Abstract
This study examines the influence of trait anxiety on working memory (WM) in safety and threat. Interactions between experimentally induced anxiety and WM performance (on different cognitive loads) have been reported in healthy, nonanxious subjects. Differences in trait anxiety may moderate these interactions. Accordingly, these interactions may be potentiated by high trait anxiety (HTA), or show a resilient pattern that protects cognitive performance. HTA and low trait anxiety (LTA) were defined by a median split of scores on the trait component of the state-trait anxiety inventory. Sustained anxiety was evoked by a probabilistic exposure to an aversive scream, and was measured by eyeblink startle and self-report. WM was tested using an n-back task (1-, 2-, and 3-back). Results revealed that, as expected, the HTA group reported greater anxiety during the task. However, trait anxiety did not impact the modulation of WM performance by induced anxiety. Notably, HTA influenced anxiety-potentiated startle (startle during threat minus startle during safe; APS) differently as a function of memory load. Accordingly, APS decreased with increasing WM load, but HTA antagonized this reduction. The HTA group showed no impairment on the 3-back WM task despite a higher APS. The amplified APS could be associated with the increase in effort-related cognitive arousal. Furthermore, this third replication of the interaction of induced anxiety by load on WM performance testifies to the robustness of the unique interplay between anxiety and WM.
Anxiety is a state of worry or apprehension that can manifest as an adaptive or pathological sustained response to threat. Anxiety can be modulated by cognitive performance and, reciprocally, anxiety can interfere with cognitive function (Vytal et al. 2012). These reciprocal effects depend on a number of factors, including the nature of the cognitive processes (e.g., attention, inhibition, memory), the nature of anxiety (e.g., severity, trait versus state, clinical), cognitive load (e.g., memory load), and the motivation to perform well. A better understanding of the complex anxiety–cognition interaction in healthy individuals can provide insights into the mechanisms of the potentially debilitating consequences of clinical anxiety. While focusing solely on working memory (WM), the present study manipulates both cognitive load and anxiety to address this question. Two types of anxiety, state and trait, are examined. Cognitive load is varied parametrically across three levels of WM. Therefore, this study examines the interactions among trait anxiety, state anxiety, and cognitive load on WM performance and a physiological correlate of anxiety (i.e., startle reflex).
State anxiety refers to an emotional state in response to a sustained external or internally generated threat. Furthermore, state anxiety is multifaceted and consists of three main interacting components: cognitive (worry, self-concern, diffuse attention, attention bias), emotional (negative feelings of tension, uneasiness, nervousness)/physiological (high arousal), and behavioral (avoidance, withdrawal) (Lang et al. 1993). Research on state anxiety per se is scarce and commonly uses induction strategies using distal (e.g., expectation of being tested after the main study task) (Jelici et al. 2004; Schoofs et al. 2008; Qin et al. 2009) or probabilistic threat (e.g., threat of electric shock, threat of aversive pictures, threat of aversive scream) (Shackman et al. 2006; King and Schaefer 2011; Vytal et al. 2012, 2013; Patel et al. 2016). State anxiety is usually investigated in tandem with trait anxiety, with the intent of uncovering deficits in trait anxiety not easily detected during neutral states. Trait anxiety is a temperament characterized by a high propensity to experience anxiety (state anxiety), without reaching clinical levels. Individuals with high trait anxiety (HTA) tend to be risk- and novelty-avoidant (e.g., Maner et al. 2007). Notably, trait anxiety represents a significant risk factor for the development of anxiety disorders (Watson et al. 1995; Plehn and Peterson 2002), thus providing a window into the vulnerabilities of these disorders. In other words, trait anxiety is the level of the propensity to experience fearful apprehension, whereas state anxiety is the actual fearful apprehension. Anxiety disorders by definition have high levels of trait anxiety, which cause impairments. However, individuals with high trait anxiety do not necessarily meet criteria for an anxiety disorder.
Previous findings on the interplay of anxiety and WM have been inconsistent in studies, which have used multiple tasks (e.g., spatial WM versus verbal WM) (e.g., Ikeda et al. 1996; Shackman et al. 2006; Vytal et al. 2013). For example, some studies find slower reaction times (RTs) on WM tasks in HTA compared with low trait anxiety (LTA) groups (Elliman et al. 1997; Ladouceur et al. 2009). Other studies find no influence of trait anxiety on working memory (e.g., Salthouse 2012). Yet, others report improved working memory (reading span, recognition, word recall) (Sorg and Whitney 1992; Terry and Burns 2001; Walkenhorst and Crowe 2009; Ferrari and Balconi 2011). Decreased WM performance, that is, longer RT, is proposed to reflect impaired cognitive efficiency in HTA individuals due to the diversion of attention toward threat-related representations (Eysenck and Calvo 1992; Eysenck and van Berkum 1992). Studies have argued that the cognitive component of anxiety (i.e., self-concern, worry), rather than the emotional/physiological, is mostly responsible for reducing WM capacity (Hayes et al. 2008; Leigh and Hirsch 2011). The present study can shed some light onto this notion.
