A heat-induced metabolic response independent of the genetically programmed heat-shock response increases heat resistance of Arabidopsis seedlings.
Abstract
High temperatures rapidly induce a genetically programmed heat-shock response (HSR) that is essential to establish short-term acquired thermotolerance. In addition, an immediate HSR-independent metabolic response is triggered, resulting in an accumulation of unsaturated triacylglycerols (TAGs) in the cytosol. The metabolic processes involved in heat-induced TAG formation in plants and their physiological significance remain to be clarified. Lipidomic analyses of Arabidopsis (Arabidopsis thaliana) seedlings indicated that during heat stress, polyunsaturated fatty acids from thylakoid galactolipids are incorporated into cytosolic TAGs. In addition, rapid conversion of plastidic monogalactosyl diacylglycerols (MGDGs) into oligogalactolipids, acylated MGDGs, and diacylglycerols (DAGs), the direct precursor of TAGs, was observed. For TAG synthesis, DAG requires a fatty acid from the acyl-CoA pool or phosphatidylcholine. Since seedlings deficient in PHOSPHOLIPID:DIACYLGLYCEROL ACYLTRANSFERASE1 (PDAT1) were unable to accumulate TAGs after heat stress, phosphatidylcholine appears to be the major fatty acid donor. Results suggest that rapid plastid lipid metabolism drives TAG accumulation during heat stress. PDAT1-mediated TAG accumulation was found to increase heat resistance, since nonacclimated pdat1 mutant seedlings were more sensitive to severe heat stress, as indicated by a more dramatic decline of the maximum efficiency of PSII and lower seedling survival compared to wild-type seedlings. In contrast, nonacclimated trigalactosyldiacylglycerol1 (tgd1) mutants overaccumulating TAGs and oligogalactolipids were more resistant to heat stress. Hence, thylakoid lipid metabolism and TAG formation increases thermotolerance in addition to the genetically encoded HSR.
Heat stress can have an adverse impact on almost all aspects of plant development, growth, reproduction, and yield (Mittler et al., 2012). As a consequence of global warming, the frequency and amplitude of heat episodes are expected to increase. Therefore, basal and acquired plant thermotolerance mechanisms will become increasingly more important to tolerate transient heat stress episodes in both crop and wild plants. Typically, plants experience a gradual temperature increase during the day, with a transient maximum during the late afternoon. While Arabidopsis (Arabidopsis thaliana) seedlings survive at moderately elevated temperatures of 32°C to 38°C for several days, they become rapidly damaged at temperatures above 40°C (Yeh et al., 2012). Within minutes after exposure to temperatures of 32°C to 38°C, a genetically highly conserved heat-shock response (HSR) is triggered in Arabidopsis via the four master regulators of the heat shock factor A1 family (HSFA1). The HSR is characterized by induction of a battery of heat-shock proteins (HSPs) primarily functioning as molecular chaperones important to maintain proteins in the correctly folded state as well as antioxidant enzymes and few metabolic enzymes (Liu et al., 2011). After induction of the HSR, Arabidopsis seedlings survive temperatures as high as 45°C for more than 2 h, a condition that is lethal for nonacclimated seedlings. This phenomenon is termed short-term acquired thermotolerance (SAT), while the ability to survive high temperatures without preacclimation is termed basal thermotolerance (Yeh et al., 2012). In response to heat, lipidomic and molecular analysis of Arabidopsis seedlings recently revealed a HSFA1/HSR-independent metabolic response characterized by a strong accumulation of triacylglycerols (TAGs). TAG accumulation in Arabidopsis seedlings was induced—similar to the HSR—at temperatures above 32°C and reached a maximum between 38°C and 42°C (Mueller et al., 2015). After return to normal growth temperatures, accumulated TAGs were rapidly degraded (Higashi et al., 2015; Mueller et al., 2015). However, the biochemical pathways leading to heat-induced TAG accumulation as well as the function of this metabolic response are not known.
Generally, the most important pathway in TAG synthesis is the Kennedy pathway, involving sequential acylation of glycerol 3-phosphate using fatty acyl residues from the acyl-CoA pool by glycerol 3-phosphate acyltransferase and lysophosphatidic acid acyltransferase to phosphatidic acid (PA), which after dephosphorylation yields diacylglycerol (DAG; for review, see Bates, 2016). The last step in TAG synthesis involves acylation of DAG either by diacylglycerol acyltransferase (DGAT) or phospholipid:diacylglycerol acyltransferase (PDAT), which use acyl-CoAs or phosphatidylcholines (PCs) as acyl donors, respectively. Three different classes of DGAT isozymes have been identified in Arabidopsis, comprising DGAT1, DGAT2, and a soluble DGAT3 (Routaboul et al., 1999; Saha et al., 2006; Shockey et al., 2006; Hernández et al., 2012), from which DGAT1 appears to be most relevant for TAG biosynthesis in developing and senescent leaves of Arabidopsis (Slocombe et al., 2009). Alternatively, DAGs can be acylated using PCs as acyl donors by PDAT (Dahlqvist et al., 2000). The endoplasmic reticulum (ER)-localized enzyme PDAT directly transfers an acyl group from the sn-2 position of PC to the sn-3 hydroxyl of DAG, producing TAG and lysophosphatidylcholine that can be reacylated by acyl-CoA:lysophophatidylcholine acyltransferase (Zhang et al., 2009; Xu et al., 2012). DGAT1 and PDAT1 have both been shown to be involved in TAG synthesis in young Arabidopsis leaves (Fan et al., 2013a; Tjellström et al., 2015).
Apart from de novo synthesis, DAGs can also be derived from PCs or monogalactosyl diacylglycerols (MGDGs). PC-derived DAG production can be catalyzed by cytidine-5′-diphosphocholine:diacylglycerol cholinephosphotransferase (CPT) under conditions of de novo synthesis of PC, by phospholipase C through cleavage of the phosphocholine headgroup, or by the combined action of phospholipase D and phosphatidic acid phosphohydrolase (PAH; for review, see Bates, 2016). Disruption of two PAHs, PAH1 and PAH2, has been shown to result in substantial decrease in TAG content in Arabidopsis leaves (Fan et al., 2014). In addition, the phosphatidylcholine:diacylglycerol cholinephosphotransferase (PDCT) enzyme (encoded by the REDUCED OLEATE DESATURATION1, ROD1, gene) plays an important role in catalyzing the exchange of the DAG moiety between DAG and PC in Arabidopsis seeds. Hence, CPT and PDCT may equilibrate the mainly saturated DAG pool originating from de novo synthesis and the predominately highly unsaturated DAG pool derived from lipid remodeling within PCs (Lu et al., 2009).
Under different stress conditions involving dehydration, such as freezing, drought, and salt stress, a highly unsaturated pool of DAGs for extraplastidic TAG production can be produced by remodeling MGDGs in plastids (Moellering et al., 2010; Mueller et al., 2015; Wang et al., 2016). During freezing stress, lipid remodeling initiated by posttranslational activation of a galactolipid:galactolipid galactosyltransferase takes place at the outer chloroplast membrane. The enzyme is encoded by the SENSITIVE TO FREEZING2 (SFR2) gene that transfers galactosyl residues from the abundant MGDG to different galactolipid acceptors, thereby producing oligogalactolipids and DAGs (Moellering et al., 2010). Using plant lines deficient in SFR2, it has been shown that MGDG remodeling at the outer chloroplast membrane is required for freezing tolerance in Arabidopsis (Moellering et al., 2010) and aides in resilience to salt and drought stress in tomato (Wang et al., 2016). The non-bilayer-forming lipid DAG is synthesized as a byproduct of lipid remodeling and converted to cytosolic TAGs. Wounding, heat, freezing, and other stresses associated with membrane damage have also been shown to activate an acyltransferase, the ACYLATED GALACTOLIPID ASSOCIATED PHOSPHOLIPASE1 (AGAP1), which transfers a fatty acid from MGDG to Gal residues in galactolipids, thereby producing acyl-MGDG and lyso-MGDG (Nilsson et al., 2015).
