Abstract
CTLA-4 is a co-receptor that modulates the threshold of T cell activation and autoimmunity. We previously showed that CTLA-4 reverses the TCR-mediated stop signal needed for T cell/APC interactions [Schneider et al., Science 2006, 313: 1972]. In this study, using a different T cell system, we show that CTLA-4 expression changed the behavior of T8.1 T cells by reducing the contact time between T cell and APC, preventing re-inforced contacts, and reducing the contact area at the immunological synapse. This led to a major reduction in Ca2+ influx/mobilization and interleukin-2 production. Further, anti-CD3/CTLA-4 increased T cell motility on antibody-coated glass slides, concurrent with an abrogation of ZAP70 microcluster formation. Our findings further support a role for CTLA-4 in limiting the interaction between T cell and APC that is needed for optimal activation.
Keywords: Conjugation, CTLA-4, Intracellular calcium, ZAP70 microclusters
Introduction
CD28 and CTLA-4 binding to CD80/86 on antigen-presenting cells (APC) play central roles in determining the outcome of T cell activation [1–4]. CTLA-4 binds with higher avidities than CD28 and bivalent CTLA-4 dimers can bridge bivalent CD80 homodimers [5]. CTLA-4 deficient (Ctla-4−/−) mice show a massive lympho-proliferative disorder, increased numbers of activated T cells and autoimmune disease with organ destruction [6, 7]. Similarly, lentiviral-induced siRNA mice have shown a concurrent reduction in CTLA-4 and a more rapid onset of diabetes [8]. Co-receptor expression is needed for anergy induction [9]. In this manner, CTLA-4 can modulate the threshold for T cell activation where it has been linked to the onset of autoimmune disorders such as type-1 diabetes [10].
Several mechanisms have been proposed to account for the molecular mechanism by which CTLA-4 generates inhibitory signals. These include ectodomain competition for CD28 binding to CD80 and CD86 [11], disruption of CD28 localization at the immunological synapse (IS) [12], modulation of phosphatases PP2A and SHP-2 [13–15] and interference with lipid raft expression [16–19]. CTLA-4 engagement of CD80 and CD86 on dendritic cells can induce the release of indoleamine 2,3-dioxygenase [20, 21].
Recently, we demonstrated that anti-CTLA-4 can reverse the anti-TCR stop signal for motility, and that CTLA-4-positiveprimarycellsshowreducedcontacttimes with APC [22]. In this study, we extend this model by showing that the mere expression of CTLA-4 can markedly change the interaction between T cells and APC. Using a T cell hybridoma reactive to tetanus toxin (Ttox) peptide, we show that the expression of CTLA-4 in T cells reduced the T cell/APC dwell time, prevented the formation of an enforced contact region with APC (i.e. instead cells remained tethered) and reduced the contact area at the IS. This in turn led to a major reduction in Ca2+ influx/mobilization and IL-2 production. Further, we show for the first time that anti-CD3/CTLA-4 abrogated anti-CD3-induced ZAP70 microcluster formation, while increasing motility on antibody-coated glass slides. Our findings further support a role for CTLA-4 in limiting T cell/APC conjugation as well as preventing microcluster formation needed for T cell activation.
Results and discussion
CTLA-4 interferes with long-term T cell/APC conjugation
T8.1 cells express human CD4 and a chimeric human-mouse TCR specific for Ttox (830–843) peptide, and lack expression of CTLA-4 [23]. The TCR reacts to Ttox peptide as presented by L625.7 cells expressing the appropriate MHC (HLA-DR*1102) and CD80/86. These cells have been used to demonstrate a requirement for Lck in T cell/APC conjugation and T cell polarization [24]. The transfectants express low levels of surface CTLA-4 without an effect on CD28 and TCR expression, as previously described [25, 26]. Using in vitro migration assays and in vivo two-photon microscopy, we previously reported that anti-CTLA-4 over-rides the TCR/CD3-induced stop signal for motility [22].
To assess the interaction between T cells and APC in more detail using a different cell system, T8.1 WT and T8.1-CTLA-4 cells were incubated with Ttox peptide-loaded L625.7 cells and monitored for in vitro conjugation (Fig. 1A). T cells normally undergo various changes in their interaction with APC – an initial tethered-like contact over the first 1–2 min followed by an enforced contact with spreading at the contact region between the T cell and APC at 2–10 min [27, 28]. Individual T cells were monitored for the duration and frequency of an interaction over a 1200-s incubation period.