As mentioned above, cognitive load is a critical component of the interaction of anxiety and WM. Prior studies in our laboratory specifically addressed this question in healthy adults and adolescents (Vytal et al. 2012; Patel et al. 2016). These studies revealed that WM accuracy was impaired by threat manipulation during low- and medium-load WM tasks, but not high load. At the same time, physiological anxiety (anxiety-potentiated startle [APS]) diminished as WM load increased. These findings reflected a detrimental influence of induced anxiety on WM performance in conditions that required fewer cognitive resources. This pattern was interpreted from the perspective of the limited cognitive resources theory (Kahneman 1973; Vytal et al. 2012). Accordingly, under lower WM loads, cognitive resources were sufficient to process the threat stimulus, which then could interfere with performance. In contrast, at the heaviest load, WM performance depleted cognitive resources, blocking the processing of the threat stimulus and preventing it from intruding on WM. As a consequence, anxiety did not interfere with performance under high WM load. This interpretation was consistent with the blockade of physiological anxiety (APS) under high load. Collectively, these findings suggest that high cognitive-demand tasks, compared with low cognitive-demand tasks, are given priority over threat processing, i.e., cognitive resources are allocated preferentially to cognitive performance at the expense of the processing of induced anxiety.
Of note, the lack of developmental differences (adolescents versus adults) in the patterns of interaction of induced anxiety with cognition (Patel et al. 2016) suggests that the mechanisms regulating the anxiety–cognition interplay are robust and already in place in adolescence. The present work uses the same experimental design as described in Patel et al. (2016) to investigate the effects of trait anxiety on anxiety–cognition interactions. The present study can also be informed by a recent study (Vytal et al. 2016), which used a similar paradigm to compare patients with generalized anxiety disorder (GAD) to healthy adults. Findings revealed that despite the absence of group differences in anxiety (i.e., physiological responses during threat and safe conditions), performance on the high-load task was not effective in blocking the effect of anxiety in the GAD group as found in the healthy group.
The present study examines the extent to which trait anxiety influences the interplay between induced anxiety and cognition. To this end, two groups of healthy subjects, defined as HTA (n = 20) and LTA (n = 20) (Table 1), completed an n-back WM task under a threat condition (unpredictable exposure to a shrieking scream) and safe condition (no threat) (Fig. 1). The task consisted of interleaved blocks of threat and safe conditions. Two possible hypotheses are considered. In the first, HTA might be associated with impaired performance, even on a high-load task, as seen with GAD patients (Vytal et al. 2016). In the second, HTA individuals might not show the interference of anxiety on cognitive function, in contrast to GAD. However, in both alternatives, APS is expected to decrease with higher cognitive demands in both HTA and LTA groups (Vytal et al. 2012, 2016; Patel et al. 2016).
Table 1.
Trait anxiety group demographics
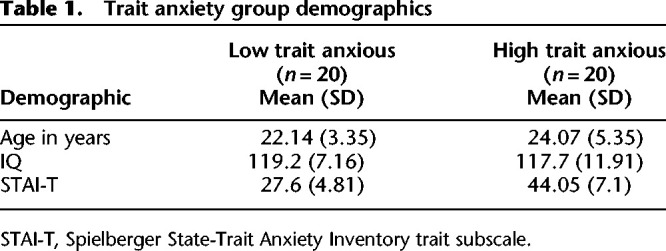
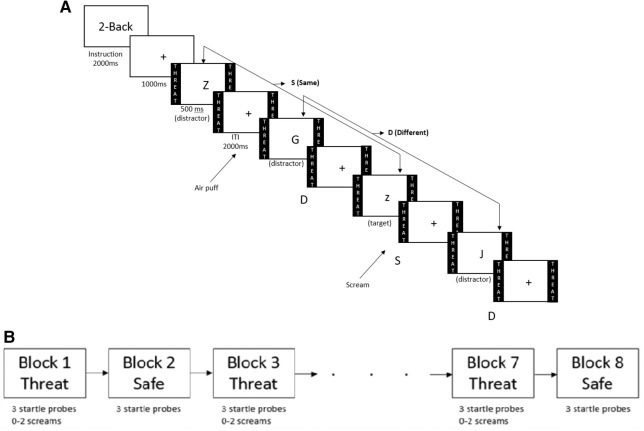
Figure 1.
N-back verbal working memory-threat task. (A) Illustration of two-back task trials in the threat condition. Participants respond to every letter pressing either the S key for “same” (target) as the letter presented two letters back, or D for “different” (distractor) from the letter presented two letters back. In the example here, same is “z,” and different is J and G. The startle probe (air puff) and threat stimulus (shrieking scream) were presented during the intertrial intervals (ITIs). (B) Illustration of one run (eight blocks) with alternating threat and safe conditions of the working memory-threat task. Blocks were alternated between the threat and safe conditions. Three startle probes (air puff) were presented during each block and 0 to 2 shrieking screams were presented during the threat blocks.
Results
Anxiety manipulation (threat of scream)
The subjective anxiety (“how anxious”) responses collected at the end of each run were analyzed using a three-way repeated-measures analysis of variance (r-ANOVA) with Group (LTA, HTA) as the between-subjects factor, and Condition (safe, threat) and Run (run 1, run 2, run 3, run 4) as the within-subjects factors. Results revealed a main effect of Group (F(1,32) = 8.9, P = 0.006), with significantly higher anxiety scores in the HTA than the LTA group, and a main effect of Condition (F(1,32) = 63.8, P < 0.0001), with significantly higher scores reported for the threat than safe condition (Fig. 2). Run had no significant effects. Interactions were not significant.
Figure 2.