However, it remains to be clarified by which mechanisms TAGs are produced under heat-stress conditions and if lipid metabolism contributes to basal or acquired thermotolerance in plants. To address these questions, we used a lipidomic approach to study TAG synthesis in transgenic Arabidopsis lines deficient in DGAT1, PDAT1, PDCT, PAH1/PAH2 or different fatty acid desaturase (FAD) genes. Analyses revealed that under heat-stress conditions, MGDG remodeling contributes to DAG formation required for TAG synthesis, while PDCT and PAH1/PAH2 appear not to be required for heat-induced DAG and TAG formation. PDAT, transferring a fatty acyl moiety from PC to DAG, was shown to be essential for heat-induced TAG accumulation. Finally, we show that pdat1 mutant lines unable to accumulate TAGs under heat-stress conditions display normal short-term acquired thermotolerance but are compromised in basal thermotolerance.
RESULTS
Heat-Induced TAG Accumulation Requires PDAT1 Activity
In order to study the relevance of TAG accumulation for thermotolerance, we first aimed to identify transgenic Arabidopsis lines deficient in heat-induced TAG accumulation as well as lines with constitutively high TAG levels. We focused on potential enzymes involved in the last step of TAG synthesis that is the acylation of DAGs by DGAT and/or PDAT enzymes. After a heat shock (45°C for 90 min), we compared TAG levels of wild type and the transgenic dgat1-1 (Zou et al., 1999) as well as pdat1-2 (Zhang et al., 2009) and pdat1-3 (SALK_032261C) lines. In addition, we analyzed the Arabidopsis tgd1-1 line that displays a point mutation in a permease-like component of an ABC transporter complex and is defective in the transport of ER-derived lipid precursors into plastids (Xu et al., 2003). The tgd1-1 mutation causes an accumulation of triacylglycerols, oligogalactolipids, and phosphatidic acids (Xu et al., 2005).
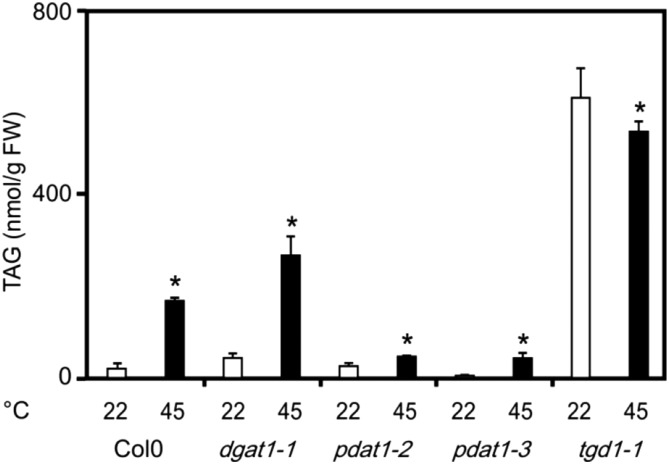
As shown in Figure 1, basal levels of the four most abundant TAGs (displaying the acyl combination 54:9, 54:8, 54:7, and 54:6) were comparable in wild type, dgat1, and pdat1 seedlings, while TAG levels were constitutively high in the tgd1 line (30-fold higher levels when compared with the wild type). After a heat shock at 45°C for 90 min, wild type and dgat1 TAG levels increased 8- and 9-fold, respectively. In contrast, there was only a minimal (1.8-fold) increase in the pdat1 lines reaching only 43 to 46 nmol g−1 FW, while the wild type displayed 170 nmol g−1 FW. In the tgd1 line, no further increase of TAG levels could be observed, but TAG levels were still 3-fold higher than the heat-induced levels in the wild type. Experiments suggest that heat-induced TAG accumulation requires PDAT1 activity and, thus, PCs are the acyl donors for acylation of DAGs to TAGs.
Figure 1.
Heat-induced TAG accumulation in wild-type, dgat1, pdat1, and tgd1 Arabidopsis seedlings. Levels of the four most abundant TAGs with the acyl composition 54:9, 54:8, 54:7, and 54:6 were determined in 2-week-old dgat1-1, pdat1-2, pdat1-3, and tgd1-1 seedlings kept at 22°C (white bars) or 45°C for 90 min (black bars). Data represent means ± sd, n = 4. Asterisks denote statistically significant differences (P < 0.05) from 22°C. FW, Fresh weight.
In the Kennedy pathway of de novo TAG synthesis, PAs produced through sequential acylation of glycerol-3-phosphate are a major source for DAG formation (Bates, 2016). PAs can be converted by PAH1 and PAH2 to DAGs, and it has been shown that disruption of PAH1 and PAH2 results in a substantial decrease in TAG content in leaves but not in seeds (Fan et al., 2014). To test the involvement of the two PAHs in heat-induced lipid metabolism, we determined PA, DAG, and TAG levels in leaves of pah1pah2 double knockout lines. However, PA and TAG levels were not different, and DAG levels were even higher compared to the wild type after a severe heat shock (Supplemental Fig. S1), suggesting that PAH1/PAH2 are not involved in heat-induced TAG formation.
Analysis of fad7/8 and fad3 Seedlings Reveals Heat-Induced Export of Polyunsaturated Fatty Acids from Chloroplasts for Cytosolic TAG Assembly
In our previous work, we could already show that levels of total fatty acids (free and esterified) did not significantly change during heat acclimation, suggesting that TAG accumulation is not driven by massive de novo fatty acid synthesis (Mueller et al., 2015). Alternatively, heat-induced TAG accumulation could be triggered by membrane lipid metabolism, resulting in channeling of fatty acids from structural lipids to TAGs. However, total levels of the major membrane lipid classes did not significantly change after the heat treatment (Mueller et al., 2015). Notably, heat-induced TAGs accumulate only 1% to 8% of all cellular fatty acids in leaves and, therefore, limited heat-induced degradation of abundant membrane lipids might have escaped detection.
To elucidate the origin of fatty acids used for TAG synthesis, we analyzed the fatty acid composition of neutral lipids (TAGs, and to a minor extent DAGs) in fad3 and fad7/8 mutant seedlings before and after heat treatment at 45°C. fad3 mutants are deficient in the ER-localized ω-3 desaturase, display reduced levels of linolenate (18:3) and correspondingly elevated levels of linoleate (18:2) in extraplastidic lipids (Browse et al., 1993). fad7/8 double mutants are deficient in two chloroplastic ω-3 desaturases, completely lack 16:3 and display reduced 18:3 levels while 18:2 and 16:2 levels are high in plastidic lipids compared to the wild type (McConn et al., 1994). Since some fatty-acid exchange between chloroplasts and ER occurs, loss of ω-3 desaturase activity in the ER (fad3) or plastids (fad7/8) does not lead to complete deficiency of 18:3 in plastids or the ER, respectively.
