Abstract
Objective
The human prohormone chromogranin A (CHGA), an index member of the granin family is processed to generate catestatin, a peptide that is hypotensive in action and modulates catecholamine release within the sympathoadrenal system. Hypertensive patients with excess sympathetic activity have diminished catestatin. Often the study of physiological consequences of human genetic variation is confounded by elements such as other variations in obligatory linkage disequilibrium with the variant being studied. Also the phenotype of the variant may be influenced by genetic background that varies amongst individuals. This study addresses the effects of a human catestatin polymorphism (rs9658667) using humanized CHGA mouse models.
Methods
We created pertinent humanized mouse models wherein the mouse Chga gene locus was replaced by the human ortholog wild-type and the variant versions. This allowed for probing of the effects of catestatin variation in vivo with controls for other variations and global genetic background.
Results
Both the wild-type and variant human catestatin expressing mouse models were normotensive. The variant catestatin mouse model recapitulated physiological influence of the polymorphism on autonomic traits. These mice had diminished catecholamine, attenuated stress response and increased baroreceptor slopes that would suggest reduced risk of developing hypertension. Elevated plasma glucose, a trait observed in humans was not observed in mice expressing the variant catestatin.
Conclusion
This functional genomics approach of creating humanized mouse models to study rs9658667 polymorphism recapitulated and validated many of the human trait associations. This approach can also be applied in the study of other human gene polymorphisms.
Keywords: blood pressure, catestatin, chromogranin A, functional genomics, humanized chromogranin A mice, rs9658667, single nucleotide polymorphism, spontaneous baroreceptor sensitivity
INTRODUCTION
Advancements in blood pressure (BP) genomics have identified many loci and variants having significant influence on BP [1–3]. To ascertain the mechanism by which these polymorphisms influence BP trait, in-vivo models are needed. Since human chromogranin A gene (CHGA) and its variant influence BP and metabolic traits in diverse ethnic groups, they were chosen as candidates for this study [4,5]. We created humanized mouse models to unequivocally resolve the functional impact of human genetic polymorphism on traits. Genetically humanized CHGA mouse models representing the wild-type and variant allelic form of the human CHGA gene [NC_000014.9] were assessed for the effect of a single nucleotide polymorphism (rs9658667) on pleiotropic autonomic and metabolic trait associations.
Chromogranin A (CHGA) is a proprotein ubiquitously stored in dense core granules of neuroendocrine cells and is important for biogenesis of the granules itself [6,7]. CHGA was first implicated in the pathogenesis of human essential hypertension when heritable increases in plasma CHGA were observed in hypertensive subjects [8,9]. In rodents the CHGA prohormone is involved in BP homeostasis centrally and peripherally [10]. CHGA is cleaved into several biologically active peptides with diverse regulatory influence on cardiovascular, endocrine and immune systems [11]. Of particular relevance in BP regulation is the peptide hormone catestatin derived from the carboxy terminus of the CHGA protein [12]. Catestatin functions as an antagonist of nicotinic cholinergic receptors and inhibits the release of catecholamine from granules of chromaffin cells and noradrenergic nerves [13]. The pleiotropic biological actions of catestatin including its cardio-protective role have recently been reviewed by Mazza et al. [14].
A direct causal relationship between CHGA and hypertension was demonstrated in Chga knockout mice. These mice have a hypertensive and hyper-adrenergic phenotype that can be rescued transiently by catestatin treatment or permanently by stable genomic integration and expression of the human CHGA transgene [6]. Functional genetic variations at the human CHGA locus, in both the proximal promoter and the 3′-untranslated region are associated with essential hypertension [15,16] as well as hypertensive renal disease [17]. Several nonsynonymous amino acid variants of catestatin have been reported in the human populations [4,5] that change the potencies of this peptide hormone [4,18,19].
The nonsynonymous CHGA variant rs9658667 was first reported to have effects on autonomic activity in an urban San Diego population and strongly influences metabolic traits such as reduced catecholamine, elevated HDL and triglycerides in the Indian population study [4,5]. This polymorphism located in the catestatin region of CHGA is speculated to reduce the risk of men developing hypertension. Therefore, we generated a humanized CHGA mouse model representing the human CHGA allelic variant and used it to validate effects of polymorphism on physiological traits. The strain representing the wild-type or major allele CHGAGly364 (referred to henceforth as the Gly/Gly mouse model) was developed by transgenesis using the bacterial artificial chromosome (BAC) cloneRP11-862G15 [6,20]. The second strain representing the minor allelic variant CHGA-Gly364Ser (referred to as Ser/Ser mouse model) was generated by recombineering the original BAC RP11-862G15 by Red-ET recombination method [21] and transgenesis. These mouse models that mimic the human polymorphism allowed us to do detailed functional analysis of effects of the variation on physiological traits. As in humans, the CHGA364Ser polymorphism had a significant effect on autonomic function in humanized Ser/Ser mice vastly improving spontaneous cardiovagal baroreflex sensitivity (BRS) and stress response. They also had elevated HDL and triglycerides and reduced circulating catecholamine levels. Thus certain phenotypic trait associations observed in CHGA364Ser humans are validated in mice whereas others such as elevated blood sugar appear to be an association that is not seen in the humanized mouse model and may in humans be an association due to linkage disequilibrium.
METHODS
Animal husbandry
Mice were housed under pathogen-free conditions, maintained on a normal murine chow-diet, and allowed to have water ad libitum. They were kept in a 12-h light (0600–1800 h) and 12-h dark cycle. Experiments were carried out in accordance with the IACUC at the University of California at San Diego with the guidelines adopted by the National Institutes of Health. All possible steps were taken to avoid distress to animals during experimentation. Mice were euthanized by deep anaesthesia in isoflurane and while the animals were unconscious, blood was collected through a transthoracic ventricular puncture in EDTA-tubes. Low speed centrifugation (660 rcf for 10 min) was used to separate the plasma and it was stored at −70°C until further use. Adrenal glands and other tissue samples were dissected and suspended in RNA later (Life Technologies, Carlsbad, California, USA) for subsequent RNA extraction. To prepare samples for protein immunoblots, the tissues were suspended in RIPA-lysis buffer supplemented with protease inhibitor cocktail (Roche, Indianapolis, Indiana, USA) and homogenized with tissuemizer as described previously [20]. Supernatants of the homogenates were stored at −70°C until further use.
