Abstract
This study investigated the risk of lung and bladder cancers in people residing in proximity of a coal-oil-fired thermal power plant in an area of north-eastern Italy, covered by a population-based cancer registry. Incidence rate ratios (IRR) by sex, age, and histology were computed according to tertiles of residential exposure to benzene, nitrogen dioxide (NO2), particular matter, and sulfur dioxide (SO2) among 1076 incident cases of lung and 650 cases of bladder cancers. In men of all ages and in women under 75 years of age, no significant associations were observed. Conversely, in women aged ≥75 years significantly increased risks of lung and bladder cancers were related to high exposure to benzene (IRR for highest vs. lowest tertile: 2.00 for lung cancer and 1.94 for bladder cancer) and NO2 (IRR: 1.72 for lung cancer; and 1.94 for bladder cancer). In these women, a 1.71-fold higher risk of lung cancer was also related to a high exposure to SO2. Acknowledging the limitations of our study, in particular that we did not have information regarding cigarette smoking habits, the findings of this study indicate that air pollution exposure may have had a role with regard to the risk of lung and bladder cancers limited to women aged ≥75 years. Such increased risk warrants further analytical investigations.
Keywords: air pollution, coal-fired thermal power plant, oil thermal power plant, geocoded, lung cancer, bladder cancer, north-eastern Italy
1. Introduction
Agents classified as Group 1 lung carcinogens by the International Agency for Research on Cancer (IARC) include personal habits and occupational and environmental exposures. Although in many populations cigarette smoking is the main cause of lung cancer, other recognized risk factors may have a relevant impact under local circumstances. Such factors include exposure to identified physical and chemical agents and their mixtures and occupational and environmental activities. The latter may entail exposures occurred in or in proximity of some industrial facilities, air pollution due to road traffic and home heating [1,2,3,4,5,6]. Sufficient or convincing evidence about causes of bladder cancer is associated with cigarette smoking, radiation, exposure to aromatic amines, arsenic and inorganic arsenic compounds, Schistosoma haematobium, and work in occupations such as aluminum production, painting, and rubber production [7,8,9]. However, a positive association between bladder cancer and outdoor air pollution has recently emerged [10]. With specific reference to the carcinogenic effect of combustion of coal, burning coal inside the home for the purpose of heating or cooking produces particulate and gas emissions that are lung carcinogens (IARC Group I). Additional substances derived from coal, also recognized as carcinogens, are: coal-tar pitch, soot, diesel engine exhaust, and related occupations (i.e., coal gasification, coke production, iron and steel founding). However, the extension and impact of the carcinogenic effects of emissions from coal and coal-oil-fired plants is a matter of debate, especially when such effects are compared with other local sources of air pollution and with alternative modes of energy production.
2. Materials and Methods
The city of Monfalcone is located in the Friuli Venezia Giulia region, northeastern Italy, and shares with 13 surrounding municipalities a concentration of industries (a power plant, a large shipyard, a paper mill, and other manufacturing industries) and several transport infrastructures such as a port, airport, and highways.
The coal-fired and oil thermal power plant is located near the city center of Monfalcone since 1965; over time, the power plant has undergone several additions and changes. Until 1969, there was only one coal-fired power generator with a 60-m high smokestack. In 1970, another coal-fired power generator was added along with another 90-m high smokestack. In 1984, two oil power generators were added and the smokestacks were replaced by one single 154-m high smokestack. In 1990, to reduce the suspension of coal dust, the coal conveyor belt was depressurized.
Throughout the years, the residents of the 14 municipalities have set up citizens committees for environmental controls over the emissions of the thermal power plant, and have solicited epidemiological monitoring of the potential adverse health effects associated with air pollution. In 2014, to respond to these public concerns, the Friuli Venezia Giulia Region implemented the “Health & Environment Regional Observatory”. Point and diffuse emissions, within the 14 municipalities, are measured by the Regional Environmental Protection Agency (ARPA-FVG). The coal-fired and oil thermal power plant, other industries, port, airport, home heating, and highways in the study area contribute pro rata to the overall atmospheric concentrations of fine particulate matter (PM10), benzene (C6H6), nitrogen dioxide (NO2), and sulfur dioxide (SO2). The study area was defined according to a deposition model of the specific emissions of NO2 of the coal-fired thermal power plant.
Since 1995, the incidence of cancer in the whole population of the Friuli Venezia Giulia region (1,200,000 inhabitants) is being recorded by the population-based cancer registry (CR-FVG) [11].