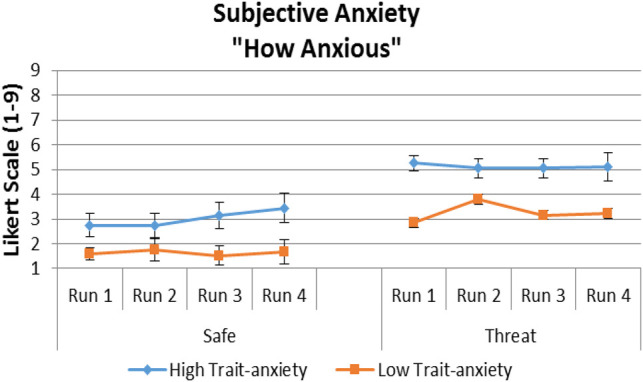
Group means (SEM) of subjective reports during each run of the safe and threat condition.
Additionally, as expected, startle responses were stronger during the threat than safe conditions (F(1,38) = 40.826, P < 0.001), further supporting the notion that the scream was anxiogenic.
Unpleasantness of the scream
Across runs, unpleasantness of the scream was rated at a moderate/high range by the HTA group and a mild/moderate range by the LTA group (HTA: 6.3 ± 1.9, LTA 4.07 ± 1.8 (mean ± SD)). Unpleasantness ratings were significantly higher for the HTA than LTA group (t(1,38) = 3.8, P = 0.0005). Correlations of unpleasantness with the outcome variables (threat minus safe) of startle, RT, and accuracy were examined. No significant correlations were found, therefore, we did not include this variable in our analytical models.
Startle response, APS
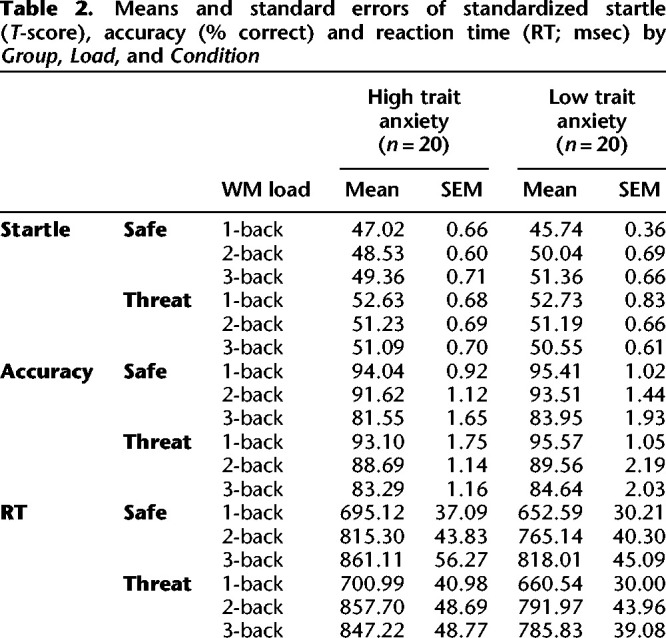
Means and SEM of the standardized startle magnitude are presented in Table 2 by Group, Condition, and Load.
Table 2.
Means and standard errors of standardized startle (T-score), accuracy (% correct) and reaction time (RT; msec) by Group, Load, and Condition
The APS scores, that is, threat startle minus safe startle, were analyzed using a two-way r-ANOVA with Group as the between-subjects factor, and Load (1-back, 2-back, 3-back) as the within-subjects factor. Findings revealed a significant Group difference in the linear trend of APS across Loads (F(1,38) = 6.06, P = 0.018). Figure 3, which displays APS means across loads, illustrates a steeper APS reduction in the LTA group compared with the HTA group. This effect was reflected as significantly higher APS across the 2-back and 3-back loads in the HTA group compared with the LTA group (F(1,38) = 4.45, P = 0.04).
Figure 3.
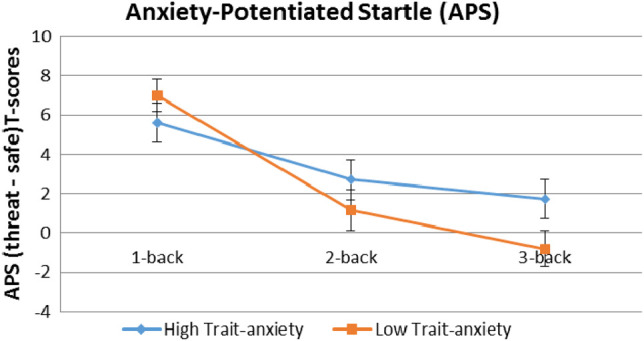
Group means (SEM) of anxiety-potentiated startle (threat startle T-scores minus safe startle T-scores).
As expected, the main effect of Load was highly significant, reflecting progressive APS decreases with increasing loads (F(2,76) = 21.46, P = 0.0001).
Working memory performance
Means and SEM of the performance measures (accuracy and RT) are presented in Table 2 by Group, Condition, and Load.
Group had no effects on WM performance (accuracy or RT), as a main effect or interaction. However, the paradigm showed strong main effects of Load. As expected, higher load was associated with lower accuracy (F(2,76) = 69.9, P < 0.0001) and slower RT (F(2,76) = 40.1, P < 0.0001). However, this main effect of Load was subsumed under a significant interaction of Load by Condition on accuracy (F(2,76) = 6.4, P = 0.003) and RT (F(2,76) = 5.6, P = 0.006).