Bacterial artificial chromosome transgenesis to create the humanized CHGAGly364 (wild-type: Gly/Gly) and CHGASer364 (variant: Ser/Ser) mice
The two humanized CHGA mouse models representing wild type and variant in the catestatin domain were generated by BAC transgenesis. The methodology used to generate the mouse model representing the wild-type major allelic variant Gly364 using the human BAC clone (RP11-862G15, chromosome 14 contig) and its preliminary characterization has been detailed previously [6,20]. The same clone was mutated in vivo to 364Ser by a single point mutation (G/A) using the counter-selection BAC modification kit according to the manufacturer’s instructions (Gene Bridges, Heidelberg, Germany). Supplemental Figure 1, http://links.lww.com/HJH/A531, shows the steps involved in mutating the BAC clone by counter-selection. Subsequently, the 364Ser mutant RP11-862G15 BAC construct was injected into the pronucleus of the fertilized ova of hybrid CB6F1strain. Genomic DNA was extracted from the tail snips of the transgenic pups that were born and analysed for the presence and stable germ-line transmission of the entire 210 869 bp BAC insert. The 364Ser founder line 3040 was then mated with wild-type CB6F1 mice and the F1 mice with the human transgene were mated with Chga−/−mice (50%C57BL/6J, 50%129/SvJ background). Brother–sister mating of the CHGA+/−;Chga+/− mice was carried out for ~12 generations until the genotype was CHGA+/+;Chga−/−. The background of both Gly364 and 364Ser catestatin variants models was determined by genome scan allele typing (Jackson Labs, Bar Harbor, Maine, USA).
Verification of CHGA expression in the transgenic mouse models
To explore the functionality of the CHGA transgene in the humanized Gly/Gly and the variant Ser/Ser mice in vivo, the expression of the transgene was monitored by RT-PCR assay in various neuroendocrine tissues using human CHGA specific primers [20]. CHGA protein expression was compared in adrenal and brain stem protein extracts of humanized Gly/Gly and Ser/Ser mice. The total protein in tissue extracts was estimated by Bradford’s assay (Biorad, Hercules, California, USA). The CHGA protein was probed for in immunoblots using primary polyclonal antibody generated against the human catestatin peptide (CHGA352–372) [20].
Phenotyping transgenic mice for blood pressure, heart rate, catecholamine, lipid profile, blood sugar
A noninvasive tail-cuff method was employed to monitor BP of mice using the BP-2000 Blood Pressure Analysis System (Visitech Systems Inc., Cary, North Carolina, USA) [20]. The BP and HR in mice were also continuously recorded by intra-arterial telemetry using the Data Sciences International (DSI; TransomaMedical, St. Paul, Minnesota, USA) PhysioTel telemetry system. Adult male mice (12–16 weeks of age) were anesthetized with isoflurane (5% for induction and 2% for maintenance) and implanted with a catheter coupled to a TA11PA-C20 (DSI) transmitter in the left carotid artery. Telemetry signals were received by an antenna below the cage that relayed the data to a signal processor (DataQuest A.R.T. Gold, version 2.3; DSI) connected to a desktop personal computer (Hewlett-Packard, Portland, Oregon, USA). After the implantation surgery, the animals were rested for 10–12 days for normalization of the diurnal pattern of BP before recording BP in these conscious mice fitted with DSI transmitters. Baseline BP and HR were determined by averaging 10 consecutive seconds of data every 5 min for 24 h.
Epinephrine and norepinephrine levels were quantified in both the plasma and urine samples by a 2-CAT Plasma ELISA kit (LDN, Germany). Standards were run along with the samples according to manufacturer’s protocol to estimate the absolute amounts of the catecholamine in the plasma and urine, and the results were expressed in ng/ml (plasma) and ng/mg creatinine (urine).
Total cholesterol, HDL and LDL/VLDL from plasma samples was measured using a commercially available kit (Abnova, Germany) following manufacturer’s protocol. EnzyChrom Triglyceride assay kit (BioAssay System, California, USA) was used for assaying triglyceride level in the mouse plasma.
Tail vein blood was sampled from mice and the random blood glucose was measured using ContourTs blood glucose meter (Bayer, Japan).
Evaluation of acute stress response and spontaneous baroreflex function in transgenic mice
The telemetry protocol used for BP and HR recordings was also used for stress studies. Mice were allowed to recover for 10–12 days after transponder implantation surgery and the BP recorded for 24 h to ensure return to circadian BP and HR rhythm. Acute immobilization stress was applied to both strains of mice for a period of 2 h, an experimental procedure comparable to the cold pressor test in humans [22]. This was done using an individual rodent restraint device made of Plexiglas fenestrate, restraining all physical movement without causing pain (Universal Restrainer apparatus; Braintree Scientific, Massachusetts, USA). The BP was continuously recorded for an hour prior to stress, the stress period and for 2 h postimmobilization.
Next we tested the impact of the polymorphism on spontaneous BRS in unrestrained mice. We used the sequence method to determine spontaneous BRS from 2 min of beat-to-beat telemetrically recorded SBP and HR [23–25]. Briefly, the slope of the linear regression lines between the SBP and the subsequent pulse intervals were calculated for all sequences where SBP and pulse interval values either increased or decreased concomitantly within three or more consecutive beats and with a pulse interval delay of 0, 1 or 2 heartbeats by using custom made Lab-VIEW program (LabVIEW8.5; National Instruments, Austin, Texas, USA). Because the highest number of sequences had the pulse interval delay of zero in both Gly/Gly and Ser/Ser type, we calculated BRS only with pulse interval values with zero delay. BRS was calculated from sequences with at least three consecutive increases or decreases of SBP of at least 0.5 mmHg/beat accompained with at least three consecutive pulse interval lengthenings or shortenings of at least 5 ms/beat if the correlation coefficients were more than 0.85 with P value more than 0.05. The average BRS was calculated as the mean value of all up and down BRS sequences within 2 min long recording obtained during the 11 : 00 h corresponding to sleep time in mice.
Statistics
Values are given as mean ±SEM. Numbers of experimental replicates are given in the figure legends. Statistical analyses were performed with SPSS-17 (SPSS Inc., Chicago, Illinois, USA) by t-test or two-way ANOVA, as appropriate, after inspection of the data distribution. Differences were considered significant at P less than 0.05.