In this study we obtained the structure of the residential population (by sex, age in quinquennia, and calendar year) using the same methodology applied in our previous investigation [12]. In the study area, 96.3% of residential addresses, extracted from regional healthcare population database between 1995 and 2009, were geocoded. For each person we considered the various exposures to air pollutants, in the study area, due changing residence in the period 1995–2009. The computerized structure of CR-FVG and regional healthcare population database allows a full integration of the two databases. We extracted from the population-based CR-FVG all incident cases of lung (i.e., 817 men and 285 women) and bladder cancers (i.e., 505 men and 157 women) diagnosed during 1995–2009 among the population residing in the 14 municipalities. Twenty-six cases of lung cancer (16 men and 10 women) and 12 cases of bladder cancers (eight men and four women) were excluded from the study, due to missing valid addresses recorded in the regional healthcare population database. We disentangled incidence data by age (in quinquennia), sex, calendar year of diagnosis, and histological subtype (for lung carcinoma). We used the International Classification of Diseases [13] to identify lung cancer (C33, C34) and bladder cancer (C67, D09.0, D41.4). We used the International Classification of Disease for Oncology (ICDO-3) [14] for the classification of histologic subtypes of lung cancer: adenocarcinoma (ADK) squamous cell carcinoma (SCC), and other and unspecified morphologies (OLC). Age-standardized incidence rates (ASRs) on 2001 EU standard population were computed for cancer incidence in the study area and for the estimated cancer incidence derived from the pool of 8 cancer registers (1995–2007) [15].
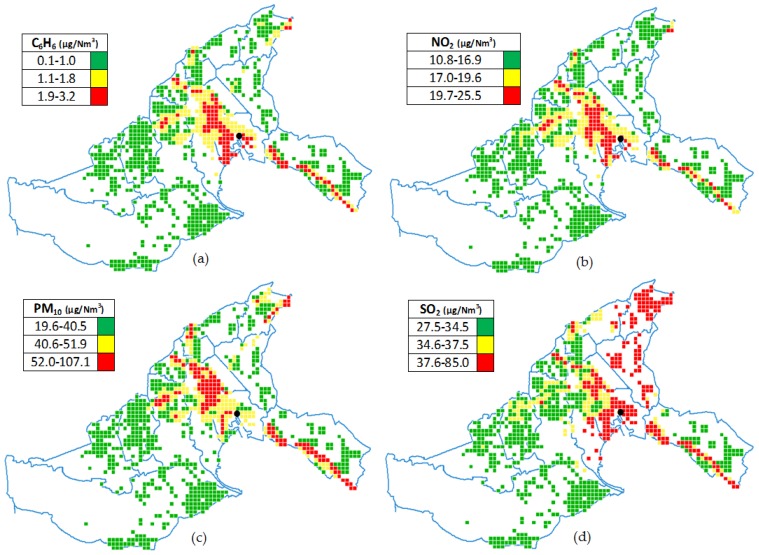
The residential exposure was defined a priori by ARPA-FVG that recovers the point emission data of C6H6, NO2, PM10, SO2 reported by individual industries and data on road traffic, port, airport, home heating, and local environmental monitoring system. We considered concentrations of pollutants for which older data were available (1998). Data on pollutants refer to 1998, a year that ARPA-FVG considers as a good proxy of exposure in the study period, taking into account the vehicle fleet, emissions from the power plant, and other industries, and type of home heating. In fact, there is a smooth decrease of anthropogenic emissions over the study area during the past years. The residential exposure was obtained through an integrated approach based on punctual observations and numerical simulated fields [16]. This approach merges punctual monitoring (i.e., precise evaluation of concentration) and the advantages of numerical modeling (i.e., homogeneous fields obtained keeping into account both local meteorological drivers and emission sources). In detail punctual data were obtained through the monitoring network active in the study area. The suite SPRAY (version 3; Arianet Srl, Milan, Italy) is the numerical model applied to this integrated approach [17]. The suite SPRAY is a three dimensional model designed to simulate the airborne pollutant dispersion, able to take into account the spatial and temporal heterogeneity of both the mean flow and turbulence. Concentration fields generated by point, area, or volume sources can be simulated by the model. The trajectory of the airborne pollutant is simulated through virtual particles: the mean motion is defined by the local wind and the dispersion is determined by solving the Langevin stochastic differential equations for the velocity fluctuations, reproducing the statistical characteristics of the turbulent flow. Different portions of the emitted plumes can therefore experience different atmospheric conditions, allowing realistic reproductions of complex phenomena, such as low wind-speed conditions, strong temperature inversions, flow over topography, land use and terrain variability. The merging of the numerical fields and punctual data has been achieved by kriging geospatial technique [18], which is based on prior covariances and can supply the best linear unbiased description of the interpolated values. Then, the residential exposure to air pollution was modelized for the overall area on a 400 × 400 m grid (Figure 1).
Figure 1.
Mathematical modeling of residential exposure to air pollutant in study area, stratified to tertile of exposure: (a) Benzene exposure; (b) Nitrogen dioxide exposure; (c) Particular matter exposure; (d) Sulfur dioxide exposure. 400 × 400 m cells correspond to area populated by one or more inhabitants in 1995–2009 period. The blue line corresponds to the boundaries of individual municipalities. The black point corresponds to the location of the thermal power plant.
Geocoded lung and bladder cancer cases and the population were linked to this grid using Geomedia software, a geographic information system (GIS) (version desktop 2015; Hexagon Geospatial, Stockholm, Sweden). Cases and population were thus exported to compute indicators by a SAS program. An exploratory high exposure (i.e., the highest tertile versus the lowest tertile) analysis was conducted to assess the risk of lung and bladder cancers according to residential exposure to the above mentioned four pollutants.