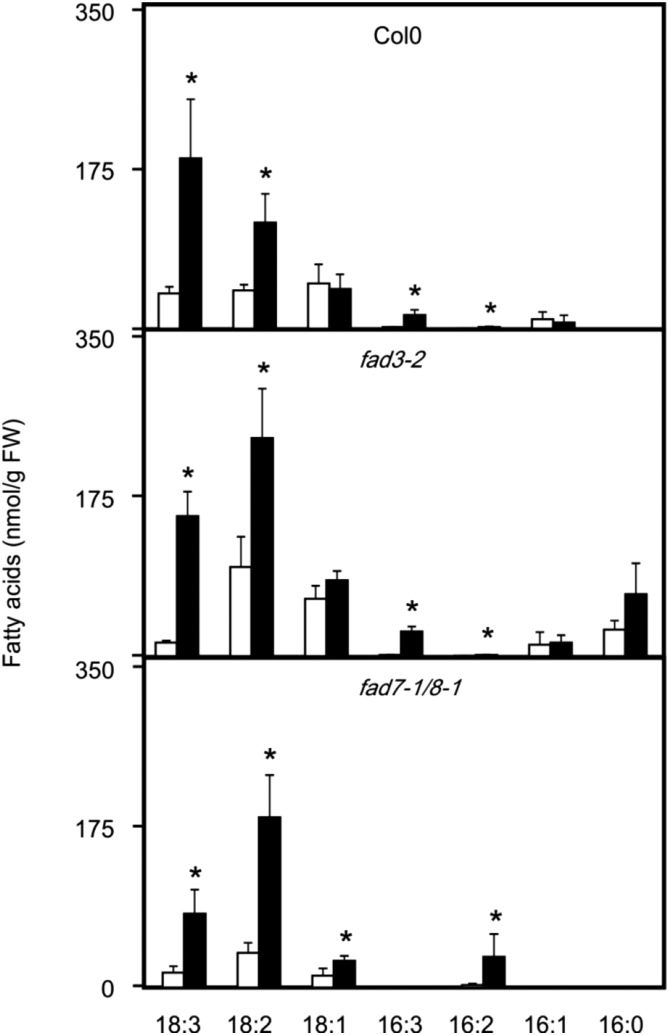
After lipid extraction of wild-type and mutant seedlings, lipid classes were separated by solid-phase extraction. Neutral lipids comprising predominately TAGs (and to a lesser extent DAGs) were isolated and hydrolyzed, and the fatty acid composition was determined (Fig. 2). Under basal conditions (22°C), fatty-acid analysis of fad3 mutants revealed more than two times higher 18:2 levels and very low levels of 18:3 in neutral lipids compared to the wild type, while in fad7/8 seedlings, 18:2 levels were only slightly higher and 18:3 levels slightly lower. This suggests that ER- and plastid-derived fatty acids both contribute to basal TAG synthesis at 22°C.
Figure 2.
Fatty acid levels in neutral lipids in wild-type, fad7/8, and fad3 seedlings. Seedlings were kept at 22°C (white bars) or treated with a heat shock (45°C for 90 min, black bars). Thereafter, neutral lipids were separated and hydrolyzed, and fatty acids were determined. Data represent means ± sd, n = 4. Asterisks denote statistically significant differences (P < 0.05) from 22°C. FW, Fresh weight.
After heat treatment (45°C, 90 min), 18:2 levels in neutral lipids increased 2- to 3-fold in fad3 and wild-type seedlings, while fad7/8 seedlings displayed a 5-fold increase of 18:2. Moreover, after heat stress, 18:3 levels in neutral lipids were similar in fad3 and wild-type plants, while fad7/8 seedlings displayed strongly reduced 18:3 levels. Most strikingly, no accumulation of 16:3 in fad7/8 seedlings could be detected, while we observed a 9- to 17-fold increase in fad3 and wild-type plants, respectively. These analyses suggest that under heat stress, polyunsaturated fatty acids are delivered to a major part from plastids for assembly of TAGs. However, fatty acids desaturated in PCs also appear to be incorporated into heat-induced TAGs to a minor extent, as indicated by the altered fatty acid composition in fad3 seedlings.
Heat-Induced Increase of the TAG Precursors DAG and PC with the Fatty Acyl Composition 18:3/16:3 and 18:2/16:3 in Wild-Type and pdat1 Seedlings
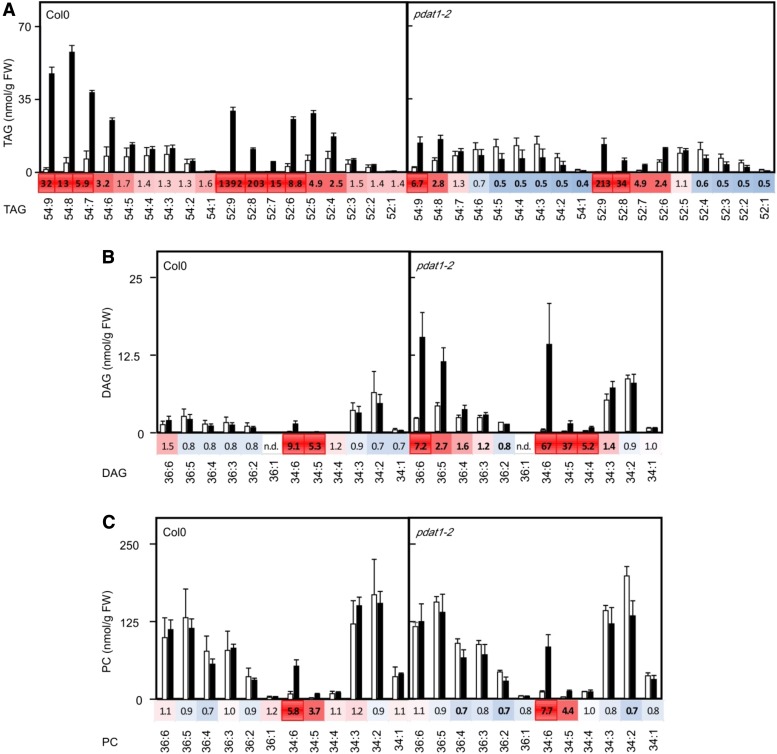
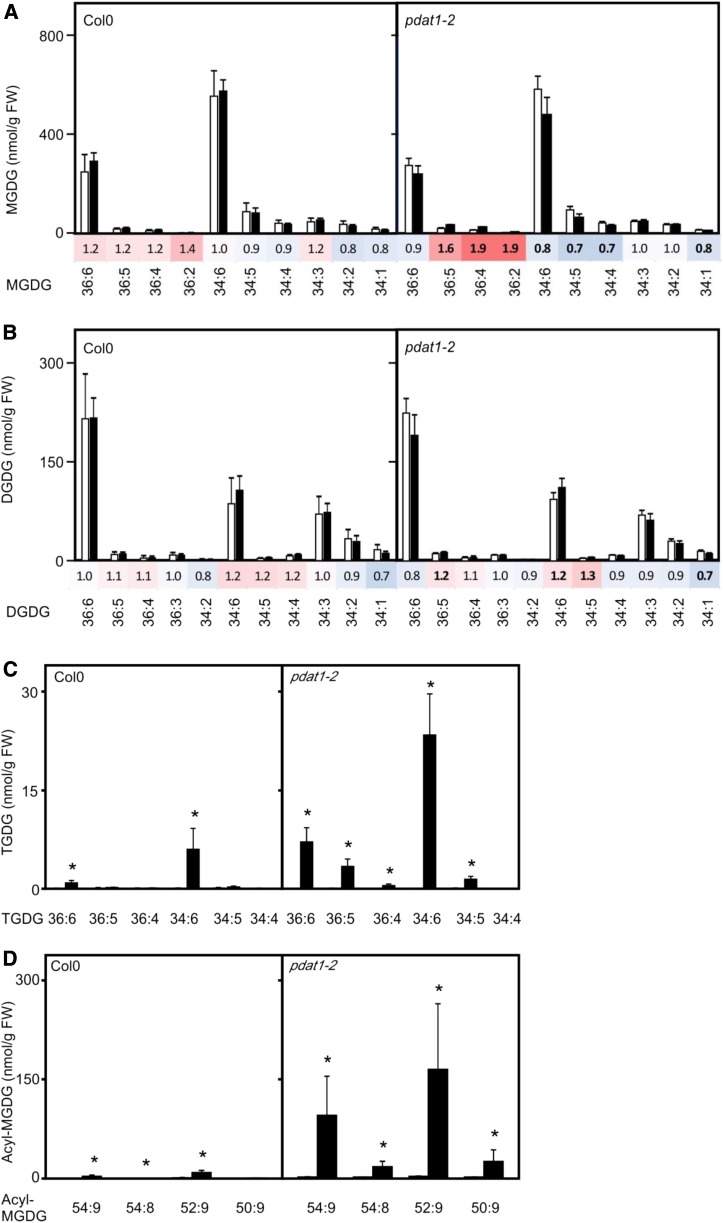
In order to study lipid remodeling in more detail, we investigated the compositions of lipid classes in 2-week-old Arabidopsis wild-type and pdat1 seedlings without (22°C) and after a heat shock (45°C, 90 min). As shown in Figure 3, highly unsaturated TAG species were most induced and abundant in wild-type seedlings, while almost no increase of TAG species was observed in pdat1 plants after the heat treatment. Interestingly, the TAG species 52:9 (total number of acyl carbons:total number of double bonds) containing two 18:3 and one 16:3 fatty acids displayed the highest induction. Under basal conditions, 16:3 is confined to plastids and occurs predominantly esterified in MGDGs and, at lower levels, in digalactosyl diacylglycerols (DGDGs) and phosphatidylglycerols (McConn et al., 1994), indicating that fatty acids are channeled from plastid lipids to TAGs. Furthermore, analysis of DAGs, the direct precursors of TAGs, revealed that DAG levels did not significantly change in heat-treated wild-type seedlings except for DAG34:6 (18:3/16:3) and DAG34:5 (18:3/16:2 and 18:2/16:3) with a 9- and 5-fold increase, respectively. In pdat1 seedlings, unable to convert DAGs to TAGs, DAG levels were higher than in the wild type. DAG species with the acyl combination 18:3/18:3 (DAG36:6), 18:3/16:3 (DAG34:6) as well as 18:3/16:2 and 18:2/16:3 (DAG34:5) most strongly accumulated with a 7-, 67-, and 37-fold increase, respectively. Since the acyl combination 18:3/18:3 occurs predominately and the acyl combination 18:3/16:3 almost exclusively in MGDGs and DGDGs, this finding suggests that heat-induced DAGs are either directly derived from these major plastid lipids or incorporate fatty acids from these lipids and also excludes the possibility that DAGs are generated through the Kennedy pathway through de novo synthesis.