RESULTS
Generating the humanized CHGAGly364Ser mouse model
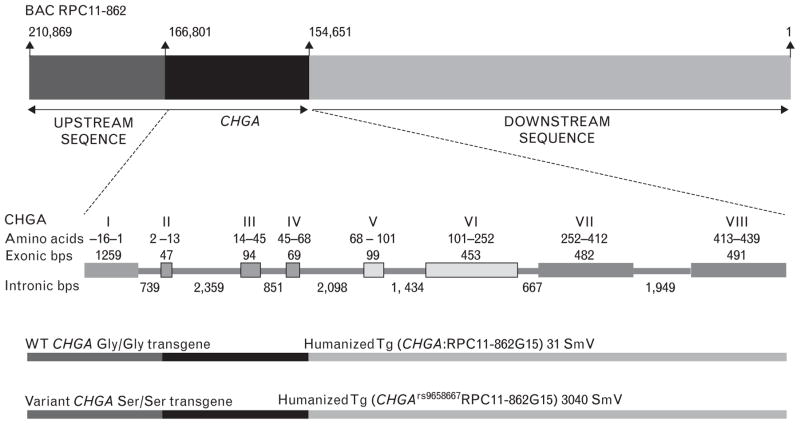
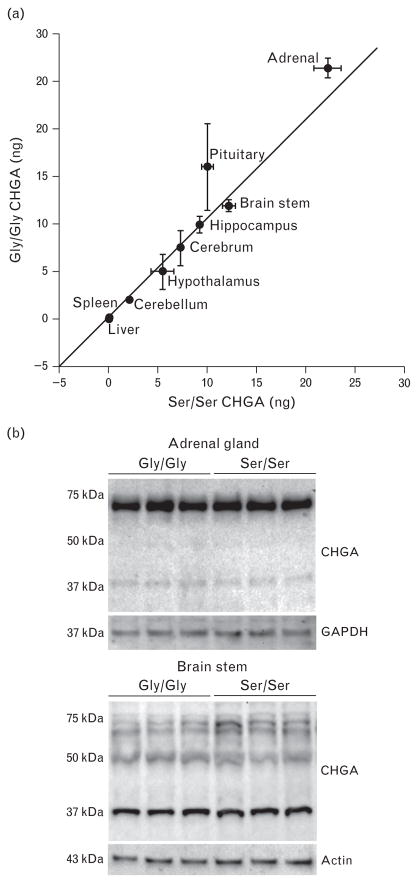
To determine how the human CHGA polymorphism rs9658667 might influence phenotype, we created the humanized CHGA mouse model representing the minor allelic variant CHGAGly364Ser (Ser/Ser mice). BAC transgenesis allowed us to create mouse models wherein a heritable ~210 kbp contig of the human chromosome 14 was stably integrated into the mouse genome. Contained in this integrated human contig, along with the CHGA transgene were large native flanking genomic sequences (~44 kbp upstream and 155 kbp downstream). Therefore all cis-regulatory elements required for expression of CHGA were co-integrated. Also the CHGA transgene had sufficient buffering from the mouse sequences at the site of integration to prevent influence of site on CHGA transgene expression. The humanized strain CHGAGly364 (Gly/Gly mice) representing the wild-type or major allele characterized previously was used as the wild-type or major allele control [20]. The Ser/Ser transgenic mouse strain was generated by BAC transgenesis. First the bacterial artificial chromosome (BAC) clone RP11-862G15 (used to generate the original humanized CHGA Gly/Gly strain) was mutated in vivo by a single point mutation (g/A) to encode for Ser instead of Gly at position 364 in the mature CHGA protein. The integrity of the entire BAC transgene insert was determined by PCR amplification and sequencing. Of the three transgenic founder lines generated with the clone BAC RP11-862G15 mutant 364Ser integration, line 3040 was chosen for further studies. This mouse line had a single copy of the complete BAC RP11-862G15 insert. Our previous studies have determined that the complete CHGA flanking native chromosome 14 sequences contained in the BAC RP11-862G15 insert are required for true fidelity of CHGA expression [20]. The schematic in Fig. 1 depicts the transgenes of the humanized mouse models Tg(CHGA:RPC11-862G15)31Smv expressing the wild-type catestatin and Tg(CHGArs9658667RPC11-862G15)3040Smv expressing the variant 364Ser catestatin. Both the mouse models demonstrated a pattern of tissue-specific expression mimicking that of the endogenous mouse Chga [26]. The mice were bred to homozygosity for the humanized CHGA transgene locus. CHGA was expressed at comparable levels in all neuroendocrine tissues with slightly higher expression in the adrenal and pituitary tissues of Gly/Gly mice as compared with Ser/Ser (Fig. 2a). No CHGA transcript was detected in the liver and spleen. The CHGA protein expression was comparable in the tissues (adrenal and brain stem) tested for both transgenic strains (Fig. 2b). Both models had identical backgrounds (~50% C57BL/6J: 50% 129/SvJ) as determined by 150 SNP panel genome scan allele typing (χ2 statistic = 2.4319, DF = 198, P = 1). In both these mouse models the mouse Chga alleles were lacking and exclusively the homozygous humanized CHGA transgenes were expressed. Consequently the phenotypic features exhibited by both the Gly/Gly and Ser/Ser mouse models were due to qualitative and not quantitative differences in the catestatin peptide expression. The Gly/Gly mice expressed the wild-type catestatin peptide (SSMKLSFRARAYGFRGPGPQL) whereas the Ser/Ser mice expressed the variant catestatin peptide (SSMKLSFRARAYSFRGPGPQL). Supplemental Figure 2, http://links.lww.com/HJH/A531, shows partial CHGA exon 7 sequence electrophoretograms of the Gly/Gly and Ser/Ser mice, with the nucleotide g and A respectively at position 93399050 (chromosome14).
FIGURE 1.
Schematic depicting the bacterial artificial chromosome construct RPC11-862G15 insert and the transgenes of wild-type Gly/Gly and variant Ser/Ser transgenic mouse models. The BAC insert contains the complete CHGA gene and substantial flanking native human chromosome 14q32 sequence. The relative size of the sequence is not drawn to scale. In both the wild-type Gly/Gly and variant Ser/Ser transgenic mouse models the complete ~210 kb of the BAC insert was integrated stably in the mouse genome. Thus in addition to the complete CHGA gene (~12 kb), the transgene contained ~44 kb upstream and ~155 kb downstream chromosome 14 sequence. In the case of the variant Ser/Ser transgenic mouse model the transgene carried a single point mutation in exon 7 of CHGA gene (G9559A) representing the minor allelic variant rs9658667. As a result the catestatin peptide in this strain of mice has Ser (364) instead of Gly. The exonic organization of CHGA is also shown.
FIGURE 2.
Comparable expression of CHGA in wild-type Gly/Gly and variant Ser/Ser transgenic mouse models: (a) Similar transcription of the CHGAGly364 and CHGASer364 transgenes was observed in the humanized mouse models. Expression of the CHGA transgene was quantified by RT-PCR assay using human CHGA gene specific primers. The mRNA expression was normalized against 18S RNA. Error bars indicate SEM. (b) Western blot analysis of CHGA expression: total protein extracts of adrenal (3 μg) and brain (20 μg) tissue of the homozygous Gly/Gly and Ser/Ser mouse models were probed for expression levels of CHGA protein. The blots were stripped and probed with housekeeping protein GAPDH or actin. Both the allelic variants of CHGA express the transgene protein at comparable levels.
