Dear Sir,
The uptake of carbon dioxide through the stomata inexorably leads to transpirational water losses, and evolutionary theories hold that plants balance this trade-off by optimizing carbon gain per unit water lost. However, the physiological mechanisms underpinning optimal stomatal regulation remain to be elucidated. In particular, a key assumption is that assimilation and stomatal conductance (gs) are tightly coordinated at diurnal time scales, but recent reviews highlight that whether and how such coordination is achieved remain elusive (Matthews et al., 2017). Circadian regulation is emerging as an additional mechanism potentially affecting the synchrony of the photosynthesis (A) and gs responses.
The circadian clock is an endogenous timer of plant metabolism that regulates the diurnal pattern of gene expression. Among other processes, A and gs are under circadian control (Fig. 1). However, there is not a single “master rhythm” that leads to a unique temporal pattern of gas exchange in plants. On the contrary, clocks in plants are, to a certain degree, tissue specific, and different clocks are entrained with different phases, amplitudes, and periods. For instance, guard cell clocks often show longer periods than mesophyll clocks (Yakir et al., 2011). In turn, circadian regulation in A is independent from circadian regulation in gs (Dodd et al., 2004), and the existence of these different, and to a degree mutually independent, clock types could explain why.
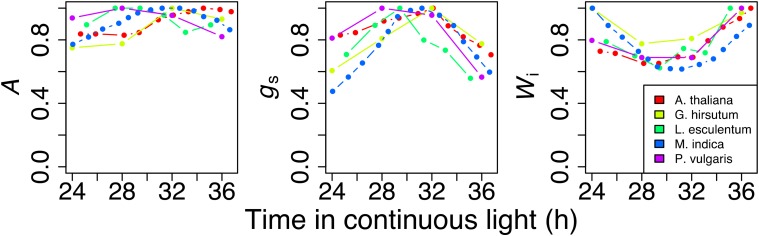
Figure 1.
Patterns of carbon assimilation (A), stomatal conductance (gs), and intrinsic water use efficiency (Wi) under continuous light conditions for different species. Each point represents the mean of at least three replicates that were measured in the second subjective day under continuous illumination in Arabidopsis (Dodd et al., 2005), Gossypium hirsutum (Resco de Dios et al., 2016), L. esculentum (Corlett et al., 1998), M. indica (Allen et al., 2000), and P. vulgaris (Resco de Dios et al., 2016). Data originates from different studies, but the entrainment phase always consisted of 12 h of day and of night (except Allen et al. [2000], where daylength was 11 h). There were differences in radiation and temperature across studies leading to differences in flux rates across species. Consequently, plotted values represent normalized values of A, gs, and Wi. Normalization was done by dividing each value by the maximum value for that species.
I digitized published records of circadian oscillations in A and gs measured for different C3 crop species under continuous light to informally assess potential in circadian regulation as a driver of diurnal gas exchange (Fig. 1). The overall variation during the “subjective day” was 15% to 25% in A and 30% to 35% in gs, respectively, across the analyzed species. Importantly, this degree of variation was comparable to that observed in response to diurnal changes in temperature or vapor pressure deficit (Resco de Dios et al., 2017). Furthermore, the temporal pattern in A and gs was decoupled in, for instance, Arabidopsis (Arabidopsis thaliana), where gs increased in the subjective morning and decreased in the subjective afternoon, while A increased at subjective midday and stayed high through the subjective afternoon. Additionally, peaks in A, gs, and Wi (intrinsic water use efficiency) varied in a species-specific manner, although the environment did not change. For instance, Wi peaked in the subjective early morning in Mangifera indica and in the subjective late afternoon for Phaseolus vulgaris and Lycopersicon esculentum. Consequently, a significant proportion of the diurnal variation in A and gs may be largely recreated when solely examining circadian regulation, and the amplitude of the circadian control over A is relatively smaller than over gs.
Afternoon declines in A and gs have also been investigated for long, and the response has been partly attributed to feedback inhibition from photosynthate accumulation, photorespiration, and hydraulic feedbacks, among other process. Data from Figure 1 was always collected under levels of photosynthetically active radiation that limit A (between 40 μmol m−2 s−1 in Arabidopsis and 500 μmol m−2 s−1 in cotton and bean) and well-watered conditions, where carbohydrate build-up, photorespiration, or hydraulics would have exerted a limited impact. Alternatively, circadian “gating,” whereby the effect of a stimulus depends on time of day, presents an alternative, yet much less explored, explanation for understanding afternoon declines in gas exchange. For instance, Mencuccini et al. (2000) observed how, for a given level of leaf water potential and of abscisic acid (ABA) concentrations, gs was lower in the afternoon than in the morning. This is because stomatal closure is more sensitive to ABA in the afternoon than in the morning due to interactions between the clock component TIMING OF CAB EXPRESSION1 and ABA-related genes (Seo and Mas, 2015). There are additional gated responses in stomata and also additional mechanisms that could explain how circadian regulation would lead to an afternoon decline in gas exchange. A full review is not intended here, but these examples may suffice to illustrate how circadian rhythmicity may help to better understand afternoon declines in A and gs.