Yearly age-standardized (to the 2001 European population) incidence rates (ASRs-EU) per 100,000 inhabitants were calculated for the whole examined period in both sexes and in two age groups (<75 and ≥75 years, i.e., the approximate median age), according to tertiles of exposure. ASRs and their corresponding 95% confidence intervals (CI) were calculated using SAS Enterprise Guide (version 7.1: SAS Institute, Cary, NC, USA). Incidence Rate Ratios (IRRs) and 95% confidence intervals (CI) [19] were computed from the ASRs, considering the lowest tertile of exposure to different pollutants as the reference category.
We calculated annual percent change (APC) [20,21] of incidence rates using Joinpoint software, for the whole 1995–2009 period in both sexes and by the two age groups (<75 and ≥75 years) according to tertile of exposures. Statistical significance (p < 0.05) of annual APC was calculated using a Student’s t-distribution [12,20,21].
3. Results
Table 1 reports the number of overall incident lung and bladder cancer cases, the ASRs with corresponding 95% CI, according to age and sex. Different incidence rates were observed in lung cancer by histological types in women of all ages with ASR of adenocarcinoma greater than squamous cell ones, and in men aged 75 years or older (i.e., the squamous cell carcinoma -SCC- type was more frequent than the adenocarcinoma -ADK- type).
Table 1.
Number of incident cases of lung and bladder carcinomas, age-standardized incidence rates (ASR) with corresponding 95% confidence intervals (CI), by sex and age group. 1995–2009 in 14 municipalities.
| <75 Years | ≥75 Years | All Ages | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||||||||
| N | ASR | 95% CI | N | ASR | 95% CI | N | ASR | 95% CI | N | ASR | 95% CI | N | ASR | 95% CI | N | ASR | 95% CI | |
| All cancer * | 3813 | 442.2 | (427.9–456.6) | 2716 | 314.9 | (302.5–327.4) | 1892 | 3383 | (3228.8–3537.3) | 1599 | 1593.4 | (1512.4–1674.4) | 5705 | 559.9 | (544.8–575) | 4315 | 366.1 | (353.7–378.5) |
| Bladder cancer | 304 | 34.3 | (30.4–38.2) | 79 | 8.6 | (6.7–10.6) | 193 | 339.5 | (291–388) | 74 | 74.2 | (56.6–91.8) | 497 | 46.5 | (42.3–50.8) | 153 | 11.4 | (9.2–13.3) |
| Lung cancer | ||||||||||||||||||
| All lung carcinoma | 527 | 58.4 | (53.4–63.5) | 153 | 16 | (13.4–18.7) | 274 | 496.5 | (437–555.9) | 122 | 126 | (102.8–149.1) | 801 | 75.9 | (70.5–81.3) | 275 | 20.4 | (17.7–23.1) |
| Adenocarcinoma | 131 | 14.6 | (12.1–17.2) | 58 | 6.5 | (4.8–8.2) | 55 | 100.7 | (73.8–127.6) | 24 | 26.8 | (15.7–37.8) | 186 | 18.1 | (15.4–20.8) | 82 | 7.3 | (5.6–9) |
| Squamous cell | 135 | 14.7 | (12.2–17.2) | 23 | 2.3 | (1.3–3.3) | 69 | 129.8 | (98.9–160.7) | 17 | 19.9 | (10.2–29.6) | 204 | 19.3 | (16.6–22) | 40 | 3 | (2–4) |
| Other | 261 | 29 | (25.5–32.6) | 72 | 7.2 | (5.5–8.9) | 150 | 266 | (222.9–309.1) | 81 | 79.3 | (61.4–97.2) | 411 | 38.5 | (34.7–42.4) | 153 | 10.1 | (8.3–11.9) |
Note: ASR calculated from age-standardized rates on 2001 EU population; (*): All cancers (non melanoma excluded).
Table 2 reports the number of lung cancers, the IRRs with corresponding 95% CI, according to age, sex, and tertile of exposure to C6H6, NO2, PM10, and SO2. No excess risks emerged in both sexes under 75 years of age. An increasing gradient in ASR of lung cancer, according to tertiles of exposure to C6H6, NO2, and SO2, emerged only in women aged 75 years or older (Table S1). Only in women aged 75 years or older, the risk of lung cancer increased with increasing degree of residential exposure to C6H6 (IRR = 1.86, 95%CI: 1.15–3.00 for intermediate tertile vs lowest tertile; IRR = 2.00, 95% CI: 1.23–3.25 for highest tertile), NO2 (IRR = 1.70, 95% CI: 1.07–2.71 for intermediate tertile; IRR = 1.72, 95% CI: 1.07–2.77 for highest tertile), and SO2 (IRR = 1.55, 95% CI: 0.96–2.49 for intermediate tertile; IRR = 1.71, 95% CI: 1.07–2.73 for highest tertile). The risk of lung cancer with increasing exposure to PM10 was not linear: IRR = 2.11, 95% CI: 1.33–3.33 for intermediate tertile, and IRR = 1.57, 95% CI: 0.94–2.60 for highest tertile.