Figure 3.
Heat-induced changes of lipids in wild-type and pdat1-2 seedlings. TAGs (A), DAGs (B), and PCs (C) were determined in 2-week-old seedlings kept at 22°C (white bars) or after a heat shock (45°C for 90 min, black bars). Data represent means ± sd, n = 4. The heat maps display the fold changes of lipid species (characterized by their headgroup; total number of acyl carbons:total number of double bonds) after the heat shock compared to control seedlings at 22°C. Statistical significant changes of lipid levels (P < 0.05) are indicated by bold numbers. FW, Fresh weight.
The other direct precursors of heat-induced TAGs are PCs. Similar to DAGs, the most abundant PCs did not display a significant change in both wild-type and pdat1 seedlings after the heat treatment, except for PC34:6 (18:3/16:3) and PC34:5 (18:2/16:3) with an about 6- and 4-fold increase, respectively. This indicates that the PC pool is largely kept in equilibrium during the flux of fatty acids through PCs.
The DAG Building Block for Heat-Induced TAG Synthesis Is Not Derived from PCs via PDCT
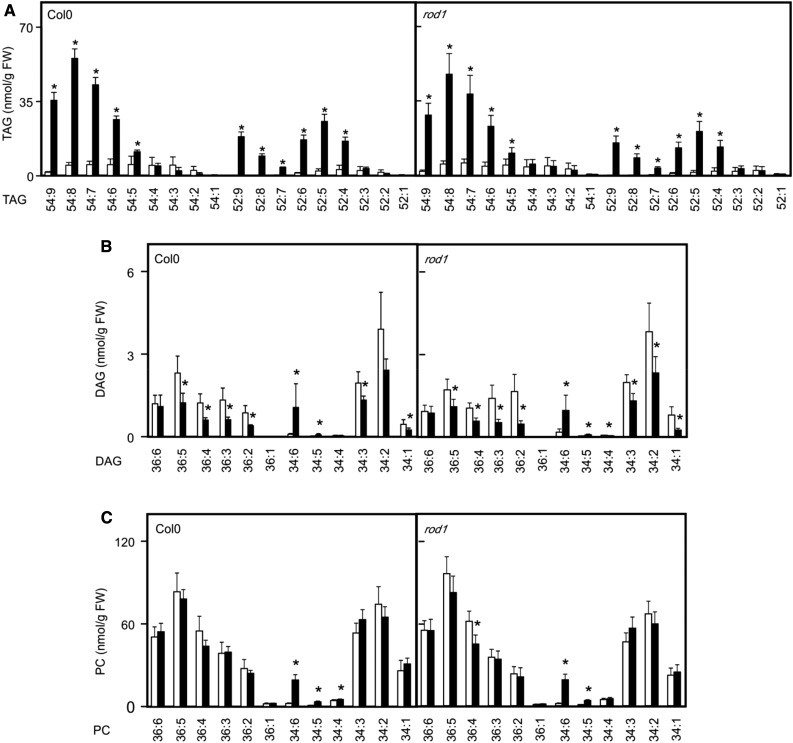
Defect of heat-induced TAG synthesis in pdat1 proves that the fatty acid at sn-3 is derived from PCs. It has long been known that the DAG moiety within PC can also be liberated from PC that serves as a major source of DAG for TAG synthesis (Bates, 2016). The PDCT enzyme (encoded by the ROD1 gene) utilizes DAG and PC as substrates and transfers the phosphocholine headgroup from PC to DAG, creating a new molecular species of PC and DAG. For TAG synthesis in seeds, PDCT appears to control the majority of acyl fluxes through PC (Bates et al., 2012). When inspecting rod1 seedlings, heat-induced TAG levels were similar to the wild type, as were the acyl compositions of TAG, DAG, and PC lipid species after heat exposure (Fig. 4). Alternatively, the CPT could also equilibrate the DAG moiety between the PC and DAG pools, or DAG could be produced from PCs by phospholipases (Bates, 2016). Another possibility is that the DAG moiety is directly generated through degradation of galactolipids.
Figure 4.
Heat-induced changes of lipids in wild-type and rod1 seedlings. TAGs (A), DAGs (B), and PCs (C) were determined in 2-week-old seedlings kept at 22°C (white bars) or after a heat shock (45°C for 90 min, black bars). Data represent means ± sd, n = 5. Asterisks denote statistically significant differences (P < 0.05) from 22°C. FW, Fresh weight.
Heat-Induced MGDG Remodeling by SFR2 and AGAP1
MGDGs display an acyl composition that resembles the acyl composition of heat-induced DAGs in pdat1 seedlings and therefore could represent a direct source of DAG for TAG synthesis. During freezing stress, MGDG remodeling initiated by posttranslational activation of SFR2 results in the transfer of galactosyl residues from MGDG to galactolipids, thereby producing on the one hand DGDGs, trigalactodiacylglycerols (TGDGs) and higher oligogalactosyldiacylglycerols as well as DAGs that can serve as direct precursors for TAG synthesis (Moellering et al., 2010). We observed an accumulation of TGDGs, indicating that severe heat stress (45°C, 90 min) activates SFR2 (Fig. 5). Notably, heat-induced increases of TGDGs (Fig. 5C) and DAGs (Fig. 3B) were much more pronounced in pdat1 seedlings unable to convert DAGs into TAGs.
Figure 5.