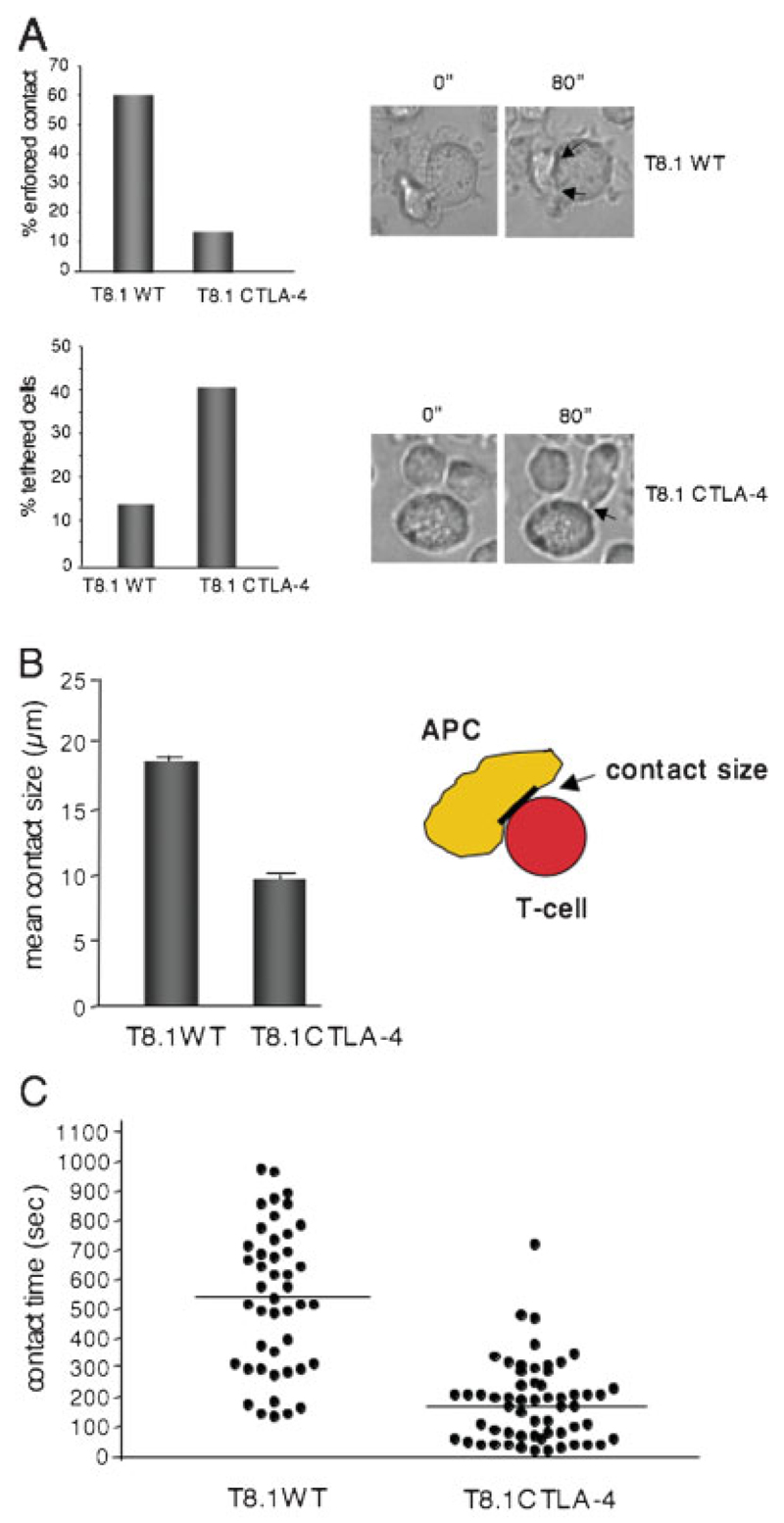
Figure 1.