Characterizing phenotypic traits of Gly/Gly and Ser/Ser mice
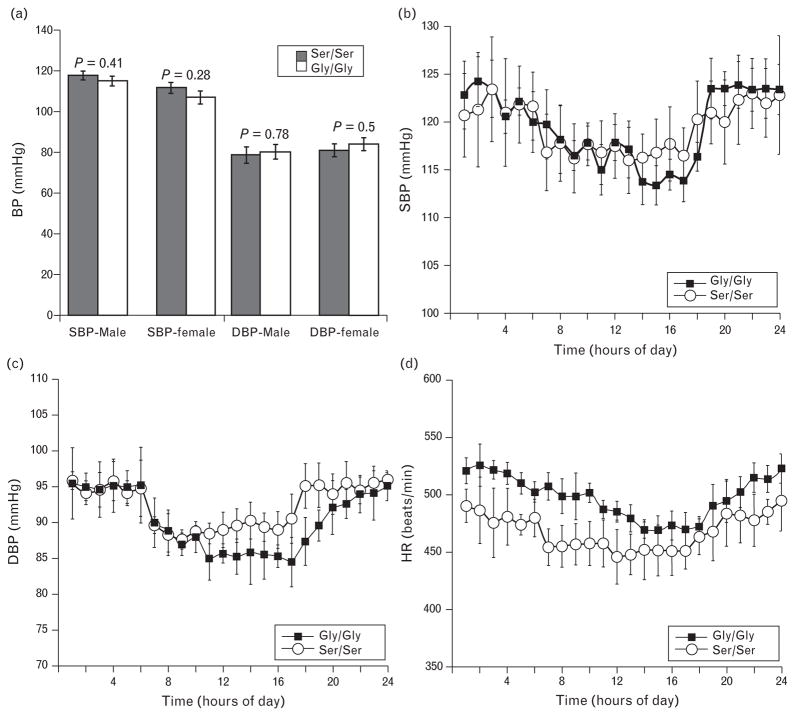
In the Southern California human study the CHGA364Ser polymorphism shows association with lower DBP, phenotype observed in two independent cohorts [5]. However this influence on the DBP phenotype is not observed in the Indian population. The Indian study compared the SBP and DBP of both homozygous and heterozygous carriers of CHGA364Ser allele with individuals homozygous for the major wild-type allele CHGAGly364. Individuals heterozygous for wild-type and variant alleles were normotensive with no significant differences in BP [4]. To resolve these differences in observation, we initially measured the BP in both strains of mice by tail-cuff method and both the strains were normotensive with similar range in SBP and DBP (Fig. 3a). Stratifying by sex yielded no difference in BP in either strain therefore, further characterization of Gly/Gly and Ser/Ser mice was performed in male mice only. The BP and HR were also recorded by telemetry, shown in Fig. 3b–d. Although the Ser/Ser mice showed lower HR, the trend was not statistically significant. The tail-cuff BP findings were confirmed in that no significant influence of strain is observed on BP.
FIGURE 3.
Blood pressure (BP) and heart rate (HR) of Gly/Gly and Ser/Ser mice are similar. (a) BP was measured in both strains of mice homozygous for the CHGA transgene by noninvasive tail cuff method. No significant difference was observed in SBP and DBP between either strains of either sex (males: n =12 Gly/Gly, 15 Ser/Ser, and females n = 14 Gly/Gly and 13 Ser/Ser). Values are expressed as mean ± SEM. Continuous 24 h telemetric recordings of BP and HR was carried out in male mice of both Gly/Gly (n = 8) and Ser/Ser (n =7) strains. Data for both strains is represented in the graphs. The average SBP/DBP/HR values for the hour are plotted and the error bars represent mean ± SEM for the entire group of mice. (b) SBP (121.4 ± 2.2 Gly/Gly vs. 120.7 ± 2.4 Ser/Ser), (c) DBP (90.9 ± 1.9 Gly/Gly vs. 94.4 ± 2.1 Ser/Ser), (d) HR (495 ± 13 Gly/Gly vs. 463 ± 14 Ser/Ser). Statistics were done by repeated measures ANOVA using linear mixed model. No significant difference in the overall 24 h SBP (P =0.82), DBP (P = 0.24) and HR (P =0.1) due to allelic difference was noted.
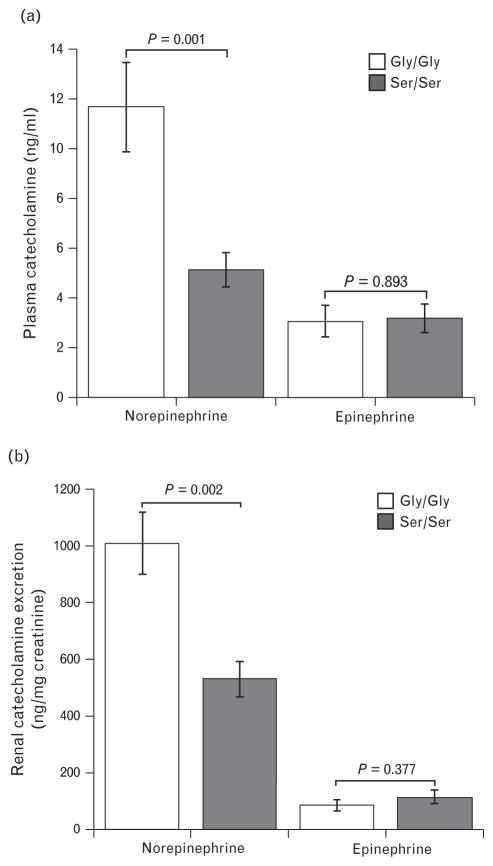
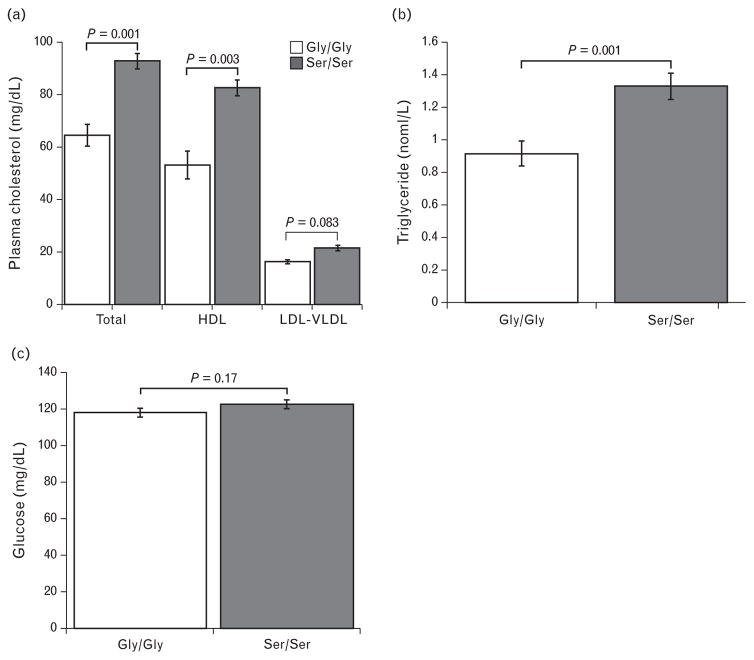
The catecholamine level was measured in mouse models since both the human studies reported diminished norepinephrine levels in CHGA364Ser allele carriers. Epinephrine levels were comparable in both strains of mice whereas norepinephrine was diminished in mice with the minor allele (Ser/Ser) (Fig. 4a). Urine norepinephrine levels that reflect integrated measurements obtained for longer periods of time also showed lower levels in Ser/Ser mice (Fig. 4b). Other metabolic traits that were reported as statistically significantly influenced by CHGA364Ser genetic variation in humans were also tested in mouse models. We measured total plasma cholesterol, which was significantly elevated in Ser/Ser mice mainly as a result of elevated HDL; LDL did not differ between strains (Fig. 5a). Plasma triglycerides were also elevated in the Ser/Ser mice (Fig. 5b). Glucose levels in the plasma of Gly/Gly and Ser/Ser mice were similar implicating lack of effect of the variant 364Ser catestatin hormone on the blood glucose trait in mice, in distinction with humans (Fig. 5c).