Heat-induced changes of TGDGs and acyl-MGDGs in wild-type and pdat1-2 seedlings. Levels of MGDGs (A), DGDGs (B), TGDGs (C), and acyl-MGDGs (D) were very low in 2-week-old seedlings kept at 22°C (white bars) and after a heat shock (45°C for 90 min, black bars). Data represent means ± sd, n = 4. Heat maps (A and B) display the fold changes of lipid species after the heat shock compared to control seedlings at 22°C; statistical significant changes of lipid levels (P < 0.05) are indicated by bold numbers. Asterisks in C and D denote statistically significant differences (P < 0.05) from 22°C. FW, Fresh weight.
In addition to SFR2, we observed a heat-induced accumulation of acyl-MGDGs that can be produced by AGAP1 that converts MGDGs to acyl-MGDGs and lyso-MGDGs. Acyl-MGDGs strongly increased after a 45°C heat shock in wild-type and, even more dramatically, in pdat1 seedlings (Fig. 5D) while accumulation of lyso-MGDGs could not be detected, possibly due to rapid turnover of lyso-MGDGs.
Since the overall accumulation of SFR2 and AGAP1 products is low compared to the accumulation of TAGs (Supplemental Fig. S3), results suggest that the contribution of precursors for TAG synthesis through these enzymes is small. We also observed that heat acclimation at 37°C (2 h) strongly increased TAG levels, although SFR2 and AGAP1 were not activated at this temperature (Supplemental Fig. S3). Notably, heat-induced MGDG turnover leading to TGDG and acyl-MGDG accumulation is not compromised in pdat1 seedlings unable to accumulate TAGs. We actually observed higher accumulation of oligogalactolipids and acyl-MGDGs in pdat1 seedlings.
PDAT1-Mediated TAG Accumulation Augments Basal Thermotolerance
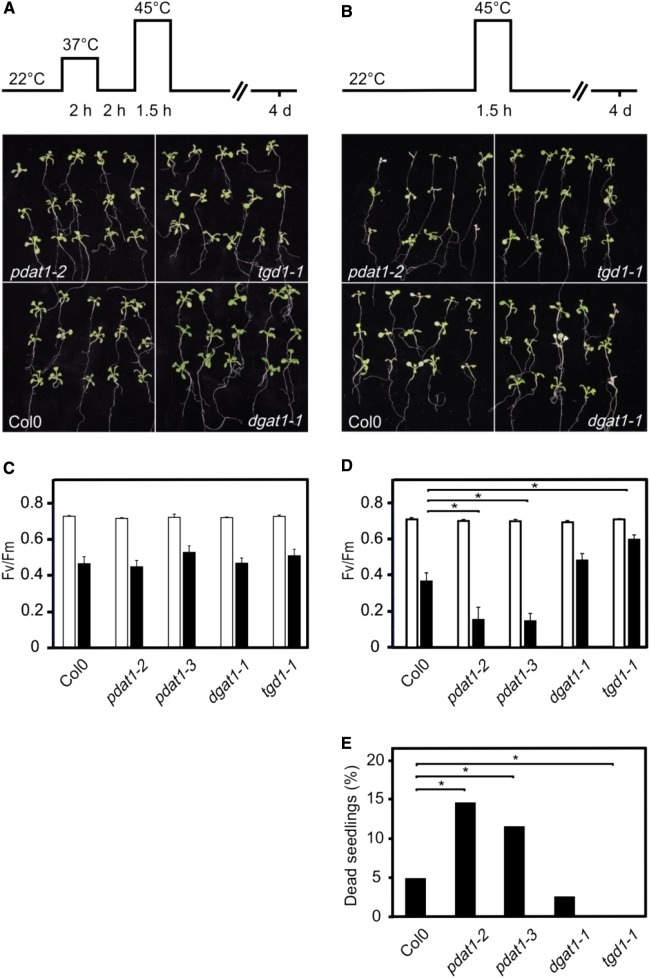
Pdat1 deficiency and lack of TAG accumulation could be associated with higher heat-stress sensitivity. To test this hypothesis, we investigated the acquired and basal thermotolerance of wild-type, pdat1, dgat1, and tgd1 seedlings. All genotypes were fully competent to acquire thermotolerance and survived a 45°C (90 min) heat shock after acclimation at 37°C (Fig. 6A). In contrast, when testing basal thermotolerance by treating nonacclimated seedlings with a 45°C heat shock, we observed significantly lower thermotolerance in both pdat1 lines and a higher thermotolerance of the TAG overaccumulating tgd1 line compared to the wild-type and the dgat1 line (Fig. 6, D and E).
Figure 6.
Thermotolerance of wild type, pdat1, dgat1, and tgd1 Arabidopsis seedlings after heat treatment. Seedlings (100 seeds/plate) grown at 22°C were exposed to 45°C with (A) or without (B) prior heat acclimation for the times indicated, and representative pictures were taken 4 d after recovery at 22°. During heat stress at 45°C, roots were cooled below 40°C. While all heat-acclimated seedlings survived the 45°C heat shock (A), survival of nonacclimated mutant lines differed (B and E). Survival rate was determined from four independent experiments (E); statistical analysis was performed on seedlings from all experiments using the χ2 test with n ≥ 85 and P < 0.05. The effect of high temperatures on the photosynthesis of acclimated (C) and nonacclimated (D) seedlings was determined in leaves that were clipped off the plants after the heat shock at 45°C (1.5 h) and a 1 h recovery period at 22°C. During heat stress at 45°C, roots were cooled below 40°C. Fv/Fm was measured in control (grown at 22°C, white bars) and heat-treated, dark-adapted leaves (black bars). Data represent means ± se, n = 10, P ≤ 0.02.
Photosynthesis is one of the most heat-sensitive functions in plant cells, rapidly declines in response to heat stress above 25°C to 28°C, and is severely damaged above 35°C to 40°C (Routaboul et al., 2012). To assess the decline of photosynthetic quantum efficiency, we determined the maximal quantum yield (Fv/Fm) of PSII photochemistry, the primary site of heat damage in the photosynthetic apparatus (Routaboul et al., 2012). This parameter describes the efficiency of electron transport inside PSII and has been shown to be linearly correlated with the quantum yield of light-limited O2 evolution and with the proportion of functional PSII reaction centers. In acclimated seedlings, we observed a similar drop of Fv/Fm in the wild-type and all transgenic lines after a 45°C heat shock for 90 min followed by a 1 h recovery period at 22°C (Fig. 6C). In nonacclimated seedlings that received the same 45°C heat shock, photosynthetic efficiency dropped stronger in pdat1 lines compared to the wild-type and dgat1 seedlings, while the tgd1 line displayed higher photosynthetic efficiency (Fig. 6D). Hence, the extent of PSII damage 1 h after the heat shock correlates well with seedling survival determined 4 d after the heat shock. Results suggest that TAGs incorporate products derived from lipid metabolism such as DAGs and fatty acids (via PCs), thereby protecting the photosynthetic apparatus and increasing thermotolerance.
DISCUSSION
In Arabidopsis, heat stress above 32°C triggers accumulation of TAGs in cytosolic lipid droplets within a few minutes (Mueller et al., 2015). The expression of many genes involved in de novo fatty acid and prokaryotic glycerolipid synthesis were shown to be repressed by heat (Higashi et al., 2015), and levels of total fatty acids (determined after total lipid hydrolysis) did not significantly change during heat stress, suggesting that fatty acids are channeled from membrane lipids to TAGs. TAG accumulation has been shown to be fully reversible after return to normal growth temperatures, and hence, TAGs appear to serve as a transient store for fatty acids (Mueller et al., 2015). In addition to lipid remodeling, temperatures above 32°C also trigger a genetically programmed HSR (Yoshida et al., 2011) that is characterized by rapid accumulation of HSPs, antioxidative enzymes, as well as galactinol synthase leading to synthesis of raffinose with similar kinetics as synthesis of TAGs (Mueller et al., 2015). After heat acclimation, SAT enables plants to survive a heat shock that is lethal for nonacclimated plants. While the importance of HSPs for thermotolerance has been well demonstrated, a function of raffinose in augmenting thermotolerance could not be shown (Panikulangara et al., 2004). Unlike heat-induced synthesis of raffinose, heat-induced accumulation of TAGs is not regulated through HSFA1 and, therefore, is a metabolic response independent of the HSR (Mueller et al., 2015). The relevance of heat-induced TAG synthesis for heat resistance is not known.