Table 2.
Number of incident cases of lung cancer, incidence rate ratio (IRR) with corresponding 95% confidence intervals (CI), by sex, tertile of exposure, and age group. 1995–2009 in 14 municipalities. Statistically significant results are reported in bold.
| <75 Years | ≥75 Years | All Ages | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||||||||
| N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | |
| C6H6 (µg/m3) | ||||||||||||||||||
| <1.1 | 175 | 1 | 52 | 1 | 97 | 1 | 25 | 1 | 272 | 1 | 77 | 1 | ||||||
| 1.1–1.8 | 181 | 1.07 | (0.87–1.32) | 53 | 0.94 | (0.64–1.38) | 89 | 0.91 | (0.71–1.18) | 51 | 1.86 | (1.15–3.00) | 270 | 1.03 | (0.87–1.22) | 104 | 1.09 | (0.81–1.46) |
| >1.8 | 171 | 1.04 | (0.84–1.29) | 48 | 0.95 | (0.64–1.41) | 88 | 0.97 | (0.75–1.26) | 46 | 2.00 | (1.23–3.25) | 259 | 1.02 | (0.86–1.21) | 94 | 1.12 | (0.83–1.52) |
| NO2 (µg/m3) | ||||||||||||||||||
| <16.9 | 172 | 1 | 49 | 1 | 95 | 1 | 28 | 1 | 267 | 1 | 77 | 1 | ||||||
| 16.9–19.6 | 184 | 1.13 | (0.92–1.39) | 57 | 1.12 | (0.77–1.65) | 88 | 0.93 | (0.72–1.20) | 50 | 1.70 | (1.07–2.71) | 272 | 1.07 | (0.91–1.27) | 107 | 1.23 | (0.92–1.65) |
| >19.6 | 171 | 1.07 | (0.86–1.32) | 47 | 0.99 | (0.67–1.48) | 91 | 1.04 | (0.80–1.34) | 44 | 1.72 | (1.07–2.77) | 262 | 1.06 | (0.89–1.25) | 91 | 1.14 | (0.84–1.54) |
| PM10 (µg/m3) | ||||||||||||||||||
| <40.6 | 181 | 1 | 58 | 1 | 94 | 1 | 26 | 1 | 275 | 1 | 84 | 1 | ||||||
| 40.6–51.9 | 183 | 1.03 | (0.84–1.27) | 51 | 0.82 | (0.57–1.20) | 97 | 1.00 | (0.78–1.28) | 61 | 2.11 | (1.33–3.33) | 280 | 1.02 | (0.87–1.21) | 112 | 1.02 | (0.77–1.36) |
| >51.9 | 163 | 0.99 | (0.81–1.23) | 44 | 0.81 | (0.55–1.20) | 83 | 1.01 | (0.78–1.32) | 35 | 1.57 | (0.94–2.60) | 246 | 1.00 | (0.84–1.19) | 79 | 0.93 | (0.68–1.27) |
| SO2 (µg/m3) | ||||||||||||||||||
| <34.6 | 184 | 1 | 52 | 1 | 99 | 1 | 27 | 1 | 283 | 1 | 79 | 1 | ||||||
| 34.6–37.5 | 175 | 1.04 | (0.85–1.28) | 47 | 0.88 | (0.59–1.30) | 91 | 1.01 | (0.79–1.30) | 45 | 1.55 | (0.96–2.49) | 266 | 1.03 | (0.87–1.22) | 92 | 0.98 | (0.73–1.33) |
| >37.5 | 168 | 0.97 | (0.79–1.20) | 54 | 1.06 | (0.72–1.55) | 84 | 0.87 | (0.67–1.13) | 50 | 1.71 | (1.07–2.73) | 252 | 0.95 | (0.80–1.12) | 104 | 1.16 | (0.87–1.56) |
The analysis of the risk of lung cancer was also carried out according to histological subtypes: SCC, ADK, and other lung cancer (OLC) types. A gradient in ASR of lung cancer, according to histological type and tertile of residential exposure to all pollutants, did not emerge in both sexes and in both classes of ages (Table S2). IRRs of lung cancer by histologic subtype (Table S3) highlighted a significantly increased risk of OLC, in women aged 75 years or older, for highest tertile of exposure to C6H6 (IRR = 2.02, 95% CI: 1.14–3.6), NO2 (IRR = 1.70, 95% CI: 0.87–3.00), PM10 (IRR = 2.00, 95% CI: 1.11–3.62), and SO2 (IRR = 2.54, 95% CI: 1.4–4.63). No excess risk of OLC emerged in women under 75 years of age and in men of all ages. A significantly increased risk of SCC was observed in women aged 75 years or older, for the intermediate tertile of exposure to C6H6 (IRR = 8.20, 95% CI: 1.06–63.52), NO2 (IRR = 8.26, 95% CI: 1.07–63.99), and PM10 (IRR = 4.99, 95% CI: 1.13–22.13). This risk was not observed for the highest tertile of exposure to all of the examined pollutants, and no excess risk of SCC emerged in women under 75 years of age and in men in both classes of ages. Moreover, no significant associations with the risk of ADK emerged in both sexes and classes of ages.
