. 2009. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress 12: 125–133. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. 1972. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning II: current research and theory (ed. Black AH, Prokasy WF), pp. 64–99. Appleton Century Crofts, New York. [Google Scholar]
- Rodrigues SM, LeDoux JE, Sapolsky RM. 2009. The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32: 289–313. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. 2009. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry 65: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. 2011. Memory modulation. Behav Neurosci 125: 797–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. 2009. Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433. [DOI] [PubMed] [Google Scholar]
- Sara SJ. 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10: 211–223. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. 2012. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76: 130–141. [DOI] [PubMed] [Google Scholar]
- Schiff HC, Johansen JP, Hou M, Bush DEA, Smith EK, Klein JE, LeDoux JE, Sears RM. 2017. β-Adrenergic receptors regulate the acquisition and consolidation phases of aversive memory formation through distinct, temporally regulated signaling pathways. Neuropsychopharmacology 42: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. 2012. Stress effects on memory: an update and integration. Neurosci Biobehav 36: 1740–1749. [DOI] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, LeDoux JE. 2013. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci 110: 20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L, Hartman BK. 1975. The central adrenergic system. An immuno-fluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-β-hydroxylase as a marker. J Comp Neurol 163: 467–505. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. 2010. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain 3: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Johansen JP. 2015. Projection specificity in heterogenous locus coeruleus cell populations: implications for learning and memory. Learn Mem 22: 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. 2002. Light-enhanced startle: further pharmacological and behavioral characterization. Psychopharmacology (Berl) 159: 304–310. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. 1999. L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci 19: 10512–10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Joëls M, Morris RG. 2010. The memory paradox. Nat Rev Neurosci 11: 837–839. [DOI] [PubMed] [Google Scholar]
- Yokoyama M, Suzuki E, Sato T, Maruta S, Watanabe S, Miyaoka H. 2005. Amygdalic levels of dopamine and serotonin rise upon exposure to conditioned fear stress without elevation of glutamate. Neurosci Lett 379: 37–41. [DOI] [PubMed] [Google Scholar]