Figures 4 and 5 illustrate the threat-related changes of the WM performance variables. For both accuracy and RT, the strongest effect of threat was related to the 2-back and 3-back tasks. Specifically, by n-back load, threat (versus safe) had no effect on the 1-back load, significantly worsened performance on the 2-back load (worse accuracy: F(1,39) = 13.32, P = 0.0008; slower RTs: F(1,39) = 8.44, P = 0.006), and relatively improved performance on the 3-back load (accuracy: F(1,39) = 9.89, P = 0.003; RT: F(1,39) = 7.17, P = 0.011). The deleterious effect of threat on performance was blocked for the 3-back load.
Figure 4.
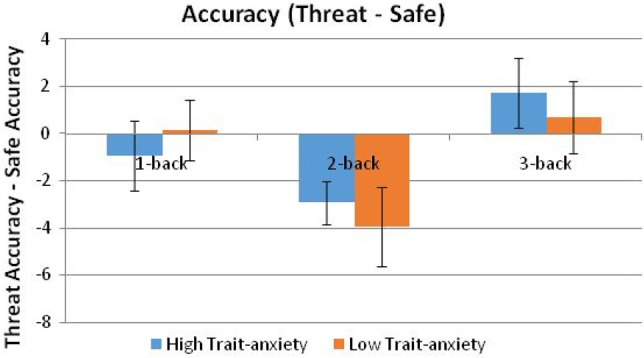
Mean (SEM) accuracy (correct responses; %) of the [threat accuracy minus safe accuracy] difference scores.
Figure 5.
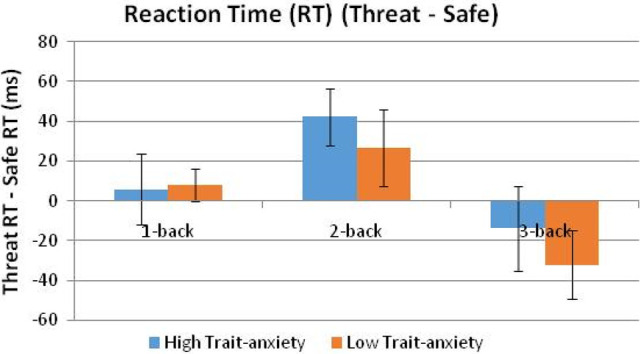
Mean (SEM) reaction time (msec) of the [threat reaction time minus safe reaction time] difference scores.
Correlations among working memory performance and startle measures
Accuracy was not correlated with RT for any of the WM loads, reflecting the absence of a speed accuracy trade-off. In addition, the [threat−safe] difference scores showed no significant correlations between performance (accuracy, RT) and APS.
Discussion
A better understanding of the emotional–cognitive correlates of trait anxiety is important to shed light on the mechanisms of the propensity for experiencing anxiety, and the risk for maladaptive clinical anxiety. The propensity for experiencing anxiety can reflect either enhanced emotion processing (bottom-up mechanism), which overcomes cognitive processes that regulate emotion, or deficits in these cognitive processes (top-down mechanism), which cannot adequately regulate emotion expression, or both. The present study manipulates both cognitive processes (WM load) and anxiety state (threat of shrieking scream) to examine which mechanism might predominate in HTA. On the one hand, increasing cognitive recruitment, via increasing WM load, might potentiate the modulation of anxiety and thereby decrease anxiety. On the other hand, experimentally induced anxiety might reduce cognitive control and impair performance.
Indeed, while trait anxiety represents a proneness to experience anxiety and is a risk factor for developing an anxiety disorder, clinical anxiety differs from trait anxiety with the presence of everyday levels of impairment and suffering. The shift from trait anxiety to clinical anxiety might reflect a breakdown of the compensatory mechanisms that provide resilience to individuals with high trait anxiety. To inform this idea, the present study examines the extent to which trait anxiety influences the interplay between induced anxiety and cognition by taking advantage of well-characterized patterns of WM-by-threat interactions in healthy subjects (Vytal et al. 2012, 2013; Patel et al. 2016). This interaction consists of a unique pattern of threat interference on WM performance, and conversely, a progressive inhibition of physiological anxiety with increasingly difficult WM performance (Vytal et al. 2012, 2013; Patel et al. 2016). Typically, threat interferes with cognitive performance, especially at moderate loads, but spares or even benefits performance at high cognitive loads.
First, it is important to underscore that the threat manipulation in the present study was successful. The “how anxious” self-report scores, which were collected retrospectively after each of the four runs, indicated that both HTA and LTA groups were more anxious during the threat than the safe condition. In addition, the “how anxious” scores did not differ across the four runs, reflecting the absence of habituation to the threat condition with time. Finally, the HTA group was overall significantly more anxious than the LTA group on the “how anxious” self-report. This supports the validity of the trait anxiety grouping, which was based on an independent measure of trait anxiety, the Spielberger Trait Anxiety Inventory (Spielberger et al. 1983).
The present study tested two alternative models for the effect of trait anxiety on the aforementioned threat–cognition interactions. On the one hand, trait anxiety might amplify the disruptive effects of threat on cognition, while also preventing high-load WM from blocking the effects of threat on performance. Alternatively, trait anxiety might not modulate cognitive performance under threat, which could putatively represent a potential marker for the resilience of trait anxiety and contribute to the protection of HTA of shifting into clinical anxiety. In both HTA and LTA groups, physiological anxiety, indexed by APS, was expected to decrease as WM load increased in line with previous work (Vytal et al. 2012; Patel et al. 2016).