Table 3 reports the number of incident cases of bladder cancer, the IRRs according to age, sex, and tertiles of exposure. An increasing gradient in ASR of bladder cancer, according to tertiles of exposure to all pollutants, emerged only in women of all ages, for example from 9.3 cases/100,000/year with lowest exposure to C6H6 to 13.4 cases/100,000/year with highest exposure. A similar gradient in ASR of bladder cancer emerged for other pollutants (Table S4). No excess risk emerged in both sexes under 75 years of age, and only in women aged 75 years or older the risk of bladder cancer increased according to increasing degree of exposure to C6H6 (IRR = 2.39, 95% CI: 1.29–4.44 for intermediate tertile; IRR = 1.94, 95% CI: 1.01–3.74 for highest tertile) and NO2 (IRR = 1.97, 95% CI: 1.07–3.63 for intermediate tertile; IRR = 1.94, 95% CI: 1.03–3.65 for highest tertile). The risk of bladder cancer by increasing levels of residential exposure to PM10 or SO2 was not linear.
Table 3.
Number of incident cases of bladder cancer incidence rate ratio (IRR) with corresponding 95% confidence intervals (CI), by sex, tertile of exposure, and age group. 1995–2009 in 14 municipalities. Statistically significant results are reported in bold.
| <75 Years | ≥75 Years | All Ages | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |||||||||||||
| N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | N | IRR | 95% CI | |
| C6H6 (µg/m3) | ||||||||||||||||||
| <1.1 | 102 | 1 | 25 | 1 | 65 | 1 | 14 | 1 | 167 | 1 | 39 | 1 | ||||||
| 1.1–1.8 | 110 | 1.12 | (0.86–1.47) | 25 | 0.89 | (0.51–1.55) | 65 | 0.96 | (0.70–1.31) | 35 | 2.39 | (1.29–4.44) | 175 | 1.07 | (0.87–1.33) | 60 | 1.16 | (0.77–1.73) |
| >1.8 | 92 | 0.95 | (0.71–1.25) | 29 | 1.33 | (0.78–2.26) | 63 | 1.05 | (0.77–1.44) | 25 | 1.94 | (1.01–3.74) | 155 | 0.98 | (0.79–1.22) | 54 | 1.44 | (0.95–2.17) |
| NO2 (µg/m3) | ||||||||||||||||||
| <16.9 | 100 | 1 | 25 | 1 | 66 | 1 | 15 | 1 | 166 | 1 | 40 | 1 | ||||||
| 16.9–19.6 | 108 | 1.13 | (0.86–1.49) | 22 | 0.79 | (0.44–1.40) | 61 | 0.92 | (0.67–1.27) | 32 | 1.97 | (1.07–3.63) | 169 | 1.07 | (0.86–1.33) | 54 | 1.02 | (0.68–1.54) |
| >19.6 | 96 | 1.02 | (0.77–1.35) | 32 | 1.44 | (0.85–2.43) | 66 | 1.10 | (0.80–1.49) | 27 | 1.94 | (1.03–3.65) | 162 | 1.04 | (0.84–1.30) | 59 | 1.53 | (1.03–2.29) |
| PM10 (µg/m3) | ||||||||||||||||||
| <40.6 | 96 | 1 | 27 | 1 | 72 | 1 | 15 | 1 | 168 | 1 | 42 | 1 | ||||||
| 40.6–51.9 | 114 | 1.18 | (0.90–1.55) | 27 | 0.91 | (0.53–1.55) | 69 | 0.92 | (0.68–1.26) | 37 | 2.39 | (1.31–4.35) | 183 | 1.10 | (0.89–1.36) | 64 | 1.16 | (0.78–1.71) |
| >51.9 | 94 | 1.06 | (0.80–1.41) | 25 | 1.10 | (0.64–1.90) | 52 | 0.88 | (0.63–1.23) | 22 | 1.78 | (0.92–3.44) | 146 | 1.00 | (0.80–1.25) | 47 | 1.21 | (0.80–1.84) |
| SO2 (µg/m3) | ||||||||||||||||||
| <34.6 | 98 | 1 | 24 | 1 | 68 | 1 | 18 | 1 | 166 | 1 | 42 | 1 | ||||||
| 34.6–37.5 | 115 | 1.28 | (0.98–1.68) | 24 | 1.03 | (0.58–1.81) | 54 | 0.89 | (0.64–1.24) | 32 | 1.75 | (0.98–3.12) | 169 | 1.16 | (0.94–1.44) | 56 | 1.19 | (0.80–1.78) |
| >37.5 | 91 | 0.97 | (0.73–1.29) | 31 | 1.43 | (0.84–2.43) | 71 | 1.14 | (0.84–1.55) | 24 | 1.27 | (0.69–2.34) | 162 | 1.02 | (0.82–1.27) | 55 | 1.39 | (0.93–2.08) |
The APCs (% per year) of incidence rates of lung cancer for the period 1995–2009 are shown in Table S5. Among men residing in areas with the lowest residential exposure to each pollutant, significantly negative APCs emerged, whereas non-significant APCs emerged among women (Table S5). Men under 75 years of age showed significantly negative APCs in areas with the lowest exposure to all pollutants and in areas with intermediate exposure to C6H6 (APC = −5.29%; 95% CI: −9.2; −1.2), NO2 (APC = −5.50%; 95% CI: −9.6; −1.2), and SO2 (APC = 4.8% per year; 95% CI: −7.0; −2.6). Temporal trends showed no variability in APCs among women. The APCs (% per year) incidence rates of bladder cancer for the period 1995–2009 are reported in Table S6. APCs variability was not observed in both sexes and in classes of age or in overall age groups.