CTLA-4 expression alters T8.1 T cell adhesion to APC. (A) T8.1-CTLA-4 cells fail to undergo enforced contact with APC. T8.1 WTand T8.1-CTLA-4 cells were incubated with L625.7 cells pulsed with Ttox (830–843) peptide and more than 100 cells were analyzed for tethered vs. stable interactions with APC. Upper panel, left: histogram showing the percentage of cells with enforced contact with APC. Upper panel, right: light transmission images of a typical conjugate formed between T8.1 WT cells and an APC, showing enforced contact. Lower panel, left: histogram showing the percentage of cells with tethered contact with APC. Lower panel, right: light transmission images of a typical conjugate formed between T8.1-CTLA-4 cells and an APC, showing a tethered contact. (B) T8.1-CTLA-4 and T8.1 WT cells differ in the size of the contact area at the IS. T8.1 WT and T8.1-CTLA-4 cells were incubated with L625.7 cells pulsed with Ttox (830–843) peptide and more than 100 cells were analyzed for the size of the contact area with APC. (C) T8.1-CTLA-4 cells show reduced contact times with APC. T8.1 WT and T8.1-CTLA-4 cells were incubated with L625.7 cells pulsed with Ttox (830–843) peptide (1.0 µg/mL) and at least 50 cells were monitored for the duration and frequency of binding to APC over a 1200-s incubation period. Similar results were obtained from two other independent experiments.
The first observation was that while 60% of WT cells underwent initial contact followed by an enforced contact with APC, only 10–15% of T8.1-CTLA-4 cells underwent enforced contact with APC over the 20-min incubation period (upper panel). An example of T cells developing an enforced contact at 0–80 s with APC is seen in the upper right panels. Cells with a T cell/APC contact greater than 12 µm were designated as having enforced contact. Conversely, some 15% of WT cells remained tethered without evidence of enforced contact (lower panel). By contrast, 40–45% of T8.1-CTLA-4 cells became tethered (lower panel). Cells with a T cell/APC contact less than 12 µm were designated as tethered cells. Examples of T cells with a tethered APC contact are seen in the images at 0 and 80 s in the lower right panels. These cells would often continue to move circuitously at the point of contact with the APC.
A direct measurement of the maximum length of the T cell/APC contact area confirmed that WT cells formed a more extended region of contact with APC than cells expressing CTLA-4 (i.e. 18 µm vs. 10 µm) (Fig. 1B). Similarly, WT cells showed longer-term interactions with APC than T8.1-CTLA-4 cells (Fig. 1C). Dot plot analysis showed heterogeneity in the length of time individual cells interacted with APC (i.e. from 100 to 1000 s); however, the mean time of interaction of T8.1 WT cells with APC was 540 s compared to 180 s for T8.1-CTLA-4 cells. These observations indicate that the mere expression of CTLA-4 alters the ability of T cells to stably interact with APC, a result consistent with our previous report documenting a reversal of the TCR stop signal by CTLA-4 in the same cells [22].
CTLA-4 effects on reduced T cell/APC dwell times result in reduced Ca2+ influx/mobilization and IL-2 production
The next question was whether reduced stability of T cell adhesion to APC resulted in a reduced response to Ttox (Fig. 2). For this, Fura-1/AM-loaded T8.1 WT and T8.1-CTLA-4 cells were incubated with Ttox-pulsed L625.7 cells and monitored for an increase in intracellular calcium. Transmitted light images were measured in 10-s intervals for intracellular calcium (340 and 380 nm excitation, 510 nm emission) (Fig. 2; Supporting Information Fig. 1, movies). While the addition of Ttox induced a significant Ca2+ response over 1500 s (Fig. 2A, upper movie panel and lower panel), little response was detected in T8.1-CTLA-4 cells (Fig. 2B, upper and lower panels). The level was only slightly above the basal calcium level. These data clearly indicated that the mere expression of CTLA-4 resulted in a major reduction in the rise of intracellular calcium that correlated with the less stable or shorter dwell times between T8.1-CTLA-4 cells and APC. Further correlated with this observation was a reduction in IL-2 production in response to Ttox at 24 h (Fig. 2C), which was also observed at 48 h (data not shown).
Figure 2.