Our findings support the second hypothesis, indicating that trait anxiety did not influence the effects of threat on WM performance. HTA individuals, similarly to those with LTA, demonstrated threat-related perturbations of WM performance at low (1-back) and moderate (2-back) loads, while showing a blockade of the interference of threat on the highly demanding WM task (3-back). With regard to the effect of cognitive engagement on physiological anxiety (APS), as expected, findings revealed a reduction of APS with increasing WM loads. However, this effect was blunted in the HTA group, resulting in significantly higher APS during the 2- and 3-back task performance in the HTA compared with the LTA group. Taken together, these findings have two major implications. First, they suggest different dynamics of threat–WM interplays in trait anxiety compared with clinical anxiety (GAD). Second, they consolidate previous findings of how cognitive engagement influences threat response and vice versa.
The present findings on trait anxiety differ from the previous study of Vytal et al. (2016). The previous study, which used a similar paradigm to compare patients with GAD to healthy adults, revealed that despite the absence of group differences in anxiety, performance on the high-load task was not effective in blocking the effect of anxiety in the GAD group as found in the healthy group. Findings in GAD differed from the present ones in two ways. First, physiological anxiety (APS) was increasingly inhibited by the progressive increase in cognitive load in HTA than LTA subjects. This is in contrast to GAD patients whose APS showed normal decrements with higher cognitive load. Second, WM performance and the effects of threat on WM did not differ between the HTA and LTA groups. Specifically, both HTA and LTA groups showed the typical blockade of the detrimental effect of threat on high-load task performance (Vytal et al. 2012; Patel et al. 2016). In contrast, GAD patients exhibited a threat-related decrement on the performance of the 3-back task, that is, a failure of the high-load task to overcome interference from threat (Vytal et al. 2016). Caution is warranted when comparing findings from the present study with those from Vytal et al. (2016) in GAD. Indeed, the two studies used different types of threat, namely, shock in GAD and shrieking scream herein. Previously, our laboratory showed that shocks were more aversive than shrieking screams (Grillon et al. 2004). However, we are not aware of any study that has investigated the impact of threat of different aversiveness on the anxiety by cognition interaction. Hence, any difference between trait anxiety and GAD could be due to the difference in threat used.
Regarding APS, the blunting of the cognitive effect on APS (lack of decreased APS) in HTA might reflect either a potentiation of anxious arousal or an effect of heightened cognitive arousal (Westbrook and Braver 2015), that is, an enhanced cognitive effort to perform optimally in the most demanding task. In the first case, cognitive performance would be expected to be worse in HTA, particularly on the high load task. In the second case, cognitive performance of HTA subjects might be preserved in the high load task. The second option fits the observed data. This interpretation would also explain the discrepancy in findings between the GAD study (Vytal et al. 2016) and the current trait anxiety study. Patients with GAD may not be able to raise physiological arousal to perform optimally on the task, and therefore, would exhibit threat-related performance deficits even at high cognitive load. This interpretation is predicated on the nature of the startle reflex, a measure of physiological arousal. Physiological arousal is consistently used to measure anxiety or fear responses, but it can also reflect cognitive effort (Westbrook and Braver 2015). That said, a word of caution is in order. Overall, the proposed interpretation of findings in trait anxiety and GAD are clearly tentative and await validation, optimally in a study directly comparing groups of GAD, HTA, and LTA individuals.
The preserved performance in HTA individuals is in line with the highly consistent profile of the interplay between anxiety and WM across multiple studies using healthy adult and adolescent samples (Vytal et al. 2012, 2013; Patel et al. 2016). Threat exposure to a sustained period of unpredictable aversive stimuli (i.e., electric shock, shrieking scream) impairs WM performance in low-load and moderate-load, but not in high-load WM tasks. Conversely, cognitive performance reduces anxious arousal (APS) scaled to the cognitive load level, that is, a steeper reduction is seen with higher cognitive loads. The present study suggests that HTA is associated with a prioritization of cognitive processing over threat processing, made possible through the mounting of physiological arousal to support the cognitive effort. In contrast, previous work suggests that such compensatory mechanisms would not be available to GAD patients (Vytal et al. 2016). This interpretation should be tested in future work.
This study comes with strengths and limitations. Among the strengths is the use of an established anxiety induction method (Grillon and Baas 2003). This method allows a within-subjects design, avoids the issues of inter-individual variability and permits subjects to serve as their own control. Another strength is the WM load manipulation, which allowed a parametric modulation of the cognitive task. In terms of limitations, the present findings cannot be generalized to other kinds of WM (e.g., spatial), or other cognitive processes (e.g., inhibition, attention). In fact, the identification of differences in the effects of anxiety as a function of the nature of the cognitive process would provide interesting leads for mechanisms underlying specific functional properties of anxiety. Another limitation is that the relatively limited sample size did not provide sufficient power to reliably examine correlations among performance, self-report and physiological measures. A potential confounding factor was the unpleasantness of the scream. Although it correlated with anxiety, it was not associated with any of the variables of interest. It might be possible in the future to titrate the ratings of the unpleasantness of the scream to a 6–7 range by varying the loudness or emotional quality of the scream. Finally, subjective ratings were not counterbalanced for the order of threat and safe conditions, such that questions always started with the threat condition followed by the safe condition. This may have introduced an expectancy bias.