4. Discussion
According to IARC, air pollution is a Group 1 carcinogen, which causes lung cancer and is associated with an increased risk of bladder cancer [10]. This population-based study assessed the risk of lung and bladder cancers among people residing in the Monfalcone area, northeastern Italy. The area is covered by a population-based cancer registry and it is characterized by the emissions, among other sources, of pollutants from a coal-fired and oil thermal power plant located near the city center.
In men, the incidence rates of lung and bladder cancer for all ages in the study area (Table 1) are lower than the corresponding national incidence rates (82.6 per 100,000 inhabitants for lung cancer; 51.3 per 100,000 inhabitants for bladder cancer). Conversely, in women the local incidence rates are higher than the national incidence rates (18.0 per 100,000 inhabitants for lung cancer; 9.2 per 100,000 inhabitants for bladder cancer).
An excess risk of lung cancer was associated with residential exposure to the highest tertile of C6H6, NO2, PM10, and SO2 only in women aged 75 years or older—an excess risk restricted to the lung cancer types other than SCC or ADK. Conversely, no excess risk was observed in men of all ages and women under 75 years of age. With regard to bladder cancer, in these women an excess risk was associated with the highest tertile of exposure to C6H6 and NO2 and with an intermediate tertile of exposure to C6H6, NO2, and PM10.
The study findings seem to support the hypothesis that the residential exposure to high levels of air pollutants may be associated with an excess risk of incidence of lung and bladder cancers, though the reported excess risks were restricted to older women. The lack of evidence relative to intense exposure to air pollution and lung and/or bladder cancers in men might be explained by heavy smoking habits in both sexes [22] and/or by occupational activities in men (e.g., shipbuilding industries) that put them at high risk of these cancers [7,23,24,25].
The analysis by histological subtypes of lung cancer shows some peculiarities. It is well-known that air pollution in urban areas is a risk factor for ADK [26], while smoking, occupational exposure, and living near pollutant sources may play a more specific role in the etiology of SCC and small cell carcinoma [1,27]. Excess of OLC in women aged 75 years or older living in areas with high exposure to the studied atmospheric pollutants may be due in part to smoking habits, the main cause of small cell carcinoma. It could be also explained by the reduced variability between the lowest and the highest tertile of exposure to all pollutants. Moreover, the association with exposure to C6H6 or NO2 suggests that the excess risk of lung and bladder cancers, if attributable to air pollution, is due in particular to traffic, as it is the main source of emission of C6H6 and NO2 [28] while thermal power plant, home heating and diesel-engines are the main sources of emission of SO2 [6]. Recent research has shown a possible association between exposure to high level of nitrogen dioxide and excess risk of lung cancer [29]. The contribution of the various sources of pollution is confirmed by the technique of apportionment, i.e., road traffic is responsible for 62% of C6H6, 55% of NO2, and 87% of PM10.
This descriptive investigation has some limitations, common to other observational studies. A first important factor not considered in this study is the occupational exposure, and in particular, the exposure to asbestos related to shipbuilding [30], which is associated with increased risks of mesotheliomas and ADK [31,32]. Occupational exposure could affect results differently between males and females.
Secondly, information on smoking, an important confounder, was lacking in this investigation. This variable was, thus, not included in the analysis, because the regional healthcare population database does not report information on this habit. We defined subgroups by sex and age group with different proportions of smokers to minimize smoke confounding. However, this indirect method cannot rule out a tobacco confounder residual effect in the results.
Thirdly, no information was available about the daily time spent in each risk area with different levels of exposures. Finally, the presence of other industries, port, airport and high traffic road near the residential study areas may hamper interpretation of results on the role of air pollution produced by the thermal power plant alone.
An individual human biomonitoring study, with a questionnaire investigating smoking habits, lifestyles, and occupational exposures, is currently underway in the population of the study area. This will allow an evaluation of the actual prevalence of smoking habits in both sexes and classes of age. It is also worth stressing that for lung and bladder cancer cases diagnosed from 1995 to 2009, the relevant period of residential exposure dates at least 30 years before diagnosis (in this case, residential exposures that have occurred between 1965 and 1994). We have therefore assumed that the exposures measured in 1998 were representative of that time period.