CTLA-4 expression reduces calcium mobilization concurrent with impaired interaction with APC. (A) T8.1 WT cells increase intracellular Ca2+ in response to antigen. L625.7 cells pulsed with 1 µg/mL of Ttox (830–843) peptide were incubated with 1 µM Fura-2/AM-loaded T8.1 WT cells. Transmitted light images were taken every 10 s in turn with intracellular calcium measurement (340 and 380 nm excitation, 510 nm emission; pseudocolor images corresponding to 340/380 ratios). Shown are typical images extracted from movies (see Supporting Information Fig. 1). Mean calcium responses of several individual cells are shown in the lower panels for T8.1 WT (n=39) and T8.1-CTLA-4 cells (n=64). Only cells making contacts with conjugates were analyzed. Upper panel:movies showing the change in Fura-2/AM color emission for T8.1 WT cells. Lower panel: figure showing the analysis of change in levels of intracellular Ca2+. (B) T8.1-CTLA-4 cells show impaired Ca2+ response to antigen. L625.7 cells pulsed and incubated with T8.1-CTLA-4-positive cells as described above. Upper panel: movies (see Supporting Information Fig. 1) showing the change in Fura-2/AM color emission for T8.1 WT cells. Lower panel: figure showing the analysis of change in levels of intracellular Ca2+. (C) T8.1-CTLA-4 cells show impaired IL-2 production in response to Ttox. T8.1 WTand T8.1-CTLA-4 cells were co-cultured with L625.7 cells (1:1) pulsed with different concentrations of Ttox peptide. After 24 h, supernatants were taken and IL-2 production was measured by ELISA.
CTLA-4 co-ligation markedly reduces ZAP70 microcluster formation induced by anti-CD3
To assess further the function of CTLA-4 in a different context, we monitored the behavior of T8.1 WT and T8.1-CTLA-4 cells on antibody-coated glass slides as described by others [29]. For this, antibodies were attached to the surface of slides followed by the overlaying of settling cells and their observation at the interface between cell and slide surface [29]. In the presence of anti-CD3, T cells (T8.1 WT and T8.1-CTLA-4) underwent little movement when compared to resting cells (Fig. 3A, lower left histogram). By contrast, anti-CD3/CTLA-4 co-ligation allowed for extensive movement of T8.1-CTLA-4 cells. While anti-CD3-ligated cells moved slowly at less than 4 µm/min, anti-CD3/CTLA-4-ligated T8.1-CTLA-4 cells achieved velocities of 20 µm/min. Examples of the movement of individual cells are shown in XY tracker charts (upper and right panels). Serum proteins such as fibronectin may also contribute to motility. This observation confirms, using a different system (i.e. T8.1 cells on antibody-coated slides), that CD3/CTLA-4 can reverse the stop signal for T cell motility.
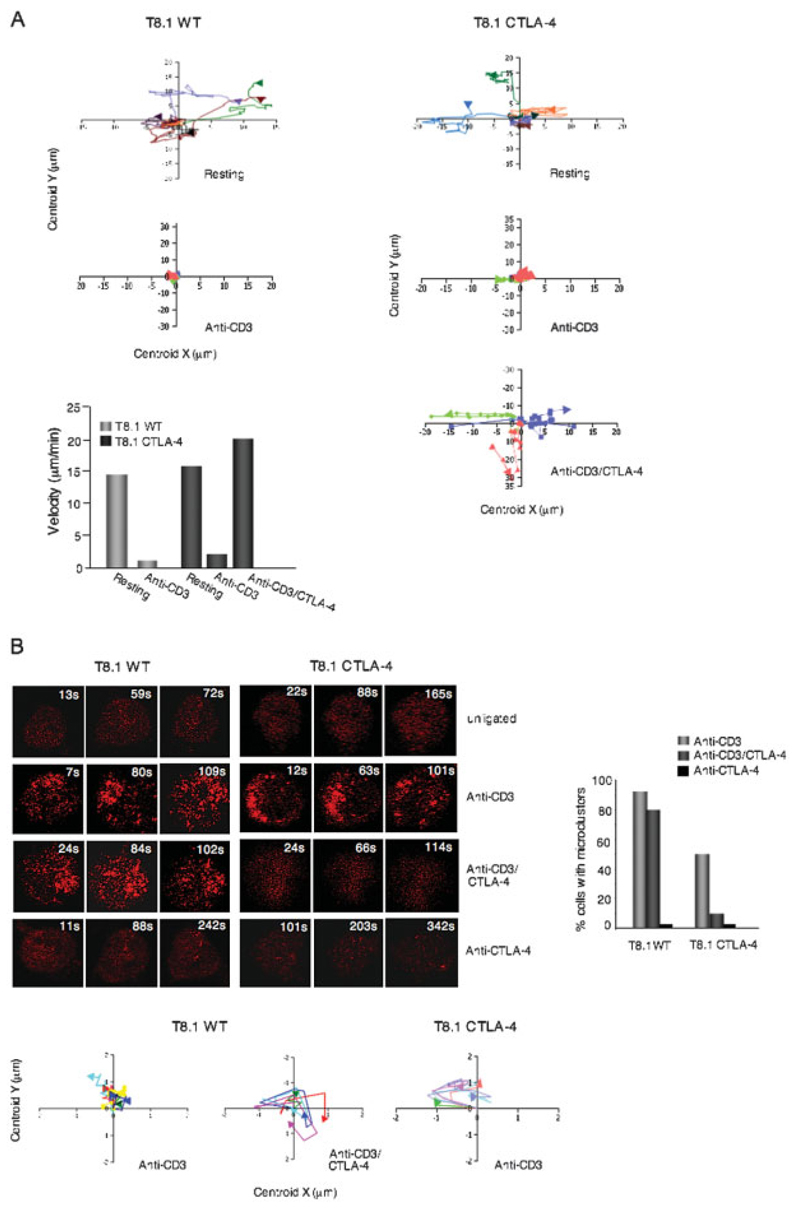
Figure 3.