In conclusion, this study suggests a mechanism that could serve to protect individuals with HTA from the cognitive deficits reported in clinically anxious patients. These findings need to be replicated and HTA individuals should be compared directly with clinically anxious patients. Finally, given the robust behavioral pattern in response to the threat–WM paradigm, applying this work in the functional neuroimaging domain might shed light onto the neural mechanisms underlying these effects.
Materials and Methods
Participants
Forty healthy adults, recruited through general advertisements at a local university, completed the study. Of note, this sample did not overlap with the samples of Vytal et al. (2012) or Patel et al. (2016). Six additional adults were excluded because of incomplete responses and insufficient startle eyeblink data. Participants were divided into LTA (n = 20; mean age 22.1 yr; 5 male) and HTA (n = 20; mean age 24.1 yr; 6 male) groups using a median split of scores on the trait component of the State-Trait Anxiety Inventory (STAI-T) (Spielberger et al. 1983). The STAI-T was used as the measure of severity of trait anxiety (Spielberger et al. 1983). The normative STAI-T score for male college students is 38.3 (mean; SD = 9.18) and female college students is 40.4 (mean; SD = 10.2) (Spielberger et al. 1983). For the general population, the mean STAI-T score is 36.3 (SD 11.4) (Crawford et al. 2011). Finally, individuals with an anxiety disorder, such as GAD, tend to have STAI-T scores above 50 (e.g., Lissek et al. 2014). The mean STAI-T score for the current study was 27.6 for the LTA group and 44.1 for the HTA group. In the present sample, the STAI-T is normally distributed (Shapiro–Wilk: P = 0.19, kurtosis = −0.46, skewness = 0.42). The two groups did not differ on sex distribution, age, socioeconomic status, or IQ (Ps > 0.1) (see Table 1).
Subjects were not taking any psychoactive medications and did not have a current psychiatric disorder as determined by the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1995). All subjects had an IQ score greater than 80 (Vocabulary and Matrix reasoning subtests; Wechsler 1999). Since IQ did not differ between groups and was not related to any variables of interest, it was not included as a covariate in further analyses.
The study was approved by the National Institute of Mental Health and the American University Institutional Review Boards. All subjects signed an informed consent form after a detailed explanation of the study. Subjects were compensated for their participation.
Procedure
The experiment was comprised of three components: (1) an n-back WM task (2) an aversive shrieking scream to manipulate state anxiety and (3) the collection of a startle eyeblink response to measure anxious arousal. PsychLab (Contact Precision Instruments) was used to present the startle probe and threat stimulus, and to record the eyeblink response. Presentation software (Version 0.70, www.neurobs.com) was used to present the n-back task and manage the presentation of the probe and stimulus.
N-back WM task
The verbal WM task (Fig. 1A,B) was a letter-based n-back task similar to that used by Vytal et al. (2012). This task had three levels of cognitive load (1-back [low-load], 2-back [medium-load], and 3-back [high-load]). A passive viewing task was also included but was not analyzed because of the passive quality of this task, which did not provide any performance data. The task consisted of four experimental runs. Each run included eight alternating threat and safe n-back blocks (i.e., four threat and four safe blocks; Fig. 1B). Each block was about 48 sec long, and there were 8 sec between blocks. N-back blocks were presented in random order during the first run so that no level of the n-back task was sequential (e.g., a 1-back task followed by a 1-back task). The remaining runs were counterbalanced using a Latin square model. Participants were reminded of the ongoing condition (threat or safe) via colored borders and the word THREAT or SAFE written within the colored border on the right and left sides of the screen (see Fig. 1A for an example of the threat condition).
Each of the four runs started with three startle habituation probes (see below). This was followed by a 2-sec instruction screen indicating the WM load and a 1-sec fixation cross. Letters (18 in each block) were presented for 500 msec, separated by fixation intertrial intervals (ITIs) of 2000 msec (±250 msec). Upper and lowercase letters were both used in order to limit reliance on perceptual information. Participants responded to each letter by pressing the “S” key if the letter was the same as the letter presented 1-, 2-, or 3-letters back, for the 1-, 2-, and 3-back tasks, respectively, and the “D” key if the letter was different. Similar to prior research using the n-back task, ∼35% of the trials were targets (i.e., “same” responses) (Braver et al. 1997; Carlson et al. 1998; Ragland et al. 2002).
Aversive shrieking scream
The threat condition was implemented via the unpredictable presentation of an aversive shrieking scream (95 dB, 1000 msec) through headphones. Zero to two shrieking screams were presented during an ITI of the threat condition, with a total of two shrieking screams per run. The shrieking scream has been used successfully in conditioning studies with adolescents (Lau et al. 2008; Britton et al. 2011) and in studies with adults (Lissek et al. 2005; Massar et al. 2011). This aversive stimulus was used here instead of electrical shock to be compatible with prior similar work comparing adolescents and adults (Patel et al. 2016).