To study the functional significance of TAG synthesis, we searched for mutants that lacked the capacity for heat-induced TAG synthesis. In Arabidopsis, the final step of TAG assembly, the acylation of DAG to yield TAG, is catalyzed by DGAT and PDAT at normal growth temperatures (Fig. 1). However, under heat-stress conditions, we show that the transfer of a fatty acid from PC to DAG catalyzed by PDAT1 is essential for TAG assembly (Figs. 1 and 3). In wild-type seedlings, the direct precursors of TAGs, PCs and DAGs, do not overaccumulate except for little but significant accumulation of PCs and DAGs with the acyl combination 18:3/16:3 (34:6) and 18:2/16:3 (34:5) under heat stress (Fig. 3), indicating that fatty acid flux in and out of these precursors is largely in steady state. In pdat1 seedlings, fatty acid flux is disturbed, resulting in DAG but not PC accumulation. The acyl composition of DAGs largely resembles the acyl composition of PCs, indicating that not only the fatty acids required for TAG synthesis but also the DAG precursor could be derived from PCs. However, we noted that in pdat1 seedlings, the most abundant and most strongly induced DAGs displayed the acyl combination 18:3/18:3 and 18:3/16:3, which are predominantly found in MGDGs (Fig. 3).
The presence of 16:3 in both PCs and DAGs reveals that fatty acids originating from MGDGs are used for TAG synthesis. In addition, analysis of desaturase mutants indicates that the majority of fatty acids originate from MGDGs (Fig. 2). We did, however, not observe a strong decline in the MGDG/DGDG content or change in species composition during acute heat stress (Fig. 5), which might have escaped detection due to the small pool size of heat-induced TAGs relative to the large pool size of MGDGs that potentially lies within the error of detection.
Lipidomic analyses revealed a strong and statistically significant heat-induced accumulation of MGDG metabolites (Fig. 5), indicating that partial lipid remodeling of MGDGs is catalyzed by at least two enzymes: AGAP1 and SFR2. AGAP1 occurs ubiquitously in plants and produces acyl-MGDGs and lyso-MGDGs at the expense of two MGDG molecules (Nilsson et al., 2015). In contrast to acyl-MGDGs, lyso-MGDGs do not accumulate, suggesting that lyso-MGDGs are either rapidly reacylated to MGDGs or hydrolyzed, thereby delivering fatty acids that potentially could enter TAGs via the PCs. The AGAP1 gene is highly expressed in green tissues, and several abiotic stresses such as salt, osmotic and heat stress, as well as phosphorous deficiency induced expression of the gene. Acyl-MGDGs were found to accumulate under conditions where tissue disruption occurs, such as after wounding, freeze-thawing, or microbe-induced hypersensitive cell death (Nilsson et al., 2015). Hence, AGAP1 might be activated through severe heat stress associated with cell damage. The other MGDG remodeling enzyme that is activated by severe heat stress is SFR2. The SFR2 protein occurs ubiquitously in all plant tissues, is expressed constitutively, and appears not to be transcriptionally upregulated by various stresses. Similar to AGAP1, SFR2 is associated with the outer chloroplastic membrane, where it is posttranslationally activated by severe stresses associated with cell damage and membrane leakage (Barnes et al., 2016). SFR2 produces oligogalactolipids, which remain in the plastid membrane, and DAGs, which can be used for TAG synthesis (Moellering and Benning, 2011). Although TAGs accumulate already at 37°C, SFR2 and AGAP1 were not activated at this temperature (Supplemental Fig. S3). This indicates that heat-induced TAG formation is to a major part not dependent on SFR2/AGAP1-mediated MGDG remodeling. Apparently, cell damage occurring at 45°C, both in acclimated and nonacclimated cells, activates SFR2 and AGAP1. The observed higher accumulation of SFR2/AGAP1 products (Fig. 5, C and D) in pdat1 mutants compared to the wild type may reflect the higher degree of heat damage in the mutant line.
To test the functional significance of heat-induced TAG synthesis, we checked if pdat1 seedlings can acquire SAT. After acclimation of pdat1 seedlings at 37°C, mutants survived a heat shock at 45°C for 90 min like the wild type (Fig. 6A), suggesting that the genetically encoded HSR is sufficient to acquire thermotolerance and TAG accumulation is dispensable under these conditions. However, basal thermotolerance (45°C for 90 min without prior acclimation) was found to be compromised in PDAT1-deficient seedlings while DGAT1-deficient mutants displayed wild type-like thermotolerance. Moreover, the survival rate of tgd1-1 mutant seedlings that overaccumulate TAGs was higher after the heat shock when compared with the wild type (Fig. 6, B and D). Notably, SFR2 has been shown to be constitutively activated in the tgd1-1 mutant, leading to high accumulation of oligogalactosyl diacylglycerols in addition to TAGs. In sfr2-3 tgd1-1 double knockout mutants, TAG content was about 27% lower compared with tgd1-1, indicating that SFR2 contributes to TAG synthesis by providing DAGs (Fan et al., 2014). Beside the survival rate, we also determined the immediate effect of the 45°C heat shock on photosynthetic performance. We observed that damage of PSII was greater in pdat1 seedlings and lower in tgd1-1 seedlings compared to the wild type already 1 h after the heat shock (Fig. 6D). Hence, lipid metabolism associated with TAG accumulation is beneficial for nonacclimated seedlings exposed to heat stress that leads to severe damages.
Why are AGAP1 and SFR2 activated by severe heat stress? Both enzymes catalyze a net removal of glycerolipids from plastid membranes, which reduces the total plastid surface area and thereby accommodates shrinkage of the organelle due to loss of water. However, only a small portion of MGDG is present in the outer chloroplast leaflet, and the total MGDG pool was only slightly and not significantly decreased after heat stress (Fig. 5). This suggests that MGDG consumption per se is not crucial for thermotolerance.
It is tempting to speculate that SFR2 may be important for heat protection since the tgd1 mutant, characterized by constitutive high oligogalactosylgalactolipid and TAG levels, displays higher basal thermotolerance than the wild type. Mechanistically, it has been suggested that stresses such as freezing in Arabidopsis (Moellering et al., 2010) as well as salt and drought stress in tomato (Wang et al., 2016) may result in cellular dehydration and membrane leakiness, leading to a lower cytosolic pH and an increase of the cytosolic Mg2+ concentration, which in turn activates SFR2 (Barnes et al., 2016). Activation of SFR2 by severe heat stress might involve cellular dehydration, thereby triggering a similar activation mechanism. Under conditions of severe dehydration, the formation of nonbilayer lipid structures, such as inverted hexagonal II(HII)-type structures can occur and stabilization of the lamellar membrane systems within dehydrated cells might be key for survival (Moellering et al., 2010). SFR2 converts MGDGs to oligogalactolipids that show a lower propensity for transition to HII phase structures. In addition, the polar surface area of oligogalactolipids is larger, thereby preventing fusion of apposed bilayers (Browse, 2010; Moellering and Benning, 2011). However, pdat1 seedlings display lower resistance to heat compared to the wild type (Fig. 6B) although the heat-induced oligogalactolipid content is considerably higher than in the wild type (Fig. 5A). Moreover, AGAP1 activation in parallel to SFR2 leads to accumulation of acylated galactolipids (Fig. 5B) with lipophilic surface properties, which likely increases the propensity of membrane fusions. The relevance of both enzymes for thermoprotection remains to be clarified. Yet, it cannot be excluded that heat-induced lipid remodeling by SFR2 and AGAP1 may occur accidentally as a consequence of membrane damage, leading to the formation of amphiphilic DAGs and fatty acids.