It is also poorly understood how circadian effects over diurnal gas exchange scale up to affect canopies and ecosystems and whether inclusion in models would provide significant increases in model performance. Recent studies have quantified that circadian regulation in gas exchange was responsible for 30% and 70% of the diurnal oscillations in A and gs, respectively, in bean and cotton at canopy scales (Resco de Dios et al., 2016). But the generality of these results across phylogenies and ecosystem types needs to be more broadly examined. Similarly, there are conflicting results in the literature on the importance of including circadian regulation in models of diurnal gas exchange (Resco de Dios et al., 2016). To date, only empirical models have been derived, and to make predictions under novel environmental conditions, we will need more mechanistic approximations.
It is well documented that circadian regulation exerts a major influence over the physiology and behavior of mammals, but unlike plants, where circadian regulation drives temporal expression in approximately 30% of the genome, only approximately 10% of the mammal genome is clock regulated. Paradoxically, circadian effects in the field have been examined to a much larger extent in mammals than in plants. The potential role of circadian regulation within ecological or agricultural settings is often not considered, and this may lead to incomplete descriptions of the processes regulating diurnal gas exchange. Testing circadian regulation in the field is challenging because its expression is often masked by direct physiological responses to environmental changes. However, the use of statistical and modeling techniques or of advanced experimental facilities could help overcome this problem (Resco de Dios et al., 2016). Indeed, evidence is building up that circadian regulation is an important driver of gas exchange in canopies, with potential effects on current approaches for scaling and modeling plant-atmosphere interactions (Resco de Dios et al., 2017). It is time to explicitly and broadly evaluate the role of circadian regulation as a driver of diurnal gas exchange in the field, across phylogenies and scales.
References
- Allen DJ, Ratner K, Giller YE, Gussakovsky EE, Shahak Y, Ort DR (2000) An overnight chill induces a delayed inhibition of photosynthesis at midday in mango (Mangifera indica L.). J Exp Bot 51: 1893–1902 [DOI] [PubMed] [Google Scholar]
- Corlett JE, Wilkinson S, Thompson AJ (1998) Diurnal control of the drought-inducible putative histone H1 gene in tomato (Lycopersicon esculentum Mill. L.). J Exp Bot 49: 945–952 [Google Scholar]
- Dodd AN, Parkinson K, Webb AAR (2004) Independent circadian regulation of assimilation and stomatal conductance in the ztl-1 mutant of Arabidopsis. New Phytol 162: 63–70 [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kévei E, Tóth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309: 630–633 [DOI] [PubMed] [Google Scholar]
- Matthews JSA, Vialet-Chabrand SRM, Lawson T (2017) Diurnal variation in gas exchange: the balance between carbon fixation and water loss. Plant Physiol 174: 614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencuccini M, Mambelli S, Comstock J (2000) Stomatal responsiveness to leaf water status in common bean (Phaseolus vulgaris L.) is a function of time of day. Plant Cell Environ 23: 1109–1118 [Google Scholar]
- Resco de Dios V, Gessler A, Ferrio JP, Alday JG, Bahn M, del Castillo J, Devidal S, García-Muñoz S, Kayler Z, Landais D, et al. (2016) Circadian rhythms have significant effects on leaf-to-canopy scale gas exchange under field conditions. Gigascience 5: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resco de Dios V, Gessler A, Ferrio JP, Alday JG, Bahn M, del Castillo J, Devidal S, García-Muñoz S, Kayler Z, Landais D, et al. (2017) Circadian rhythms regulate the environmental responses of net CO2 exchange in bean and cotton canopies. Agric For Meteorol 239: 185–191 [Google Scholar]
- Seo PJ, Mas P (2015) STRESSing the role of the plant circadian clock. Trends Plant Sci 20: 230–237 [DOI] [PubMed] [Google Scholar]
- Yakir E, Hassidim M, Melamed-Book N, Hilman D, Kron I, Green RM (2011) Cell autonomous and cell-type specific circadian rhythms in Arabidopsis. Plant J 68: 520–531 [DOI] [PubMed] [Google Scholar]