CTLA-4 co-ligation increased cell motility on antibody-coated glass slides while reducing the formation of ZAP70 microclusters. (A) Upper left and right panels: XY tracker charts showing examples of movement of individual microclusters formed in T8.1 WT and T8.1-CTLA-4 cells when left untreated or stimulated with anti-CD3 or anti-CD3/CTLA-4. Lower left panel: T8.1-CTLA-4 cells show increased motility. T8.1 WT and T8.1-CTLA-4 cells transfected with mRFP-ZAP70 were plated in their culture medium on glass slides coated with anti-CD3 or anti-CD3/CTLA-4 and monitored for movement using a Zeiss LSM 510 confocal microscope. Velocity software was used for measuring velocity. (B) Left upper panel: T8.1-CTLA-4 cells show defective ZAP70 cluster formation in response to anti-CD3/CTLA-4 ligation. T8.1 WT and T8.1-CTLA-4 cells transfected with mRFP-ZAP70 were plated in their culture medium on glass culture slides coated with anti-CD3, anti-CD3/CTLA-4 or anti-CTLA-4. Cells were imaged for the formation of ZAP70 microclusters at the interface for a period of time using a Zeiss LSM 510 confocal microscope. Right panel:histogram showing the percentage of cells forming ZAP70 microclusters. Left lower panels: XY tracker charts showing microcluster movement of T8.1 WT cells treated with anti-CD3 or anti-CD3/CTLA-4, and T8.1-CTLA-4 cells treated with anti-CD3. Similar results were obtained from at least two other independent experiments.
We next then examined the well-documented ability of anti-CD3 to induce ZAP70 microcluster formation in T cells (Fig. 3B). Recent studies have documented the importance of anti-CD3-induced microcluster formation for T cell signaling [29, 30]. T8.1 WT and T8.1-CTLA-4 cells were transfected with mRFP-ZAP70 and monitored at the interface for microcluster formation using a Zeiss LSM 510 confocal microscope. Cells were monitored continuously for 7–350 s following the initial contact with the slides.
mRFP-ZAP70 was diffusely distributed throughout unligated T8.1 WT and T8.1-CTLA-4 cells (top panels). Within seconds of exposure to anti-CD3, ZAP70 microclusters formed in the majority of T8.1 WT and T8.1-CTLA-4 cells (i.e. 80–90%) (upper middle panels; right histogram). At the earlier time points (i.e. 1–100 s), the ZAP70 microclusters were located mostly at the periphery of the cells with limited movement to the center, after which they began to disappear, as previously reported [29–31]. By contrast, anti-CTLA-4 co-ligation with anti-CD3 markedly reduced ZAP70 microcluster formation in T8.1-CTLA-4 cells (lower middle right panel; histogram).
Of the 5–10% of cells that still showed some microcluster formation, both the number and size of microclusters were reduced. The majority of cells showed a pattern of diffusely dispersed mRFP-ZAP70 throughout the cell (i.e. less intense signal due to dispersion). The same pattern was observed in resting cells (top panels) and in cells ligated alone with anti-CTLA-4 (lower panels). The movement of individual microclusters within the peripheral region as shown in the XY tracker charts showed similar degrees of movement in T8.1 WT and T8.1-CTLA-4 cells (lower graphs). These findings indicated using a different system (i.e. antibodies on slides) that CTLA-4 co-ligation over-rides the anti-CD3 stop signal and further there is a marked affect in reducing ZAP70 microcluster formation.