The immediate and greater damage of PSII in pdat1 seedlings compared to the wild type can potentially be attributed to a toxic accumulation of DAGs (Fig. 3) or other lipid metabolic intermediates such as fatty acids in pdat1 seedlings after the heat shock. Previously, it has been shown that PDAT1 protects against fatty-acid-induced cell death (Fan et al., 2013b). Amphiphilic, non-bilayer-forming lipids such as fatty acids and DAGs insert into membranes and can disrupt membrane integrity and function. Incorporation of DAGs in membranes, in particular polyunsaturated DAGs, increases the tendency of membranes to form HII-hexagonal phases and also results in reduced packing pressure, polar group spacing, and dehydration in the lipid-water interface (Gómez-Fernández and Corbalán-García, 2007). Hence, DAG accumulation in plastid membranes is expected to decrease membrane stability and likely affects photosynthetic efficiency.
In addition, TAGs may be beneficial during the recovery from heat stress by providing fatty acids for peroxisomal β-oxidation. Since photosynthesis is markedly compromised after a 45°C heat shock (Fig. 6, C and D), TAGs may serve as a source of energy until the photosynthetic apparatus is repaired. It has been proposed that PDAT1 and the TAG lipase SUGAR-DEPENDENT1 synergistically regulate fatty acid flow from membrane lipids toward β-oxidation through a transient TAG pool (Fan et al., 2014).
Hence, TAG accumulation may be required to avoid toxic accumulation of lipid metabolic intermediates during heat stress and to serve as a transient source of energy during recovery from heat stress.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) Col-0 and the mutant lines were grown in a growth chamber under an 8 h/16 h short-day cycle at 22 °C (160 µE) for 2 weeks. Seedlings were grown on agar plates with Murashige and Skoog medium (Murashige and Skoog medium basal mixture, including MES buffer, pH 5.7; Duchefa Biochemie B.V.) containing 3% sugar and 1.2% agarose. The T-DNA insertion mutant lines tgd1-1, dgat1-1, and pdat1-2 were kindly provided by Changcheng Xu (Xu et al., 2003; Fan et al., 2013a), the double mutant pah1/pah2 by Hiroyuki Ohta (Nakamura et al., 2009), and rod1 by John Browse (Lu et al., 2009). In addition, pdat1-3 (SALK_032261C), fad3-2 (N8036), and fad7-1/8-1 (N8034) were received from the Nottingham Arabidopsis Stock Center (Browse et al., 1993; McConn et al., 1994).
Heat Treatments
For lipid analysis, 2-week-old seedlings grown on agar plates (100 seeds per plate) at 22°C were transferred to a growth chamber set at 45°C for 90 min. For lipid extraction, all seedlings grown on a plate were harvested, immediately shock frozen with liquid nitrogen, and stored at −80°C until extraction.
For survival tests, 2-week-old seedlings were grown at 22°C on agar plates (100 seeds per plate). For heat acclimation, seedlings were treated with 37°C for 2 h and then kept at 22°C for 2 h for recovery. Acclimated or nonacclimated seedlings were transferred to a growth chamber set at 45°C for 90 min. During heat stress at 45°C, roots were cooled below 40°C. After the 45°C heat shock, agar plates were grown under standard growth conditions (8 h/16 h short-day cycle at 22°C) and evaluated after 4 d.
Lipid Analysis
Seedlings (100 mg, pooled from one plate) were shock-frozen in liquid nitrogen and extracted two times with 600 µL of chloroform/methanol/water (3:2:1, v/v/v) using a ball mill at 21 Hz for 10 min (Retsch). The organic phase was separated and evaporated in a vacuum concentrator at 40°C. The residue was resuspended in 100 µL of isopropanol for analysis.
Lipids were analyzed with an Acquity Ultra Performance Liquid Chromatography system coupled to a Synapt G2 HDMS quadrupole time-of-flight hybrid mass spectrometer (all Waters). Chromatographic separation was carried out on a BEH C18 column (2.1 × 100 mm, 1.7 μm; Waters) with a linear binary solvent gradient of 30%–100% eluent B over 10 min at a flow rate of 0.3 mL min−1. Eluent A consisted of 10 mm ammonium acetate in water: acetonitrile (60:40, v/v), and eluent B consisted of 10 mm ammonium acetate in isopropanol:acetonitrile (90:10, v/v).
After chromatographic separation, lipids were detected by a mass spectrometer. The electrospray ionization (ESI) source was operated in the positive mode. The ESI capillary voltage was set to 0.8 kV and nitrogen (at 350 °C, flow rate of 800 L h−1) was used as desolvation gas. The quadrupole was operated in a wide-band RF mode, and data were acquired over the mass range of 50–1200 D. Two discrete and independent interleaved acquisition functions were automatically created. The first function collected the low-energy data where molecule ions were acquired, while the second function collected the fragments of the molecule ion (high-energy data) by using a collision energy ramp from 15 to 35 eV (MSE). MassLynx, MarkerLynx, and QuanLynx (version 4.1; all Waters) were used to acquire and process chromatograms. By using molecule ion and fragment data, the acyl combination of lipid species could be determined.
For the targeted analysis of defined lipid species, PC34:0, MGDG36:0, DAG20:0 (2.4 µg/sample), and TAG30:0 (0.24 µg/sample) were used as internal standards (IS) for each lipid class. For semiquantitative analysis, peak areas of the analytes and IS were determined in the extracted total ion chromatogram, and lipid concentrations were calculated by using a response factor of 1 for each analyte/internal standard pair.
Fatty Acid Composition Analysis of Neutral Lipids
Lipid extracts were dissolved in 80 µL of chloroform and loaded on silica solid-phase extraction glass column (500 mg Chromabond SiOH, Macherey and Nagel) equilibrated with 5 mL of methanol and 5 mL of chloroform. Neutral lipids (TAG, DAG) were eluted with 7.5 mL of chloroform and dried in a vacuum concentrator. The residue was hydrolyzed by adding 500 µL of isopropanol containing 10% potassium hydroxide (w/v) and 5 µg heptadecanoic acid (IS). The sample was incubated at 60°C for 1 h. Thereafter, the pH was adjusted to pH 6, and the sample was analyzed by ultra performance liquid chromatography-quadrupole time-of-flight hybrid mass spectrometry (Waters) in the negative ESI mode. Free fatty acids in the sample were separated on a Ethylene Bridged Hybrid (BEH) C18 column (1.7 µm, 2.1 × 100 mm; Waters) at 40 °C using an acetonitrile (0.1% formic acid) gradient from 70% to 100% within 10 min at a flow rate of 0.3 mL min−1. Operational parameters of the mass spectrometer were the same as for the analysis of complex lipids.
Measurement of Chlorophyll Fluorescence
Pulse amplitude modulation fluorometry was used to measure chlorophyll fluorescence in seedlings. Chlorophyll fluorescence was measured with a Maxi Imaging PAM chlorophyll fluorometer (Walz GmbH) using the saturation pulse method as described (Schreiber, 2004; Bonfig et al., 2006). The optimal quantum yield of PSII (Fv/Fm) was determined using the software ImagingWin V2.41a (Walz GmbH) as described (van Kooten and Snel, 1990).