However, this study has some strengths. The incidence cases and the study population were derived from two validated regional databases (CR-FVG and healthcare population), which cover overall resident population of the study area. Furthermore, as environmental exposure is concerned, we assumed the residential location of the participants as a proxy of individual exposure. This assumption is based on geocoded residential address and use of mathematical models of air pollution distribution.
5. Conclusions
The findings of this descriptive study indicate that air pollution may have a role with regard to the risk of lung and bladder cancers, though limited to women aged ≥75 years. These persons represented approximately 11% of all the cases of lung and bladder cancers in this population. Further analytical investigations are necessary to shed light on the possible determinants of these increased risks, in particular, air pollutants from multiple industrial sources, road, port and airport traffic, home heating, as well as on the role of occupation, smoking habits, and other lifestyles.
Acknowledgments
The authors wish to thank Tiziana Angelin for recoding and validating morphology codes of lung and bladder cancers and Luigina Mei for editorial support.
Supplementary Materials
The followings are available online at www.mdpi.com/1660-4601/14/8/860/s1: Table S1: Number of incident cases of lung cancer, age standardized rates (ASR) with corresponding 95% confidence intervals (CI), by sex, tertile of exposure and, age group. 1995–2009 in 14 municipalities. Table S2: Number of incident cases of lung carcinomas, age standardized rates (ASR) with corresponding 95% confidence intervals (CI), by sex, morphology, tertile of exposure, and age group. 1995–2009 in 14 municipalities. Table S3: Number of incident cases of lung carcinomas, incidence rate ratio (IRR) with corresponding 95% confidence intervals (CI), by sex, morphology, tertile of exposure, and age group. 1995–2009 in 14 municipalities. Table S4: Number of incident cases of bladder cancer, age standardized rates (ASR) with corresponding 95% confidence intervals (CI), by sex, tertile of exposure, and age group. 1995–2009 in 14 municipalities. Table S5: Annual percent changes (APC) and corresponding 95% confidence intervals (CI), of lung cancer incidence by sex, tertile of exposure, and age group. 1995–2009 in 14 municipalities. Table S6: Annual percent changes (APC) and corresponding 95% confidence intervals (CI), of bladder cancer incidence by sex, tertile of exposure, and age group. 1995–2009 in 14 municipalities.
Author Contributions
Paolo Collarile, Ettore Bidoli, Fabio Barbone, and Diego Serraino wrote the primary manuscript; Paolo Collarile, and Ettore Bidoli performed all analyses; Paolo Collarile, Ettore Bidoli, Stefania Del Zotto, Chiara Panato, and Diego Serraino participated in the acquisition of incidence data; Paolo Collarile, Ettore Bidoli, and Stefania Del Zotto participated in the acquisition of population data; Paolo Collarile, and Loris Zanier geocoded the population; Simonetta Fuser, Fulvio Stel, and Irene Gallai calculated the deposition model of thermal power plant emissions; Diego Serraino, Fabio Barbone, and Loris Zanier promoted this study as members of the Regional Observatory of “Health and Environment”; All authors read, participated in discussions of appropriate groups for analysis and interpretation of results, and they all approved the final manuscript.
Conflicts of Interest
The Authors declare that they have no conflict of interest.
References
- 1.López-Cima M.F., García-Pérez J., Pérez-Gómez B., Aragonés N., López-Abente G., Tardón A., Pollán M. Lung cancer risk and pollution in an industrial region of Northern Spain: A hospital-based case-control study. Int. J. Health Geogr. 2011 doi: 10.1186/1476-072X-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field R.W., Withers B.L. Occupational and environmental causes of lung cancer. Clin. Chest Med. 2012;33:681–703. doi: 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sava F., Carlsten C. Respiratory health effects of ambient air pollution: An update. Clin. Chest Med. 2012;33:759–769. doi: 10.1016/j.ccm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer . Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Volume 105 IARC; Lyon, France: 2013. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. [Google Scholar]
- 5.Barbone F., Bovenzi M., Cavallieri F., Stanta G. Air pollution and lung cancer in Trieste, Italy. Am. J. Epidemiol. 1995;141:1161–1169. doi: 10.1093/oxfordjournals.aje.a117389. [DOI] [PubMed] [Google Scholar]
- 6.Nyberg F., Gustavsson P., Jarup L., Bellander T., Berglind N., Jakobsson R., Pershagen G. Urban air pollution and lung cancer in Stockholm. Epidemiology. 2000;11:487–495. doi: 10.1097/00001648-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer . Some Aromatic Amines, Organic Dyes, and Related Exposures. Volume 99. IARC; Lyon, France: 2010. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. [PMC free article] [PubMed] [Google Scholar]
- 8.Barbone F., Franceschi S., Talamini R., Bidoli E., La Vecchia C. Occupational and bladder cancer in Pordenone (North-east Italy): A case control study. Int. J. Epidemiol. 1994;23:58–65. doi: 10.1093/ije/23.1.58. [DOI] [PubMed] [Google Scholar]
- 9.Malats N., Real F.X. Epidemiology of bladder cancer. Hematol. Oncol. Clin. N. Am. 2015;29:177–189. doi: 10.1016/j.hoc.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer . Outdoor Air Pollution. Volume 109 IARC; Lyon, France: 2016. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. [Google Scholar]
- 11.International Agency for Research on Cancer. [(accessed on 11 July 2017)]; Available online: http://ci5.iarc.fr/CI5I-X/old/vol10/CI5vol10.pdf.