Overall, although we previously showed that anti-CTLA-4 reverses the TCR/CD3 mediated stop signal [22], an important issue had been to determine whether this occurs in other model systems, and whether the simple expression of CTLA-4 in a given T cell can reproduce these effects. The use of blocking reagents has been complicated by the ability of CD80/86 to bind to CD28/CTLA-4/programmed death 1 ligand [32] and the uncertain effects of mono- and bivalent antibodies on CTLA-4 function. The simplicity with T8.1 WT and T8.1-CTLA-4 cells is that they differ only by the expression of CTLA-4.
From this, we showed that CTLA-4 expression causes a major change in the behavior of T8.1 T cells in the context of multiple parameters. CTLA-4 reduced the dwell times with APC and prevented the formation of reinforced contacts at the IS (Fig. 1). Instead, these cells made initial contact with APC, but continued to move and failed to form a stable interface as was observed with WT cells (Fig. 1A). They generally remained tethered, often dangling at the contact point with APC, as described with tolerized T cells [33, 34]. This in turn was correlated with a major reduction in calcium mobilization and IL-2 production (Fig. 1, 2). While an effect in early signaling events could contribute to the impairment of conjugation, the reduction in dwell times and re-inforced contact between T cells and APC also correlated well with the major impairment of calcium mobilization and cytokine production.
In addition to these effects, we showed for the first time that anti-CTLA-4 co-ligation had a major effect on the ability of anti-CD3 to induce microcluster formation (Fig. 3). Microclusters form shortly after the TCR ligation and involve the TCR complex as well as signaling mediators such as ZAP70, Src homology 2 domain-containing leukocyte protein of 76 kDa (SLP-76) and GADS. Some of these mediators exist in overlapping clusters (i.e. GADS and SLP-76), while other complexes carrying ZAP70 can transiently interact with the TCR or SLP-76 clusters [29, 30]. Peripheral microclusters can sustain signaling that is terminated in the central supramolecular activation cluster [35].
Our findings clearly showed that CTLA-4 co-ligation blocked ZAP70 microcluster formation in the majority of cells from the earliest time point and throughout the incubation period (i.e. 5–10 min). The pattern of mRFP-ZAP70 in the CTLA-4-ligated cells was comparable to the pattern observed in resting cells where the signal was evenly dispersed throughout the cells. The co-receptor therefore has an unusually potent ability to disrupt the earliest of TCR-mediated events needed for the successful propagation of signals required for the stimulation of cells. Whether this is due to the engagement of intracellular negative regulator, and/or is the result of continuous motility, is not clear. The continuous motility induced by CTLA-4 may not permit stable enough interactions with antibody on plates to allow for microcluster formation. This may be related to the steric effects of motility on stable TCR aggregation (i.e. the need for the stop signal) and re-directed signaling. Nevertheless, the ability to over-ride the stop signal is consistent with the observed effect of the coreceptor in limiting conjugation times, inhibiting ZAP70 microcluster formation as well as calcium mobilization in the antigen presentation system. These effects are consistent with a role for CTLA-4 in anergy and tolerance induction [9]. Although the basis for the effect of anti-CTLA-4 on motility is not known, the polarization involves phosphatidylinositol 3-kinase, Vav-1, Cdc42, and myosin light chain kinase [36].
While there are advantages and disadvantages in examining CTLA-4 function in T8.1 cells relative to other cells, one advantage was the lack of involvement of regulatory T cells (Treg). None of the T8.1 cells express Foxp3 (data not shown) and yet they behave in ways comparable to separated CTLA-4-positive primary T cells that include the possible presence of Treg [37, 38]. Our findings indicate that CTLA-4 can influence T cell/APC interactions and motility independently of Treg involvement. Future studies will be needed to establish whether the formation of other microclusters is affected by CTLA-4, and relative effects of intracellular signaling vs. motility on the process.