Startle eyeblink measure
An air puff was used as the startle probe, instead of the white noise used in our previous work (e.g., Vytal et al. 2012). The selection of this probe was based on the need to deliver the startle probe in a sensory modality different from that of the threat stimulus (scream). The air puff was delivered to the forehead through a tube attached to a helmet worn by the subject. To obtain a moderate-to-large eyeblink response, the air puff was presented initially at 4 psi. If a startle response was not obtained at 4 psi, the psi was increased until an appropriate response was elicited. Responses to the air puff were measured via electromyographic recordings of the reflexive startle eyeblink response (e.g., Vytal et al. 2012; Patel et al. 2016). Recordings were taken through a ground electrode on the left arm and two electrodes beneath the left eye to record orbicularis oculi muscle activity. The bandpass filter was 30–500 Hz and the sampling rate was 1000 Hz. Prior to the task, participants were exposed to a 5-min startle habituation period, during which they received five startle probes (Lissek et al. 2005). The habituation period served to prevent an artificially high startle response at first presentation of the startle probe during the task. Similarly, for each run, three startle habituation probes were presented before the task began. Three unpredictable startle probes were presented during the ITIs of each block (3 startle probes × 8 blocks = 24 startle probes per run). Related to the shrieking scream, in order to minimize sensitization effects of it on startle, it preceded startle probes by at least 16 sec, and followed startle probes with a mean latency of ∼2 sec.
Timeline
Subjects were first set up for the threat (delivery of the shrieking scream) and startle probe (delivery of the startle probe and recording of the startle eyeblink response). They were then exposed to the startle probe. Following the selection of an effective intensity of the startle probe, a 5-min startle habituation period was implemented. Afterwards, subjects were presented with the shrieking scream to establish familiarity. Finally, subjects received the instructions for the verbal WM task. They were trained on a paper version of the WM task, and completed a short practice session on the computer. Once all of these initial prestudy steps were completed, the actual study was initiated.
Questionnaires
At the end of each of the four runs, subjects were asked to rate their subjective anxiety on “how anxious” they felt during the threat and safe conditions (on a scale of 1 = not at all to 9 = extremely), and the level of unpleasantness of the scream (on a scale of 1 = not at all to 9 = extremely).
Data analysis
Threat manipulation, assessed based on the subjective anxiety (“how anxious”) ratings, was analyzed via a r-ANOVA, with Run (run 1, run 2, run 3, run 4) and Condition (safe, threat) as the within-subjects factors, and Group (LTA, HTA) as the between-subjects factor. Run was included in this analysis to ensure that threat manipulation was present throughout the experiment.
WM performance was indexed by two variables, accuracy, that is, the number of trials in which subjects correctly indicated whether the letters matched or not, and RT for correct responses. Each post-scream trial was removed from the analysis (total of eight post-scream trials removed, two trials per run). Accuracy and RTs were analyzed using a r-ANOVA with Condition and Load (1-back, 2-back, 3-back) as the within-subjects factors, and Group as the between-subjects factor. Additionally, difference scores of performance (accuracy and RT) between threat and safe conditions (threat condition−safe condition) were calculated.
Physiological arousal was indexed by the eyeblink startle responses recorded during the 1-back, 2-back, and 3-back tasks. Startle responses were also recorded during passive viewing, and showed the expected greater magnitude during threat than safe (F(1,36) = 21.2, P < 0.0001). No group differences were found, indicating that physiological arousal was similar between groups at baseline. Startle responses during passive viewing were not analyzed further.
Prior to identifying the peak eyeblink (startle) response, the electromyographic signal was integrated with a 20-msec time constant. The peak scoring window was 20–120-msec post-startle probe. Trials with excessive (three times greater than the standard deviation of the mean) electromyographic activity during baseline (20 msec prestartle probe) were excluded. Startle scores (including 0 scores) were averaged within each condition and load. Responses were processed similarly to our previous work (e.g., Vytal et al. 2012; Patel et al. 2016). Magnitude T-scores were calculated to normalize the startle data and to account for individual differences in startle variability. Eye-blink startle responses during the safe condition were subtracted from those during the threat condition [threat−safe]. This difference score, the APS, provided a physiological measure of anxiety (Grillon et al. 1991, 2004). Analysis of APS consisted of the group comparison of the linear trend of APS changes with cognitive load.
Statistical tests used an α of 0.05. Greenhouse–Geisser corrections were applied to account for violations of sphericity in r-ANOVAs. Analyses were corrected for multiple tests by means of the Bonferroni test for multiple comparisons, and the Hochberg's procedure (Hochberg 1988) for multiple correlations.
Acknowledgments
We thank Katherine Watts for her help in the design of the study. This study was funded by the Intramural Research Program of the National Institute of Mental Health (grant MH002798, Proposal 1ziamh002781) and the American University College of Arts and Sciences Dean's discretionary account.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.044123.116.