- 12.Bidoli E., Barbone F., Collarile P., Valent F., Zanier L., Daris F., Gini A., Birri S., Serraino D. Residence in proximity of an iron foundry and risk of lung cancer in the municipality of Trieste, Italy, 1995–2009. Int. J. Environ. Res. Public. Health. 2015;12:9025–9035. doi: 10.3390/ijerph120809025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization, International Classification of Diseases, 10th Edition, 2002, Italy. [(accessed on 11 July 2017)]; Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1929_allegato.pdf.
- 14.World Health Organization, International Classification of Disease for Oncology, Third Edition, 2000, Atlanta, USA. [(accessed on 11 July 2017)]; Available online: http://codes.iarc.fr/codegroup/1.
- 15.International Agency for Research on Cancer. [(accessed on 11 July 2017)]; Available online: https://ci5.iarc.fr/CI5plus/Pages/table4_sel.aspx.
- 16.Stel F., Daris F., Giaiotti D.B., Montanari F. Atti Del Convegno “Evoluzione e Controllo della Qualità dell’Aria Sul Territorio Italiano n. 217”, Barti Edizioni. Accademia dei Lincei; Rome, Italy: 2016. Evaluation of a steel industry impacts in the Trieste area: The benzo[a]pyrene affair; p. 224. XXXII Giornata dell’Ambiente. [Google Scholar]
- 17.Tinarelli G., Anfossi D., Bider M., Ferrero E., Trini Castelli S. A new high performance version of the Lagrangian particle dispersion model SPRAY, some case studies. In: Gryning S.E., Batchvarova E., editors. Air Pollution Modelling and Its Application XIII. Volume 23. Plenum Press; New York, NY, USA: 2000. pp. 499–506. [Google Scholar]
- 18.Cressie N. Mathematical Geology. Volume 22. Springer; Berlin, Germany: 1990. The Origins of Kriging; pp. 239–252. [Google Scholar]
- 19.Rothman K.J., Greenland S., Lash T.L. Modern Epidemiology. 3rd ed. Lippincott. Williams & Wilkins; Philadelphia, PA, USA: 2008. [Google Scholar]
- 20.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19:335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. Erratum in 2001, 20, 655. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute: Joinpoint Regression Program Volume Version 4.0.4. 2013. Released 6 May 2013. [(accessed on 1 June 2013)]; Available online: https://surveillance.cancer.gov/joinpoint/revisions.html.
- 22.Istituto Superiore di Sanità, Rome, Italy: Sorveglianza Passi, 2006. [(accessed on 11 July 2017)]; Available online: http://www.epicentro.iss.it/passi/
- 23.International Agency for Research on Cancer . Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. Volume 92. IARC; Lyon, France: 2010. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. [PMC free article] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer . Painting, Firefighting, and Shiftwork. Volume 98. IARC; Lyon, France: 2010. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. [PMC free article] [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer . Chemical Agents and Related Occupations. Volume 100F IARC; Lyon, France: 2012. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. [Google Scholar]
- 26.Raaschou-Nielsen O., Andersen Z.J., Beelen R., Samoli E., Stafoggia M., Weinmayr G., Hoffmann B., Fischer P., Nieuwenhuijsen M.J., Brunekreef B., et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 27.Lubin J.H., Blot W.J. Assessment of lung cancer risk factors by histologic category. J. Natl. Cancer Inst. 1984;73:383–389. doi: 10.1093/jnci/73.2.383. [DOI] [PubMed] [Google Scholar]
- 28.Caselli M., de Gennaro G., Marzocca A., Trizio L., Tutino M. Assessment of the impact of the vehicular traffic on BTEX concentration in ring roads in urban areas of Bari (Italy) Chemosphere. 2010;81:306–311. doi: 10.1016/j.chemosphere.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Hamra G.B., Laden F., Cohen A.J., Raaschou-Nielsen O., Brauer M., Loomis D. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2015;123:1107–1112. doi: 10.1289/ehp.1408882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi C., Bianchi T. Shipbuilding and mesothelioma in Monfalcone, Italy. Indian J. Occup. Environ. Med. 2012;16:14–17. doi: 10.4103/0019-5278.99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paris C., Benichou J., Saunier F., Metayer J., Brochard P., Thiberville L., Nouvet G. Smoking status. occupational asbestos exposure and bronchial location of lung cancer. Lung Cancer. 2003;40:17–24. doi: 10.1016/S0169-5002(02)00538-X. [DOI] [PubMed] [Google Scholar]
- 32.Mollo F., Piolatto G., Bellis D., Andrion A., Delsedime L., Bernardi P., Pira E., Ardissone F. Asbestos exposure and histologic cell types of lung cancer in surgical and autopsy series. Int. J. Cancer. 1990;46:576–580. doi: 10.1002/ijc.2910460404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.