Concluding remarks
Our previous studies have shown that T cell motility and the TCR-induced stop signal can be reversed by CTLA-4 [22]. Our present study shows that the mere expression of CTLA-4 on T8.1 cells in the context of CD80/CD86-expressing APC markedly changed the ability of T cells to form stable, enforced contacts with APC and reduced the contact area at the IS as well as Ca2+ influx/mobilization and IL-2 production. Further, anti-CD3/CTLA-4 co-ligation by antibody on glass slides led to increased cell motility and disrupted ZAP70 microcluster formation that was otherwise induced by anti-CD3. Collectively, these findings further support the notion that CTLA-4 limits the dwell times and the formation of microclusters needed for optimal T cell activation.
Materials and methods
Cells and reagents
The murine hybridoma T8.1 expresses a chimeric human-mouse TCR specific for Ttox (830–843) peptide restricted by HLA-DRB1*1102. The DR-expressing murine fibroblasts L625.7 used as APC also express endogenous B7-1/2 and ICAM-2 (gifts from O. Acuto, Oxford University, Oxford, UK). Stable T8.1-CTLA-4 transfectants were generated using the human CTLA-4 gene in the pSRa vector together with pBABE containing a puromycin-resistant gene [25]. Cells were maintained in DMEM with 10% FCS, 2 mM L-glutamine, supplemented with 400 nM methotrexate, 1 mg/mL G418, and 50 µM 2-ME. L625.7 cells were cultured in complete DMEM containing 250 µg/mL G418. Anti-mouse CD3 (145-2C11) was obtained from American Type Culture Collection. Anti-human CTLA-4 (BNI3) was kindly provided by B. Broeker (Greifswald, Germany). Poly-L-lysine was purchased from Sigma, chambered coverglass from Nalge Nunc. pMXs-mZAP70-mRFP was a kind gift from T. Saito (Japan).
Single-cell calcium measurements and video imaging
L625.7 fibroblasts (3.5×105/mL) pulsed with 1 µg/mL of Ttox (830–843) peptide were plated in their culture medium on glass coverslips mounted in 25-mm Petri dishes and incubated at 37°C. T8.1 WT and T8.1-CTLA-4 cells (3.5 ×105) were incubated for 20 min at 37°C with 1 µM Fura-2/AM in 1 mL of 10 mM HEPES buffer, pH 7.5, containing 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 0.5 mM MgCl2,1 mM Na2HPO4 and 1 mg/mL glucose, washed and added to fibroblasts in a final volume of 200 µL. After 2 min to allow the T cells to deposit, acquisition was performed at 37°C on an Eclipse TE300 inverted microscope as mentioned above. Transmitted light images were taken every 10 s in turn with intracellular calcium measurement (340 and 380 nm excitation, 510 nm emission) images. Movies were made with the Metafluor software.
T cell/APC conjugation
L625.7 and T8.1 cells were treated and cultured as described above. Transmitted light images were acquired every 10 s during 20 min. T cell/APC interactions were then monitored by ImageJ software.
Imaging of cells on antibody-coated chambered glass culture slides
Poly-L-lysine-treated chambered glass culture slides were coated with 5 µg/mL anti-CD3 and/or 20 µg/mL anti-CTLA-4 antibodies for 1 h at 37°C to generate antigenic surfaces. Nonspecific binding sites on the glass surface were blocked with RPMI 1640 containing 10% FCS for 10 min prior to imaging. Cells were imaged for the interface using a Zeiss LSM 510 confocal microscope. The temperature of the sample was maintained at 37°C.
IL-2 measurement
T8.1 WT and T8.1-CTLA-4 cells were co-cultured with L625.7 cells (1:1) pulsed with different concentrations of Ttox peptide. After 24 h, IL-2 production was measured by ELISA (PharMingen).
Statistical analysis
Results are given as the mean ± the standard error of the mean. Statistical analysis was performed using either two-tailed unpaired Student’s t-test or one-way ANOVA using GraphPad Prism; p value <0.05 was considered as significant.
Supplementary Material
Supporting information for this article is available at http://www.wiley-vch.de/contents/jc_2040/2007/37423_s.html
Acknowledgements
C. E. Rudd was supported by a Programme Grant and holds a Principal Research Fellow Award (PRF) from the Wellcome Trust. H. Schneider is supported by a grant from the BBSRC (UK).