FIGURE 4.
Decreased norepinephrine levels associated with mice carrying the Ser/Ser allele. Mouse models of both genotypes showed a difference in the circulating and urine norepinephrine levels. Mice with the Ser/Ser genotype had significantly decreased norepinephrine in both plasma (a) and urine (b) whereas the epinephrine levels did not vary, n =8 for each strain.
FIGURE 5.
CHGASer/Ser genotype strongly influences lipid profile. (a) Total cholesterol in the plasma of mice with the CHGASer/Ser genotype (n = 9) was increased and this was mostly as a consequence of elevated HDL. (b) Ser/Ser mice (n =16) also had significantly higher plasma triglyceride compared with Gly/Gly (n =20) mice. (c) Tail vein sampled blood glucose level was measured in both Ser/Ser (n = 21) and Gly/Gly (n =25) mice. No difference in normal blood glucose was detected. Values are expressed, as mean ± SEM and P value of 0.17 was considered insignificant.
Stress response and spontaneous baroreflex sensitivity of Gly/Gly and Ser/Ser mice
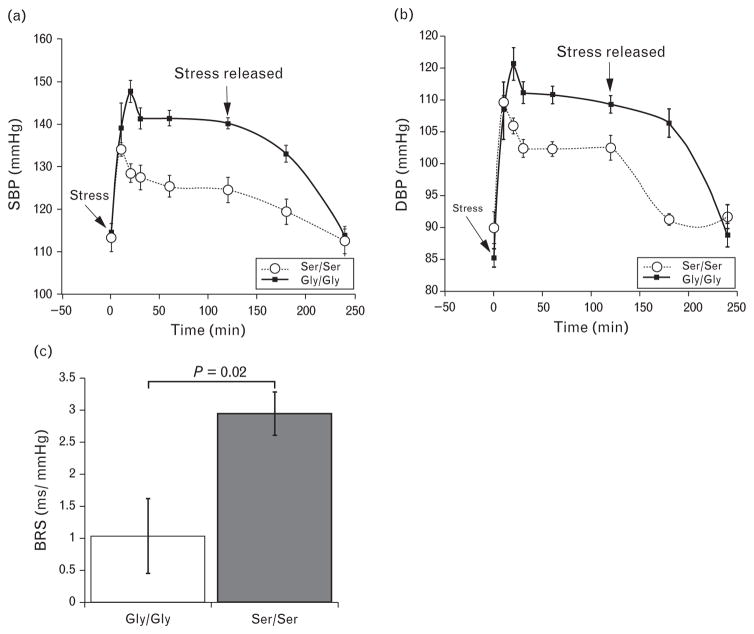
The catestatin 364Ser variant has a significant influence on the autonomic function traits of stress response and baroreflex sensitivity in humans [5]. The Gly/Gly and Ser/Ser mice were equipped with telemetric devices to monitor BP and subjected to immobilization stress for a period of 2 h. As shown in Fig. 6a and b, both strains of mice responded to stress with elevation in BP. However both the amplitude and duration of the stress-response was greater in the Gly/Gly mice. In the Gly/Gly mice the BP continued to rise for the first 20 min of immobilization stress and the delta increase in SBP/DBP was greater (36/30 mmHg) as compared with the diminished response in Ser/Ser mice. In the variant Ser/Ser strain, the maximal response to stress was in the first 10 min and resulted in 20/18 mmHg jump over basal BP. The return to normal BP poststress was also accelerated in the Ser/Ser strain of mice compared with Gly/Gly mice.
FIGURE 6.
Humanized Ser/Ser transgenic mice have a decreased response to immobilization stress and improved spontaneous BRS. Male mice of both strains (n =8 for each strain) equipped with telemetric transmitters for recording blood pressure (BP) were subjected to 2 h of immobilization stress. The time point 0 represents the start of stress and the corresponding BP the basal BP prior to stress. After 120′ the mice were freed from restraint and the BP was monitored for additional 2 h. (a) SBP and (b) DBP tracings. Values plotted are mean ± SEM. Results of telemetric BP recordings measured during the 2-h duration of stress and 2 h poststress period were evaluated by linear mixed model for repeated measures in SPSS factoring for time, strain and strain-by-time interaction: SBP (P time < 0.001, strain < 0.001 and time*strain =0.001); 0.001); DBP (P time < 0.001, strain = 0.001 and time*strain < 0.001). (c) Spontaneous BRS in Ser/Ser and Gly/Gly mice was determined by the sequence method. Overall mean of all up and down-BRS sequences is significantly lower in Gly/Gly than in Ser/Ser type mice. The slopes were calculated from telemetric recordings (n = 7Gly/Gly mice, n =6 Ser/Ser mice).
Spontaneous BRS is used as an index of vagal modulation of HR. Although average SBP and pulse interval values were similar in both Ser/Ser and Gly/Gly mice models, BRS was almost threefold greater in Ser/Ser mice 2.95 ± 0.59 ms/mmHg vs. 1.04 ± 0.34 ms/mmHg respectively (Fig. 6c).