References
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. 1997. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. 2011. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety 28: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rämä P, Salli E, Korvenoja A, Aronen HJ. 1998. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex 8: 743–752. [DOI] [PubMed] [Google Scholar]
- Crawford J, Cayley C, Lovibond PF, Wilson PH, Hartley C. 2011. Percentile norms and accompanying interval estimates from an Australian general adult population sample for self-report mood scales (BAI, BDI, CRSD, CES-D, DASS, DASS-21, STAI-X, STAI-Y, SRDS, and SRAS). Aust Psychol 46: 3–14. [Google Scholar]
- Elliman NA, Green MW, Rogers PJ, Finch GM. 1997. Processing-efficiency theory and the working memory system: impairments associated with subclinical anxiety. Pers Individ Dif 23: 31–35. [Google Scholar]
- Eysenck MW, Calvo MG. 1992. Anxiety and performance: the processing efficiency theory. Cogn Emot 6: 409–434. [Google Scholar]
- Eysenck MW, van Berkum J. 1992. Trait anxiety, defensiveness, and the structure of worry. Pers Individ Dif 13: 1285–1290. [Google Scholar]
- Ferrari C, Balconi M. 2011. DLPFC implication in memory processing of affective information: a look on trait anxiety contribution. Neuropsychol Trends 2011: 53–70. [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. 1995. Structured Clinical Interview for DSM-IV (SCID). American Psychiatric Association, Washington, DC. [Google Scholar]
- Grillon C, Baas J. 2003. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 114: 1557–1579. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. 1991. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology 28: 588–595. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. 2004. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci 118: 916–924. [DOI] [PubMed] [Google Scholar]
- Hayes S, Hirsch C, Mathews A. 2008. Restriction of working memory capacity during worry. J Abnorm Psychol 117: 712–717. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. 1988. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75: 800–802. [Google Scholar]
- Ikeda M, Iwanaga M, Seiwa H. 1996. Test anxiety and working memory system. Percept Mot Skills 82: 1223–1231. [DOI] [PubMed] [Google Scholar]
- Jelici M, Geraerts E, Merckelbach H, Guerrieri R. 2004. Acute stress enhances memory for emotional words, but impairs memory for neutral words. Int J Neurosci 114: 1343–1351. [DOI] [PubMed] [Google Scholar]
- Kahneman D. 1973. Attention and effort. Prentice-Hall, Englewood Cliffs, NJ. [Google Scholar]
- King R, Schaefer A. 2011. The emotional startle effect is disrupted by a concurrent working memory task. Psychophysiology 48: 269–272. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. 2009. Fearful faces influence attentional control processes in anxious youth and adults. Emotion 9: 855–864. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. 1993. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 30: 261–273. [DOI] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. 2008. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. J Am Acad Child Adolesc Psychiatry 47: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh E, Hirsch CR. 2011. Worry in imagery and verbal form: effect on residual working memory capacity. Behav Res Ther 49: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Baas JMP, Pine DS, Orme K, Dvir S, Nugent M, Rosenberger E, Rawson E, Grillon C. 2005. Airpuff startle probes: An efficacious and less aversive alternative to white-noise. Biol Psychol 68: 283–297. [DOI] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. 2014. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 75: 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner JK, Richey JA, Cromer K, Mallott M, Lejuez CW, Joiner TE, Schmidt NB. 2007. Dispositional anxiety and risk-avoidant decision-making. Pers Individ Dif 42: 665–675. [Google Scholar]
- Massar SAA, Mol NM, Kenemans JL, Baas JMP. 2011. Attentional bias in high and low anxious individuals: evidence for threat induced effects on engagement and disengagement. Cogn Emot 25: 805–817. [DOI] [PubMed] [Google Scholar]
- Patel N, Vytal K, Pavletic N, Stoodley C, Pine DS, Grillon C, Ernst M. 2016. Interaction of threat and verbal working memory in adolescents. Psychophysiology 53: 518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plehn K, Peterson RA. 2002. Anxiety sensitivity as a predictor of the development of panic symptoms, panic attacks, and panic disorder: a prospective study. J Anxiety Disord 16: 455–474. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernández G. 2009. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry 66: 25–32. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. 2002. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology 16: 370–379. [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. 2012. How general are the effects of trait anxiety and depressive symptoms on cognitive functioning? Emotion 12: 1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, Preuß D, Wolf OT. 2008. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology 33: 643–653. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. 2006. Anxiety selectively disrupts visuospatial working memory. Emotion 6: 40–61. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Whitney P. 1992. The effect of trait anxiety and situational stress on working memory capacity. J Res Pers 26: 235–241. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. 1983. State-trait anxiety inventory for adults: manual, instrument and scoring guide. Mind Garden, Inc. [Google Scholar]
- Terry WS, Burns JS. 2001. Anxiety and repression in attention and retention. J Gen Psychol 128: 422–432. [DOI] [PubMed] [Google Scholar]
- Vytal K, Cornwell B, Arkin N, Grillon C. 2012. Describing the interplay between anxiety and cognition: from impaired performance under low cognitive load to reduced anxiety under high load. Psychophysiology 49: 842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Cornwell BR, Letkiewicz AM, Arkin NE, Grillon C. 2013. The complex interaction between anxiety and cognition: insight from spatial and verbal working memory. Front Hum Neurosci 7: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytal KE, Arkin NE, Overstreet C, Lieberman L, Grillon C. 2016. Induced-anxiety differentially disrupts working memory in generalized anxiety disorder. BMC Psychiatry 16: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkenhorst E, Crowe SF. 2009. The effect of state worry and trait anxiety on working memory processes in a normal sample. Anxiety Stress Coping 22: 167–187. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. 1995. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 104: 3–14. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1999. Wechsler abbreviated scale of intelligence. The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Westbrook A, Braver TS. 2015. Cognitive effort: a neuroeconomic approach. Cogn Affect Behav Neurosci 15: 395–415. [DOI] [PMC free article] [PubMed] [Google Scholar]