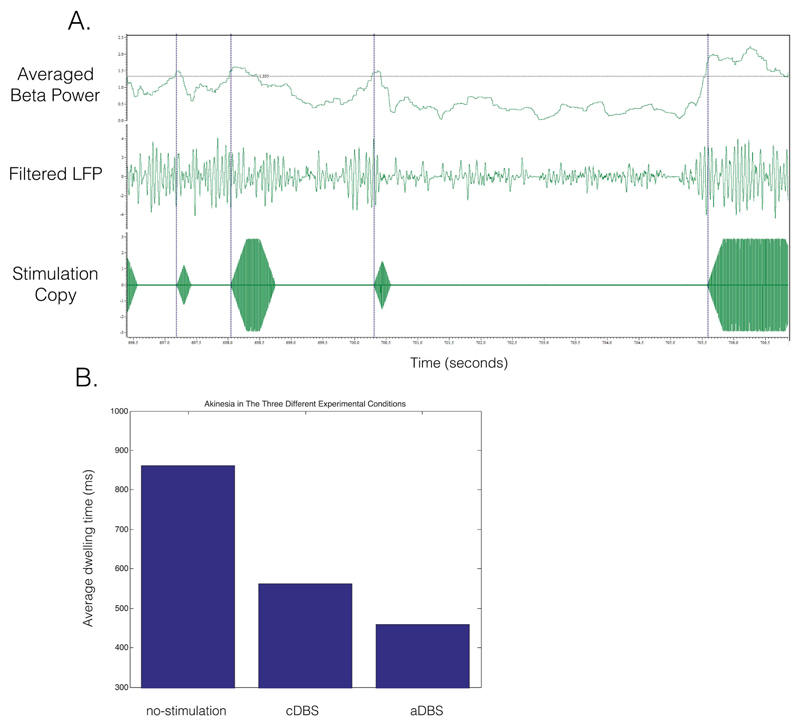
Preliminary evidence from patients with Parkinson’s disease (PD) suggests that deep brain stimulation (DBS) might work better, more efficiently, and with fewer side effects when applied in an adaptive manner (aDBS)1–4 In each of these studies aDBS was delivered according to the amplitude of beta oscillations (13-30 Hz) in the subthalamic nucleus (STN), which itself has been shown to correlate with contralateral akinesia and rigidity (AR).5 The key limitations in these clinical aDBS studies are that they have been conducted in the immediate postoperative phase. In this period, clinical testing and stimulation titration are complicated by the “stun” effect, and the optimal chronic DBS settings are not yet known. Furthermore, AR has thus far only been assessed with ordinal clinical scores, a limited and subjective rating system. To circumvent these limitations, we applied aDBS in a PD patient who had been chronically implanted with DBS and already titrated to optimal stimulation parameters and assessed bradykinesia with a validated digital task. aDBS was applied during battery replacement surgery in a 68-year-old patient with a 27-year history of Parkinson’s disease who had been implanted with bilateral STN electrodes for 14 years. The patient gave consent to the research protocol, which was approved by the local ethics committee. Bipolar local field potential (LFP) amplitude in the beta range ± 3 Hz was used as biomarker in such a way that conventional DBS was provided only when beta amplitude exceeded an estimated median value (for methods see references 1, 3, and 4). aDBS was applied for 12 minutes to the right STN (contralateral to the most affected side) with matched stimulation parameters to optimized conventional DBS (cDBS), namely, 2.8V, 60-microsecond pulse width, and 135 Hz (with 250-millisecond ramping at onset and offset). Bradykinesia was assessed using a tablet-based version of the validated bradykinesia, akinesia, incoordination (BRAIN) task.6 The BRAIN task assesses the velocity of alternating finger movements between 2 buttons and provides an average “dwell time” (milliseconds). Bradykinesia assessments were performed following dopaminergic withdrawal for stimulation conditions OFF (OFF-stim) and during the application of aDBS and cDBS. Bipolar LFP amplitude of the right STN showed a marked peak around 20 Hz that was used as a feedback signal for aDBS (Fig. 1a). aDBS was well tolerated and only induced transient contralateral paresthesia in the left hand. The average dwell times were compared for the 3 stimulation conditions and found to be lowest for aDBS compared with cDBS and OFF-stim (449, 556, and 861 milliseconds respectively; Fig. 1b).
Fig. 1.
(a) Adaptive DBS (aDBS) testing in a chronically implanted Parkinson’s patient. Upper channel shows the averaged beta power over 400-millisecond episodes (moving average). The black line demarcates the threshold for applying stimulation. The middle channel shows the LFP filtered around the dominant beta frequency (20 ± 3 Hz). The bottom channel shows the stimulation copy that starts and ends with a 250-milliseconds ramping period. The dotted blue lines illustrate the relation between the increase in beta power and the start of stimulation. (b) Average dwell time on a tablet tapping task during nostimulation, continuous DBS (cDBS), and aDBS. [Color figure can be viewed at wileyonlinelibrary.com]
Here we present the first case of aDBS in a parkinsonian patient with chronic STN-DBS treatment with parameters matched to optimized cDBS. We conclude that aDBS (1) can be applied in the chronically implanted DBS phase and (2) is at least as effective as cDBS when objectively assessed. With the development of implantable aDBS hardware capable of sensing and stimulating,7 similar studies may soon be conducted outside the operating theater, which would enable more extensive testing.
Footnotes
Relevant conflicts of interest/financial disclosures: All authors reports no disclosures concerning this research.
References
- 1.Little S, Pogosyan A, Neal S, et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann Neurol. 2013;74:449–457. doi: 10.1002/ana.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosa M, Arlotti M, Ardolino G, et al. Adaptive deep brain stimulation in a freely moving parkinsonian patient. Mov Disord. 2015;30:1003–1005. doi: 10.1002/mds.26241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little S, Beudel M, Zrinzo L, et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2016;87:717–721. doi: 10.1136/jnnp-2015-310972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little S, Tripoliti E, Beudel M, et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J Neurol Neurosurg Psychiatry. 2016;87(12):1388–1389. doi: 10.1136/jnnp-2016-313518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beudel M, Oswal A, Jha A, et al. OFF-state oscillatory beta power correlates with contralateral bradykinesia-rigidity in the parkinsonian STN. Mov Disord. 2017;32(1):174–175. doi: 10.1002/mds.26860. [DOI] [PubMed] [Google Scholar]
- 6.Noyce AJ, Nagy A, Acharya S, et al. Bradykinesia-akinesia incoordination test: validating an online keyboard test of upper limb function. PLoS One. 2014;9:e96260. doi: 10.1371/journal.pone.0096260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malekmohammadi M, Herron J, Velisar A, et al. Kinematic adaptive deep brain stimulation for resting tremor in Parkinson’s disease. Mov Disord. 2016;31:426–428. doi: 10.1002/mds.26482. [DOI] [PubMed] [Google Scholar]