Accession Numbers
The following genes referred to in the text are listed with their accession numbers: PDAT1, At5g13640; DGAT1, At2g19450; TGD1, At1g19800; AGAP1, At2g42690; SFR2, At3g06510; PAH1, At3g09560; PAH2, At5g42870; ROD1, At3g15820; FAD3, At2g29980; FAD7, At3g11170; FAD8, At5g05580.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Heat-induced changes of lipids in wild-type and pah1/pah2 seedlings.
Supplemental Figure S2. Changes of MGDG levels and species composition after a heat shock and recovery.
Supplemental Figure S3. TGDG, acyl-MGDG, and TAG levels after different heat treatments.
Acknowledgments
The authors thank Changcheng Xu for kindly providing them with the T-DNA insertion mutant lines tgd1-1, dgat1-1, and pdat1-2, Hiroyuki Ohta for providing them with pah1/pah2 seeds, and John Browse for sending them rod1 seeds. Lipid analyses were performed in the Metabolomics Core Unit of the University Würzburg. They would also like to thank Maria Lesch, Michel Mayr, Matthias Freund, and Stefan Schäbler for technical support.
Glossary
- HSR
heat-shock response
- HSP
heat-shock protein
- SAT
short-term acquired thermotolerance
Footnotes
S.P.M. was supported by a grant of the German Excellence Initiative to the Graduate School of Life Sciences, University Wuerzburg.
References
- Barnes AC, Benning C, Roston RL (2016) Chloroplast membrane remodeling during freezing stress is accompanied by cytoplasmic acidification activating SENSITIVE TO FREEZING2. Plant Physiol 171: 2140–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD. (2016) Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim Biophys Acta 1861(9 Pt B): 1214–1225 [DOI] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160: 1530–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfig KB, Schreiber U, Gabler A, Roitsch T, Berger S (2006) Infection with virulent and avirulent P. syringae strains differentially affects photosynthesis and sink metabolism in Arabidopsis leaves. Planta 225: 1–12 [DOI] [PubMed] [Google Scholar]
- Browse J. (2010) Plant science. Saving the bilayer. Science 330: 185–186 [DOI] [PubMed] [Google Scholar]
- Browse J, McConn M, James D Jr., Miquel M (1993) Mutants of Arabidopsis deficient in the synthesis of alpha-linolenate. Biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J Biol Chem 268: 16345–16351 [PubMed] [Google Scholar]
- Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97: 6487–6492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Roston R, Shanklin J, Xu C (2014) Arabidopsis lipins, PDAT1 acyltransferase, and SDP1 triacylglycerol lipase synergistically direct fatty acids toward β-oxidation, thereby maintaining membrane lipid homeostasis. Plant Cell 26: 4119–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Zhang X, Xu C (2013a) Dual role for phospholipid:diacylglycerol acyltransferase: Enhancing fatty acid synthesis and diverting fatty acids from membrane lipids to triacylglycerol in Arabidopsis leaves. Plant Cell 25: 3506–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Yan C, Xu C (2013b) Phospholipid:diacylglycerol acyltransferase-mediated triacylglycerol biosynthesis is crucial for protection against fatty acid-induced cell death in growing tissues of Arabidopsis. Plant J 76: 930–942 [DOI] [PubMed] [Google Scholar]
- Gómez-Fernández JC, Corbalán-García S (2007) Diacylglycerols, multivalent membrane modulators. Chem Phys Lipids 148: 1–25 [DOI] [PubMed] [Google Scholar]
- Hernández ML, Whitehead L, He Z, Gazda V, Gilday A, Kozhevnikova E, Vaistij FE, Larson TR, Graham IA (2012) A cytosolic acyltransferase contributes to triacylglycerol synthesis in sucrose-rescued Arabidopsis seed oil catabolism mutants. Plant Physiol 160: 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Okazaki Y, Myouga F, Shinozaki K, Saito K (2015) Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci Rep 5: 10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Liao HT, Charng YY (2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ 34: 738–751 [DOI] [PubMed] [Google Scholar]
- Lu C, Xin Z, Ren Z, Miquel M, Browse J (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA 106: 18837–18842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConn M, Hugly S, Browse J, Somerville C (1994) A mutation at the fad8 locus of Arabidopsis identifies a second chloroplast [omega]-3 desaturase. Plant Physiol 106: 1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Finka A, Goloubinoff P (2012) How do plants feel the heat? Trends Biochem Sci 37: 118–125 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: A time for remodeling. Trends Plant Sci 16: 98–107 [DOI] [PubMed] [Google Scholar]
- Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330: 226–228 [DOI] [PubMed] [Google Scholar]
- Mueller SP, Krause DM, Mueller MJ, Fekete A (2015) Accumulation of extra-chloroplastic triacylglycerols in Arabidopsis seedlings during heat acclimation. J Exp Bot 66: 4517–4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Koizumi R, Shui G, Shimojima M, Wenk MR, Ito T, Ohta H (2009) Arabidopsis lipins mediate eukaryotic pathway of lipid metabolism and cope critically with phosphate starvation. Proc Natl Acad Sci USA 106: 20978–20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson AK, Johansson ON, Fahlberg P, Kommuri M, Töpel M, Bodin LJ, Sikora P, Modarres M, Ekengren S, Nguyen CT, et al. (2015) Acylated monogalactosyl diacylglycerol: Prevalence in the plant kingdom and identification of an enzyme catalyzing galactolipid head group acylation in Arabidopsis thaliana. Plant J 84: 1152–1166 [DOI] [PubMed] [Google Scholar]
- Panikulangara TJ, Eggers-Schumacher G, Wunderlich M, Stransky H, Schöffl F (2004) Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides in Arabidopsis. Plant Physiol 136: 3148–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routaboul JM, Benning C, Bechtold N, Caboche M, Lepiniec L (1999) The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol Biochem 37: 831–840 [DOI] [PubMed] [Google Scholar]
- Routaboul JM, Skidmore C, Wallis JG, Browse J (2012) Arabidopsis mutants reveal that short- and long-term thermotolerance have different requirements for trienoic fatty acids. J Exp Bot 63: 1435–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Enugutti B, Rajakumari S, Rajasekharan R (2006) Cytosolic triacylglycerol biosynthetic pathway in oilseeds. Molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol 141: 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U. (2004) Pulse-amplitude-Modulation (PAM) fluorometry and saturation pulse method: An overview. In Papageorgiou GC, Govinjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 279–319 [Google Scholar]
- Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18: 2294–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, Gilday A, Dyer JM, Graham IA (2009) Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J 7: 694–703 [DOI] [PubMed] [Google Scholar]
- Tjellström H, Strawsine M, Ohlrogge JB (2015) Tracking synthesis and turnover of triacylglycerol in leaves. J Exp Bot 66: 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kooten O, Snel JF (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25: 147–150 [DOI] [PubMed] [Google Scholar]
- Wang K, Hersh HL, Benning C (2016) SENSITIVE TO FREEZING2 aides in resilience to salt and drought in freezing-sensitive Tomato. Plant Physiol 172: 1432–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Carlsson AS, Francis T, Zhang M, Hoffman T, Giblin ME, Taylor DC (2012) Triacylglycerol synthesis by PDAT1 in the absence of DGAT1 activity is dependent on re-acylation of LPC by LPCAT2. BMC Plant Biol 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fan J, Froehlich JE, Awai K, Benning C (2005) Mutation of the TGD1 chloroplast envelope protein affects phosphatidate metabolism in Arabidopsis. Plant Cell 17: 3094–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fan J, Riekhof W, Froehlich JE, Benning C (2003) A permease-like protein involved in ER to thylakoid lipid transfer in Arabidopsis. EMBO J 22: 2370–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Kaplinsky NJ, Hu C, Charng YY (2012) Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci 195: 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim JM, Seki M, Todaka D, et al. (2011) Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Genet Genomics 286: 321–332 [DOI] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB (2009) DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21: 3885–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19: 645–653 [DOI] [PubMed] [Google Scholar]