DISCUSSION
CHGA and its derived peptides have been extensively studied since their discovery almost 50 years ago [27]. The heritability of basal circulating CHGA concentration has been reported in twin studies and is correlated with the sympathetic tone [9,28,29]. In hypertensive individuals low circulating levels of catestatin (CHGA’s proteolytically processed fragment) is considered as an early intermediate phenotype in the eventual development of hypertension [30]. Twin studies from two continents using genome-wide linkage established the heritable determination of the autonomic trait (catestatin) contributing to BP [31]. Early on CHGA was identified as a candidate gene for autonomic dysfunction syndromes, including intermediate phenotypes that contribute to human hypertension [32,33]. However recent genome-wide association studies have not identified CHGA locus as associated with BP phenotype [1–3]. The LocusZoom analysis of a genome-wide association study found no significant trait association of CHGA SNP and BP (Supplemental Figure 3, http://links.lww.com/HJH/A531) [2,34].
Resequencing of the human CHGA gene in a diverse urban Southern California population identified nonsynonymous polymorphisms in the catestatin region of CHGA [33]. One of the polymorphisms: rs9658667 occurred at a frequency of ~3%. Most of the individuals with this minor allele CHGA364Ser were heterozygous and were extensively phenotyped along with individuals homozygous for the major CHGAGly364 allele [5]. A much larger study in the geographically and ethnically distinct Indian population identified many more heterozygousCHGA364Ser allele carriers (minor allele frequency of 15%) and a few homozygotes were identified [4]. Some traits associated with this polymorphism were corroborated in both studies and others were not. Given the role of the CHGA gene and its catestatin product in human health we chose the CHGA and its catestatin polymorphism as candidates in this study. We investigated the consequence of this polymorphism using the humanized CHGA mouse models expressing either the major allelic variant CHGAGly364 or the minor allele CHGA364Ser to corroborate trait associations.
We were able to recapitulate in Ser/Ser humanized mouse model distinctive phenotypic features of CHGASer364 humans such as diminished circulating catecholamine, elevated HDL, attenuated stress response and increased BRS. These traits would lend themselves to decreasing the risk of developing hypertension in humans. The Ser/Ser mouse model contradicted the association of the polymorphism with elevated glucose levels as noted in the Indian carries of the allele. Both mouse models of either sex were normotensive. Although in Ser/Ser mice HR trended lower compared with Gly/Gly, this effect was not significant. Physiological phenotyping of the Gly/Ser heterozygotes and Gly/Gly homozygotes in the human study that included the initial case–control UCSD population and the replication Kaiser population, showed lower DBP (5.5 and 5 mmHg, respectively) in Gly/Ser heterozygotes. Stratification by sex revealed that the difference was significant amongst males, but not females [5]. In the same UCSD study, SBP did not contrast amongst genotypes. The replication Kaiser study did not show significant genotype effect on SBP; stratifying the data by sex however showed significant gene by sex interaction in men and also a trend towards lower HR. No significant difference in DBP, SBP and HR was observed in the much larger Indian study that included substantial number of individuals with minor allele Ser364 (both heterozygous and homozygous) [4]. Therefore Rao et al.’s observation of lower DBP in CHGA364Ser allele carriers was not seen in the Indian population and also clearly not observed in the normotensive Ser/Ser mice. The BP phenotype variance in the two human studies could be attributed to demographics and ethnicity.
In the Ser/Ser mouse model norepinephrine was diminished in both plasma and urine, whereas epinephrine was at comparable levels. The effects of norepinephrine are largely mediated by the sympathetic nervous system and the effects of epinephrine are brought about by the adrenal medulla therefore, in mice expressing the variant catestatin peptide Ser364, catecholamine secretion seems to be modulated in the postganglionic fibres. The study by Rao et al. compares the phenotypes of individuals heterozygous for the 364Ser allele with homozygous Gly364 allele carriers [5]. Although the Indian study has significantly larger number of 364Ser carriers, both human studies have fewer 364Ser homozygotes, a limitation clearly overcome using humanized mouse model. In 364Ser carriers, the diminished sympathetic activity is implied by declined urine catecholamine and plasma norepinephrine levels [5]. Sahu et al. also characterized both 364Ser homozygous and heterozygous individuals with declined plasma norepinephrine levels [4]. Plasma epinephrine levels were no different in individuals of both genotypes and this was attributed to lack of or small numbers of Ser/Ser homozygotes in both human studies an argument clearly refuted by our mouse findings. Catestatin is well known to modulate various nicotinic-cholinergic processes in vitro and is an antagonist of the acetylcholine receptors blocking the release of catecholamine [4,19]. The variant catestatin peptide has less potency as an antagonist compared with the wild-type catestatin, which would suggest elevated plasma catecholamine in carriers of the minor allelic variant. However nicotinic-cholinergic processes are also subject to agonist-induced desensitization and the catestatin peptide has a blocking effect on this process as well. Again, the variant catestatin is less potent compared with the wild type at this process. The net consequence of both blocking catecholamine release and agonist-induced desensitization is diminished norepinephrine in plasma of both men [5] and as seen in this study, mice.
We evaluated the observed pleiotropic effects of the CHGASer364 polymorphism in mice. The Ser/Ser mice had elevated triglycerides and HDL. The individuals in the Indian population expressing the catestatin variant have higher plasma glucose, triglycerides, and HDL. Rao et al. did not report on these parameters [5]. Catestatin promotes lipolysis and fatty acid oxidation however the relative potencies of the variant and wild-type forms have not been studied [35]. [36] Sahu et al. speculated that catestatin involvement in the process of gluconeogenesis and lipolysis might vary in potencies for both forms of catestatin [4]. That and the attenuated catecholamine levels in CHGASer364 carriers would cause their elevated triglyceride levels. Recently a positive correlation between plasma catestatin and HDL levels has been reported [36].
Higher plasma glucose levels were not observed in Ser/Ser mice, which was also the case in the UCSD study (unpublished data). In the Indian study, individuals with the 364Ser allele with elevated blood glucose also showed strong linkage disequilibrium with the nonsynonymous SNP Gly297Ser. This SNP Gly297Ser occurs in the pancreastatin (dysglycemic peptide) domain of CHGA and would therefore have a dominant effect on the hyperglycemic phenotype [37,38]. The elevated plasma glucose trait is not recapitulated in mice expressing the minor human allele possibly due to species specificity. It should also be noted that both the Gly/Gly and Ser/Ser mouse models were created in the background of Chga−/− knockout mice that have increased insulin sensitivity phenotype attributed to lack of the dysglycemic hormone pancreastatin derived by proteolytic cleavage of CHGA [39].
Baroreflex is a critical regulatory mechanism involved in stabilization of systemic arterial pressure. Cardiovagal part of this reflex modulate HR in response to spontaneous increases and decreases of systemic arterial BP. In Ser/Ser mice increased cardiovagal BRS is also linked to lower plasma norepinephrine concentration implying reduced sympathetic outflow to blood vessels. This contrasts with the phenotype of decreased baroreceptor sensitivity in Chga knockout mice [40].