Abbreviations
- IS
immunological synapse
- SLP-76
Src homology 2 domain-containing leukocyte protein of 76 kDa
- Ttox
tetanus toxin
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Linsley PS. Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule 4 receptor during T-cell activation. J Exp Med. 1995;182:289–292. doi: 10.1084/jem.182.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone J. New perspectives of CD28-B7 mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 4.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA-4 co-receptor signaling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 5.Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 6.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 7.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Stockton J, Mathis D, Benoist C. Modeling CTLA4–linked autoimmunity with RNA interference in mice. Proc Natl Acad Sci USA. 2006;103:16400–16405. doi: 10.1073/pnas.0607854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 10.Ueda H, Howson J, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow D et al. Association of the T-cell regulatory gene CTLA-4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 11.Masteller EM, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 12.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Chuang E, Fisher TS, Morgan RW, Robbins MD, Duerr MG, Vander Heiden JP, Gardner JE, et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity. 2000;13:313–322. doi: 10.1016/s1074-7613(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee KM, Chuang E, Griffin M, Khattri R, Hong DK, Zhang W, Straus D, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 15.Marengere LEM, Waterhouse P, Duncan GS, Mittrücker H-W, Feng G-S, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase Syp association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 16.Chikuma S, Imboden JB, Bluestone JA. Negative regulation of T cell receptor-lipid raft interaction by cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2003;197:129–135. doi: 10.1084/jem.20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlington PJ, Baroja ML, Chau TA, Siu E, Ling V, Carreno BM, Madrenas J. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 potently inhibits cell surface raft expression in its regulation of T cell function. J Exp Med. 2001;194:1675–1681. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudd CE, Martin M, Schneider H. CTLA-4 negative signaling via lipid rafts: A new perspective. Sci STKE. 2002;128:PE18. doi: 10.1126/stke.2002.128.pe18. [DOI] [PubMed] [Google Scholar]
- 20.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2004;105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- 21.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 22.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 23.Blank U, Boitel B, Mege D, Ermonval M, Acuto OE. Analysis of tetanus toxin peptide/DR recognition by human T cell receptors reconstituted into a murine T cell hybridoma. J Immunol. 1993;23:3057–3065. doi: 10.1002/eji.1830231203. [DOI] [PubMed] [Google Scholar]
- 24.Donnadieu E, Lang V, Bismuth G, Ellmeier W, Acuto O, Michel F, Trautman A. Differential roles of Lck and Itk in T cell response to antigen recognition revealed by calcium imaging and electron microscopy. J Immunol. 2001;166:5540–5549. doi: 10.4049/jimmunol.166.9.5540. [DOI] [PubMed] [Google Scholar]
- 25.Schneider H, Martin M, Agarraberes FA, Yin L, Rapoport I, Kirchhausen T, Rudd CE. Cytolytic T lymphocyte-associated antigen-4 and the TcRz/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol. 1999;163:1868–1879. [PubMed] [Google Scholar]
- 26.Da Rocha Dias S, Rudd CE. CTLA-4 blockade of antigen-induced cell death. Blood. 2001;97:1134–1137. doi: 10.1182/blood.v97.4.1134. [DOI] [PubMed] [Google Scholar]
- 27.Bismuth G, Trautmann A. The immunological synapse: Models facing facts. Med Sci (Paris) 2006;22:721–726. doi: 10.1051/medsci/20062289721. [DOI] [PubMed] [Google Scholar]
- 28.Dustin ML, Carpen O, Springer TA. Regulation of locomotion and cell-cell contact area by the LFA-1 and ICAM-1 adhesion receptors. J Immunol. 1992;148:2654–2663. [PubMed] [Google Scholar]
- 29.Bunnell SC, Hong DL, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 31.Moss WC, Irvine DJ, Davis MM, Krummel MF. Quantifying signaling-induced reorientation of T cell receptors during immunological synapse formation. Proc Natl Acad Sci USA. 2002;99:15024–15029. doi: 10.1073/pnas.192573999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinselmeyer BH, Dempster J, Gurney AM, Wokosin D, Miller M, Ho H, Millington OR, et al. In situ characterization of CD4+ T cell behavior in mucosal and systemic lymphoid tissues during the induction of oral priming and tolerance. J Exp Med. 2005;201:1815–1823. doi: 10.1084/jem.20050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei B, da Rocha Dias S, Wang H, Rudd CE. CTL-associated antigen-4 ligation induces rapid T cell polarization that depends on phosphatidylinositol 3-kinase, Vav-1, Cdc42, and myosin light chain kinase. J Immunol. 2007;179:400–408. doi: 10.4049/jimmunol.179.1.400. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 38.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.