The difference between the normotensive mouse models was deconstructed showing significant variances in autonomic function. Acute immobilization stress was applied to both strains of mice, a procedure comparable to cold pressor test in humans. The intensity of the stress response in terms of BP-elevation was higher for the wild-type Gly/Gly mice and attenuated for Ser/Ser mice expressing the variant catestatin suggesting better baroreflex function in Ser/Ser mice. Therefore stress response, attenuated norepinephrine and their concomitant effects on physiological functions, such as BP, HR and lipolysis, may serve as objective indicators of lower risk for developing hypertension. Autonomic phenotyping in the Rao et al. study found that carriers of the 364Ser allele exhibited heightened baroreflex control and concluded that CHGA364Ser confers a protective role to carriers against future development of hypertension. Results from Ser/Ser mice also support this view.
The human (NM_001275) and mouse (NM_007693) genes for chromogranin A share 82% identity and the catestatin domains show 28.6% divergence [19]. At position 13 of the 21 amino acid catestatin peptide the Gly residue is invariant across species. This represents position 364 in the mature CHGA protein and is polymorphic in humans resulting in amino acid Ser instead of Gly in about 15% of the individuals. This change in sequence of catestatin results in change in structure and significant loss of functional potency [4].
With the advent of genome-wide association studies and complimentary genomic strategies, many human genetic variations that influence BP have been identified [1,3,41]. Model organisms can help us understand the functional impact of these polymorphisms and prove causality unequivocally. Recently the mouse ENCODE consortium reported that the mouse and human genomes have large divergences of sequences involved in transcriptional regulation, chromatin state and organization; therefore, the study of the mouse ortholog to understand role of the human gene may simply be not accurate [42]. Hence this approach of creating humanized mouse models with germ-line integration of the human genome region under scrutiny is promising [43].
The limitation of this study is that the CHGA transgenic models used have the BAC transgene with the wild-type/mutant CHGA gene integrated randomly in the mouse genome, a better strategy would have entailed co-placement ensuring placement of both variants of the CHGA gene at the same mouse loci. This approach was effectively done for the human angiotensinogen gene [44]. However, both the humanized CHGA strains displayed similar CHGA expression profiles mimicking mouse Chga expression, and we therefore conclude that no adverse gene disruption occurred as a result of BAC integration in both lines. Even the co-placement approach could not have controlled the integration site of the BAC. The co-integration of sizeable CHGA flanking native chromosome 14 sequences, insulates transgene from mouse genome influence.
Further studies with these mouse models involve characterization of the sympathetic and parasympathetic branches of their autonomic system. Also we speculate differences may exist in the metabolic processes such as gluconeogenesis, lipogenesis and lipolysis resulting in elevated triglycerides in Gly364Ser carriers and will be delineated.
In conclusion, in this study we provide proof-of-principle of the functionality of humanized mouse models to evaluate role of a human gene and its polymorphism. Using the humanized CHGA mouse models expressing the catestatin wild-type or Gly364Ser variant we were able to parse pleiotropic trait associations seen in human subjects. In both mice and men the Gly364Ser polymorphism conferred metabolic traits such as elevated HDL and lowered norepinephrine levels; it was associated with superior baroreflex function and therefore better response to stress. Although in Ser/Ser mice we did not see lowering of DBP as is observed in one San Diego human cohort, all of these trait associations would suggest reduced sympathetic outflow and protection due to stress-induced hypertension. This study reiterates many trait associations observed in human CHGA rs9658667 carriers.
Supplementary Material
Acknowledgments
We are indebted to late Prof. Daniel T. O’Connor who discovered this polymorphism and for his guidance in this study and appreciative of Prof. Ana Pajor for critically reviewing the manuscript. The Chga−/− knockout mice generated in Prof. S. K. Mahata’s laboratory were used to breed the humanized mice.
Funding for this research was from NIH grants (5R01HL108629-A1 & 5R01DK094894) awarded to S.M.V. T.R. received support from Satellite Healthcare, Diabetes Endocrinology Research Center (P30DK063491) and American Heart Association 15BGIA22410018.
Abbreviations
- BAC
bacterial artificial chromosome
- BP
blood pressure
- BRS
baroreflex sensitivity
- CHGA
chromogranin A protein
- CHGA
human chromogranin A gene
- Chga
mouse chromogranin A gene
- CHGAGly364
wild-type, or major allele with Gly at position 364 of CHGA
- CHGAGly364Ser
minor allele with Ser at position 364 of CHGA
- Gly/Gly
humanized CHGA transgenic mouse models carrying the major allele
- HDL
high-density lipoprotein
- HR
heart rate
- LDL
low-density lipoprotein
- Ser/Ser
humanized CHGA transgenic mouse models carrying the minor allele
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43:1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, Sim X, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahu BS, Obbineni JM, Sahu G, Allu PK, Subramanian L, Sonawane PJ, et al. Functional genetic variants of the catecholamine-release-inhibitory peptide catestatin in an Indian population: allele-specific effects on metabolic traits. J Biol Chem. 2012;287:43840–43852. doi: 10.1074/jbc.M112.407916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 6.Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim T, Tao-Cheng JH, Eiden LE, Loh YP, Chromogranin A. An ‘on/off’ switch controlling dense-core secretory granule biogenesis. Cell. 2001;106:499–509. doi: 10.1016/s0092-8674(01)00459-7. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor DT. Plasma chromogranin A. Initial studies in human hypertension. Hypertension. 1985;7(3 Pt 2):I76–179. doi: 10.1161/01.hyp.7.3_pt_2.i76. [DOI] [PubMed] [Google Scholar]
- 9.Takiyyuddin MA, Parmer RJ, Kailasam MT, Cervenka JH, Kennedy B, Ziegler MG, et al. Chromogranin A in human hypertension. Influence of heredity. Hypertension. 1995;26:213–220. doi: 10.1161/01.hyp.26.1.213. [DOI] [PubMed] [Google Scholar]
- 10.Friese RS, Altshuler AE, Zhang K, Miramontes-Gonzalez JP, Hightower CM, Jirout ML, et al. MicroRNA-22 and promoter motif polymorphisms at the Chga locus in genetic hypertension: functional and therapeutic implications for gene expression and the pathogenesis of hypertension. Hum Mol Genet. 2013;22:3624–3640. doi: 10.1093/hmg/ddt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 12.Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takiyyuddin MA, Cervenka JH, Sullivan PA, Pandian MR, Parmer RJ, Barbosa JA, et al. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. doi: 10.1161/01.cir.81.1.185. [DOI] [PubMed] [Google Scholar]
- 14.Mazza R, Tota B, Gattuso A. Cardio-vascular activity of catestatin: interlocking the puzzle pieces. Curr Med Chem. 2015;22:292–304. doi: 10.2174/0929867321666141106114928. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Rao F, Rodriguez-Flores JL, Mahata M, Fung MM, Stridsberg M, et al. Naturally occurring human genetic variation in the 3′-untranslated region of the secretory protein chromogranin A is associated with autonomic blood pressure regulation and hypertension in a sex-dependent fashion. J Am Coll Cardiol. 2008;52:1468–1481. doi: 10.1016/j.jacc.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Rao F, Rodriguez-Flores JL, Mahapatra NR, Mahata M, Wen G, et al. Common genetic variants in the chromogranin A promoter alter autonomic activity and blood pressure. Kidney Int. 2008;74:115–125. doi: 10.1038/ki.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salem RM, Cadman PE, Chen Y, Rao F, Wen G, Hamilton BA, et al. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol. 2008;19:600–614. doi: 10.1681/ASN.2007070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahu BS, Mohan J, Sahu G, Singh PK, Sonawane PJ, Sasi BK, et al. Molecular interactions of the physiological antihypertensive peptide catestatin with the neuronal nicotinic acetylcholine receptor. J Cell Sci. 2012;125(Pt 9):2323–3237. doi: 10.1242/jcs.103176. [DOI] [PubMed] [Google Scholar]
- 19.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, et al. The catecholamine release-inhibitory ‘catestatin’ fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–1191. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- 20.Vaingankar SM, Li Y, Corti A, Biswas N, Gayen J, O’Connor DT, et al. Long human CHGA flanking chromosome 14 sequence required for optimal BAC transgenic ‘rescue’ of disease phenotypes in the mouse Chga knockout. Physiol Genomics. 2010;41:91–101. doi: 10.1152/physiolgenomics.00086.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muyrers JP, Zhang Y, Stewart AF. Techniques: Recombinogenic engineering–new options for cloning and manipulating DNA. Trends Biochem Sci. 2001;26:325–331. doi: 10.1016/s0968-0004(00)01757-6. [DOI] [PubMed] [Google Scholar]
- 22.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Laude D, Baudrie V, Elghozi JL. Applicability of recent methods used to estimate spontaneous baroreflex sensitivity to resting mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:42–50. doi: 10.1152/ajpregu.00319.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254:H377–H383. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- 25.Milic M, Sun P, Liu F, Fainman C, Dimsdale J, Mills PJ, et al. A comparison of pharmacologic and spontaneous baroreflex methods in aging and hypertension. J Hypertens. 2009;27:1243–1251. doi: 10.1097/HJH.0b013e32832a6e1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahata SK, Mahapatra NR, Mahata M, Wang TC, Kennedy BP, Ziegler MG, et al. Catecholamine secretory vesicle stimulus-transcription coupling in vivo. Demonstration by a novel transgenic promoter/photoprotein reporter and inhibition of secretion and transcription by the chromogranin A fragment catestatin. J Biol Chem. 2003;278:32058–32067. doi: 10.1074/jbc.M305545200. [DOI] [PubMed] [Google Scholar]
- 27.Banks P, Helle K. The release of protein from the stimulated adrenal medulla. Biochem J. 1965;97:40C–41C. doi: 10.1042/bj0970040c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takiyyuddin MA, Baron AD, Cervenka JH, Barbosa JA, Neumann HP, Parmer RJ, et al. Suppression of chromogranin-A release from neuroendocrine sources in man: pharmacological studies. J Clin Endocrinol Metab. 1991;72:616–622. doi: 10.1210/jcem-72-3-616. [DOI] [PubMed] [Google Scholar]
- 29.Dimsdale JE, O’Connor DT, Ziegler M, Mills P. Chromogranin A correlates with norepinephrine release rate. Life Sci. 1992;51:519–525. doi: 10.1016/0024-3205(92)90029-o. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. The catecholamine release-inhibitory ‘catestatin’ region of chromogranin A: early decline in humans at genetic risk of hypertension. Ann N Y Acad Sci. 2002;971:533–535. doi: 10.1111/j.1749-6632.2002.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, et al. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin A: implications for secretion and blood pressure. Circulation. 2008;118:247–257. doi: 10.1161/CIRCULATIONAHA.107.709105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kailasam MT, O’Connor DT, Parmer RJ. Hereditary intermediate phenotypes in African American hypertension. Ethn Health. 1996;1:117–128. doi: 10.1080/13557858.1996.9961778. [DOI] [PubMed] [Google Scholar]
- 33.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, et al. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandyopadhyay GK, Vu CU, Gentile S, Lee H, Biswas N, Chi NW, et al. Catestatin (chromogranin A(352–372)) and novel effects on mobilization of fat from adipose tissue through regulation of adrenergic and leptin signaling. J Biol Chem. 2012;287:23141–23151. doi: 10.1074/jbc.M111.335877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durakoğlugil ME, Ayaz T, Kocaman SA, Kırbaş A, Durakoğlugil T, Erdoğan T, et al. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: influence on high-density lipoprotein cholesterol. Anatol J Cardiol. 2015;15:577–585. doi: 10.5152/akd.2014.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valicherla GR, Hossain Z, Mahata SK, Gayen JR. Pancreastatin is an endogenous peptide that regulates glucose homeostasis. Physiol Genomics. 2013;45:1060–1071. doi: 10.1152/physiolgenomics.00131.2013. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Margalet V, González-Yanes C, Najib S, Santos-Álvarez J. Metabolic effects and mechanism of action of the chromogranin A-derived peptide pancreastatin. Regul Pept. 2010;165:71–77. doi: 10.1016/j.regpep.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Gayen JR, Saberi M, Schenk S, Biswas N, Vaingankar SM, Cheung WW, et al. A novel pathway of insulin sensitivity in chromogranin A null mice: a crucial role for pancreastatin in glucose homeostasis. J Biol Chem. 2009;284:28498–28509. doi: 10.1074/jbc.M109.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dev NB, Gayen JR, O’Connor DT, Mahata SK. Chromogranin A and the autonomic system: decomposition of heart rate variability and rescue by its catestatin fragment. Endocrinology. 2010;151:2760–2768. doi: 10.1210/en.2009-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Consortium for Blood Pressure Genome-Wide Association Studies. Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yue F, Cheng Y, Breschi A, Vierstra J, Wu W, Ryba T, et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devoy A, Bunton-Stasyshyn RK, Tybulewicz VL, Smith AJ, Fisher EM. Genomically humanized mice: technologies and promises. Nat Rev Genet. 2012;13:14–20. doi: 10.1038/nrg3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu T, Oishi T, Omori A, Sugiura A, Hirota K, Aoyama H, et al. Identification of cis-regulatory sequences in the human angiotensinogen gene by transgene coplacement and site-specific recombination. Mol Cell Biol. 2005;25:2938–2945. doi: 10.1128/MCB.25.8.2938-2945.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.